Abstract
Remote ischemic conditioning (RIC) is a promising therapeutic approach for ischemic stroke patients. It has been proven that RIC reduces infarct size and improves functional outcomes. RIC can be applied either before ischemia (pre-conditioning; RIPreC), during ischemia (per-conditioning; RIPerC) or after ischemia (post-conditioning; RIPostC). Our aim was to systematically determine the efficacy of RIC in reducing infarct volumes and define the cellular pathways involved in preclinical animal models of ischemic stroke. A systematic search in three databases yielded 50 peer-review articles. Data were analyzed using random effects models and results expressed as percentage of reduction in infarct size (95% CI). A meta-regression was also performed to evaluate the effects of covariates on the pooled effect-size. 95.3% of analyzed experiments were carried out in rodents. Thirty-nine out of the 64 experiments studied RIPostC (61%), sixteen examined RIPreC (25%) and nine tested RIPerC (14%). In all studies, RIC was shown to reduce infarct volume (− 38.36%; CI − 42.09 to − 34.62%) when compared to controls. There was a significant interaction caused by species. Short cycles in mice significantly reduces infarct volume while in rats the opposite occurs. RIPreC was shown to be the most effective strategy in mice. The present meta-analysis suggests that RIC is more efficient in transient ischemia, using a smaller number of RIC cycles, applying larger length of limb occlusion, and employing barbiturates anesthetics. There is a preclinical evidence for RIC, it is safe and effective. However, the exact cellular pathways and underlying mechanisms are still not fully determined, and its definition will be crucial for the understanding of RIC mechanism of action.
Similar content being viewed by others
Introduction
Acute ischemic stroke (AIS) is the world’s second leading cause of mortality and the major cause of disability in adults worldwide1. The main revascularization therapies for AIS are thrombolysis with recombinant tissue-plasminogen activator (tPA) and endovascular thrombectomy. Unfortunately, many patients cannot benefit from those therapies due to mainly narrow therapeutic window and they can also induce ischemia–reperfusion injury (IRI)2,3. The development of novel therapeutic strategies is needed to extend therapeutic windows and to mitigate further brain injury.
Neuroprotective therapies have a great potential to not only increase the benefits of available reperfusion therapies but also to provide an advisable medical procedure for AIS patients who are not eligible for current treatments4. But translation of strategies targeting neuroprotection to the clinical practice has failed so far, despite extensive clinical trials5. In this scenario, a promising therapeutic approach, but insufficient traveled avenue, is the remote ischemic conditioning (RIC)6. Murry et al. first introduced the ischemic preconditioning therapy in a canine myocardial infarction model in 19867. Since then, it has been repeatedly confirmed in animal models that ischemic preconditioning is a powerful endogenous protective strategy against IRI of multiple organs, including heart, brain and kidneys8. A significant breakthrough was the discovery that ischemic conditioning induction to a remote organ from the site of severe ischemia can also protect target tissue9. RIC consists of brief episodes of ischemia/reperfusion (I/R) in a distant organ, such as a limb, that can provide protection to the ischemic brain. It can be applied either before ischemia (pre-conditioning; RIPreC), during ischemia (per-conditioning; RIPerC) or after ischemia (post-conditioning; RIPostC) in a very simple way by using a blood pressure cuff on an arm. RIC is a safe, inexpensive, feasible, well tolerated, simple and harmless therapy for stroke; so, it has practical value10. The protective effect of RIC may be mediated by cellular mechanisms that counteract numerous aspects of stroke pathogenesis11. However, the specific underlying mechanisms contributing to RIC are complex and remain poorly understood12.
The interest of RIC in AIS has emerged in the last years. Three clinical trials have evaluated different strategies of RIC among AIS with mixed results13,14,15. In parallel, several clinical trials are now ongoing to investigate the efficacy of RIC in patients with acute stroke16. This systematic review and meta-analysis investigated basic preclinical studies of RIC in animal models of cerebral ischemia. Our aim was to elucidate the overall effects and variability of RIC on infarct volume in preclinical animal models compared with control group (no RIC application). Mainly, there are a number of unanswered questions: type of RIC application, number of limbs where RIC should be applied, number and length of RIC cycles of limb I/R and anesthetic used prior ischemia. We therefore identify the most suitable animal model to study the phenomenon of RIC and propose combinational scenarios with drugs that can amplify the beneficial effect of RIC. Finally, an updated of cellular pathways involved on RIC’s types was also performed.
Material and methods
The systematic review protocol was registered on PROSPERO (CRD42020221321). The review protocol was prepared according to the preferred reporting items for systematic review and meta-analysis protocols statements (PRISMA-P)17. The systematic review report was written following the Preferred Reporting Items for Systematic review and Meta-Analysis (PRISMA) guidelines and the PRISMA checklist18. The search was first carried out in March 2020 and was repeated for an update by the first author in March 2021, in PubMed, SCOPUS and Web of Science (WOS) databases.
The search terms, strategy, and selection criteria are based on the PICOS system19 and were adapted to each database. PICOS-Parameters inclusion criteria were based on Population (animal models of ischemic stroke), Intervention (RIC: pre, per, post), Compare (RIC protocols/control/sham), Outcome (infarct volume), and Study design (experimental groups).
Articles were obtained by concatenating terms with boolean operators as follows: (“remote ischemic conditioning” OR “RIC” OR “limb ischemic conditioning” OR “Remote ischemic postconditioning” OR “remote postconditioning” OR “remote ischemic perconditioning” OR “remote ischemic preconditioning”) AND (“animal model” OR “mice” OR “rat”) AND (“stroke” OR “ischemic stroke” OR “ischaemia” OR “cerebral ischemia”). Studies were included from 2010 until present.
All studies were considered eligible if they investigated the effect of limb RIC (pre, per, or post) on cerebral ischemia animal models. No restrictions on species were applied. Studies were excluded if they did not investigate cerebral ischemia or did not apply RIC to a limb. Furthermore, studies were excluded if they specifically investigated hemorrhagic stroke model, if they applied RIC in humans and if the animal models had co-morbidities or risk factors. Reports were excluded if they were not available in English nor published in a peer-reviewed journals. Abstract articles, review articles, letters, proceedings paper or book chapters were also excluded.
One author (CT-Q) screened the title and abstract of each paper. After the screening, full texts were evaluated. For each study the following information when available was extracted: intervention, animal, gender, age, weight, animal model of ischemia, duration of ischemia, anesthetic used prior to ischemia, anesthetic used during RIC, RIC protocol, when RIC was started, RIC organ, outcomes reported, main pathway investigated and reference. For any missing or unclear data, the corresponding authors were contacted by e-mail to obtain the missing details.
CT-Q extracted the data from the selected studies. Data were manually entered into a Microsoft Excel spreadsheet (Version 14.0, 2010, Microsoft Corp., Redmond, California, USA); then reviewed, discussed and adjusted in accordance with the two reviewers (GA, FP). If needed, a consensus meeting and discussion resolved disagreement.
Data analysis
All outcomes were transformed into effect sizes by using the studies’ reported statistics, mean and standard deviation or standard error, or results from analyses including t-tests, analysis of variance, correlations, regressions, and linear mixed-effects models.
The primary outcome was defined as the percentage of volume infarct reduction between RIC and control groups. The meta-analysis was conducted using the packages ‘tidyverse’, ‘meta’, ‘metafor’ and ‘dmetar’ of the R 4.0 software20. Studies presenting mean infarct size with standard deviation (SD) or standard error of mean (SEM) values in both intervention and control groups, were extracted for the meta-analysis. The effect size included was the difference in the mean percentage change (control–intervention), presented as the mean percentage change (95% confidence interval) in the infarct size of intervention group with respect to controls. As the SEM of the difference in percentage change was not reported, we first calculate the SD for each group (SEM*√n) to obtain the SEM of the difference (√[(SD12/n1) + (SD22/n2)]). Since the characteristics and methods of the interventions used in the studies are different, a random-effects model with the inverse variance method was performed to calculate the mean effect size. Forest plots were performed to show individual and global effect sizes.
Statistical heterogeneity across studies was evaluated using the Cochran's Q test and I2 statistic. I2 estimates the percentage of variation between all studies that is due to heterogeneity rather than chance; I2 > 50% is considered as substantial heterogeneity. The function find.outliers of the 'dmetar' package was used to explore for possible outliers and the function InfluenceAnalysis was used to detect studies with a high influence on the overall results.
A Baujat Plot was performed to plot the overall heterogeneity contribution and the influence on pooled results for each study in the meta-analysis. As heterogeneity was highly presented in the study and we performed meta-regressions and subgroup analyses to explore the effects of the different characteristics on the percentage change in infarct volume. Q statistic was used to assess difference in the subgroup analysis and random-effects linear regression models were performed to assess correlations with quantitative variables. The bubble function of the package 'meta' was used to plot meta-regression outputs.
Some quantitative variables (duration of cerebral ischemia, number of cycles and cycle duration) were divided into groups to have a different approach in a subgroup analysis. The analysis was also stratified by animal species, running separate meta-analyses for checking if the effects of some characteristics on the infarct size were different according to the animal tested.
Ethical statement
This article does not contain any studies with human participants or animals performed by any of the authors.
Results
The results of the retrieved literature and selection process are presented in Fig. 1. The initial search identified 286 studies, of which 124 duplicates were removed, leaving 162 studies. After screening by title and abstract, 102 articles were rejected when exclusion criteria were applied: hemorrhagic stroke, articles not related to cerebral ischemia or limb RIC, articles related to humans, animal models with comorbidities, articles not available in English, review articles, letters, proceedings paper and a book chapter. The full text of the remaining 60 articles were read and 10 studies were excluded because there was no available data. Finally, fifty studies were included in the meta-analysis with data on 64 experiments.
The key characteristics of the included studies are presented in Table 1. Sixty-one of the 64 (95.3%) experiments we carried out in rodents: 52 used rats (81.2%) and nine studied mice (14.1%). Primates were only used in three experiments (4.7%). Almost all studies were performed in young and healthy animals. The majority of the studies used solely male (93.7%) animals and 6.3% of studies used solely female animals. The majority of publications induced transient focal cerebral ischemia (87.5%) with 20–120 min of middle cerebral artery occlusion (MCAo). Most studies included in this meta-analysis induced cerebral ischemia by intraluminal filament (84.3%) and seven studies used permanent models of middle cerebral artery (10.9%).
The most commonly RIC protocol employed was three to four repetitions of 5–15 min I/R using a pressure cuff, applied on one (37.5%) or two (60.9%) limbs to observe a neuroprotective effect. Thirty-nine of the 64 experiments studied RIPostC (61%), sixteen examined RIPreC (25%) and nine tested RIPerC (14%). In 58 studies, RIC was performed as a single application (90.6%) and six studies employed multiple applications (9.4%). The anesthetic used varied between studies, being chloral hydrate the most used (36%) (Table 1).
Infarct volume’s dependent factors
The meta-analysis included data from 941 animals (779 [82.8%] rats, 138 [14.7%] mice, 24 [2.6%] monkeys), 468 (49.7%) animals were control and 473 (50.3%) animals that underwent RIC. A random effect model showed that RIC was significantly effective when compared to control group (− 38.36%; 95% CI − 42.09 to − 34.62%; 95% PI [prediction interval], − 64.46 to − 12.25%; p ≤ 0.0001) (Fig. 2). However, high heterogeneity between studies was detected (I2 = 90.1%; Q = 635.72, df = 63, p < 0.0001). The variance of the distribution of the effect sizes in this samples was T2 = 167.06 (Table 2). Figure 3A presented results of the influence analysis. The study that contributed to a higher heterogeneity21 and the most influential study on the overall results22 were identified (Fig. 3A).
Forest plot to illustrate the efficacy of remote ischemic conditioning on infarct volume by animal model from 64 analyzed experiments. Forest plot of mean difference (MD) and their 95% CI for individual trials determined from the result of 64 trials comparing the effect of remote ischemic conditioning with control on infarct volume. Studies are grouped by species. The solid vertical line represents a mean difference of 0 or no effect. Points to the left of the line represent a reduction in infarct volume, and points to the right of the line indicate an increase. Each square around the point effect represents the mean effect size for that study and reflects the relative weighting of the study to the overall effect size estimate. The larger the box, the greater the study contribution to the overall estimate. The weight that each study contributed is in the right-hand column. MD mean difference, CI confidence interval.
Impact of studied factors on infarct volume evaluated by meta-analysis comparisons of all included species. (A) The Baujat plot shown which studies contributed to greater heterogeneity76 and what were the most influential studies on the overall result48. (B) Duration meta-regression graph. There was not greater reduction in volume to longer duration of cerebral ischemia (p = 0.465). (C) Number of cycles meta-regression graph. There was less reduction in volume with a greater number of cycles (p < 0.001). (D) Duration of cycles (min) meta-regression graph. There was a greater reduction in volume as the duration of the cycles increases (p = 0.0165). (E) Conditioning start time meta-regression graph. There was no significant volume reduction based on conditioning onset time (p = 0.205).
Subgroup analysis and meta-regression was performed using random-effects model. No significant differences were observed on the type of intervention when all species were analyzed: RIPreC (− 36.2%; 95% CI − 43.4 to − 29.1%), RIPerC (− 39.7%; 95% CI − 45.7 to − 33.7%) and RIPostC (− 38.8%; 95% CI − 44.3 to − 33.3%) (p = 0.709 between groups). In mice, the major effect was significantly observed in RIPreC (− 48.4%; 95% CI − 77.4 to − 19.5%; p < 0.001). In contrast to studies performed on rats and mice, in the three studies performed on monkeys RIPostC showed a tendency to increase the infarct volume (+ 4.4%; 95% CI − 1.96%, + 10.74%, p = 0.097). The reduction in infarct size was significantly higher in transient ischemia studies (− 40.8%; 95% CI − 44.2 to − 37.5%) than in permanent ischemia studies (− 16%; 95% CI − 33.8 to + 1.9%) (p < 0.001). However, the duration of ischemia did not show a time-dependent effect (− 0.042%; 95% CI − 0.156 to + 0.072%; p = 0.465) (Fig. 3B).
Infarct size was significantly increased when a higher number of RIC cycles were applied (+ 5.817; 95% CI + 3.571 to + 8.064%; p < 0.001) (Fig. 3C). When all species (mice, rats, monkeys) were considered, volume was significantly reduced when the cycle duration increased (− 1.282%; 95% CI − 2.321 to − 0.242%; p = 0.016) (Fig. 3D). However, in mice the observation was the opposite: studies that applied 10-min RIC cycles described higher volume reduction than studies that used 5-min RIC cycles.
When RIC was applied to one limb (− 39.1%; 95% CI − 45.7 to − 32.5%) the effect was similar to when it was applied to two limbs (− 38.8%; 95% CI − 43.2 to − 34.5%). Only in one study, which used monkeys, RIC was applied to four extremities (+ 5.0%; 95% CI − 18.4 to + 28.3%)23. The results of this study within the meta-analysis showed significant differences (p = 0.013) regarding the number of limbs. However, if this study was not included in the meta-analysis, differences were not observed on the variable number of limbs (p = 0.604). Initiation of RIC was not related with the infarct volume reduction (− 0.461%; 95% CI − 1.180 to − 0.258%; p = 0.205) (Fig. 3E). Finally, significant sex-differences were observed in experiments performed on rats but not on mice. Experiments performed on male animals obtained higher proportion of volume reduction than experiments performed on female animals (− 41.9%; 95% CI − 45.6 to − 38.2% vs. − 28.8%; 95% CI − 44.8 to − 12.8%; p = 0.002), which it might indicate a different RIC’s mechanism of action by sex.
Effect of anesthetic on infarct volume reduction
Up to nine different anesthetic strategies were used in the experiments. Among them, chloral hydrate was used in 22 experiments and isoflurane was used in 20, both were the most represented. The combination of ketamine and propofol was only used in monkey experiments. Significant differences were observed in experiments performed on mice (p < 0.001), due to anesthetic strategy. In addition, when we compared the two most frequent used types of anesthesia, chloral hydrate (− 43.37%; 95% CI − 48.73 to − 38.00%) showed a greater infarct volume reduction than isoflurane (− 34.76%; 95% CI − 40.48 to − 29.05%, (p = 0.022).
Pathophysiology of RIC effects
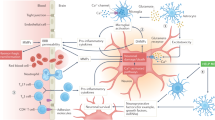
Figure 4 illustrates the schematic representation of suggested underlying mechanisms of RIPreC, RIPerC and RIPostC. Selected studies had also described molecular and cellular processes involved on RIC. Diagram showed the different mechanisms grouped by cellular processes related with ischemic damage: oxidative stress, inflammation, hemodynamics, immune response, autophagy, and apoptosis. However, many molecular pathways were described, none was translated to humans. Special consideration should be given to four spots where no data was reported: no autophagic pathway was related to RIPreC and RIPerC molecular underlying mechanisms were not described on apoptosis, oxidative stress, and immune response.
RIPreC would decrease oxidative stress through the release of endothelin-1 and the increase of H2S, Nrf2, HIF-1α, SOD1 and HO124,25,26,27,28. It would also reduce neuroinflammation by modulating the expression of HIF-1α, HIF-2α and activating the Notch1 and NF-KB pathways25,29,30. Apoptosis has been shown to be reduced when preconditioning is applied by regulating the JAK2/STAT3 signalling pathway31. Also, an improvement of brain edema and downregulation of the expression of AQP4 is observed32,33. Several studies have shown that RIPreC modulates the immune response decreasing the levels of IL-10, IL-6 and TNFα in the blood34,35,36. RIPerC would inhibit the autophagy process by increasing Bcl-2 phosphorylation37,38,39, decrease inflammation through incrementing Notch and NICD expressions40 and increase collateral circulation41,42,43,44. Finally, RIPostC would decrease brain edema and blood–brain barrier permeability via upregulating eNOS, decreasing MMP-9 and increasing claudin-5 expression41,45,46,47,48,49. Multiple preclinical studies have shown that RIPostC could reduce oxidative stress through upregulation of Nrf2 along with HO1, NQO1 and Parkin/Dj-150,51,52,53. RIPostC has been shown to protect against ischemic injury by downregulating proinflammatory pathways22,23,54,55,56,57,58 and improving the peripheral immune response36,59. Diverse mechanisms have been proposed for RIPostC-mediated autophagy, including increase of AKT/GSK3β-dependent activation, induction the mitophagy via up-regulation of Parkin/DJ-1 proteins expression and activation of the mTOR/p70S6K signaling pathway53,60. Other studies demonstrated that RIPostC treatment upregulate Bcl-2 and heat-shock protein 70 (HSP70) expression and downregulate Bax expression, attenuating apoptosis21,46,54,61,62,63.
Discussion
This systematic review and meta-analysis summarized the evidence on the protective effects of RIC on infarct volume in preclinical stroke models. A total of fifty studies with data on 64 experiments were included, which involved 941 animals. In all studies, the reduction in infarct volume in RIC groups compared to control was 38.4%. Our results suggested that RIC is more efficacious in transient than permanent ischemia, applying a smaller number of RIC cycles, using a RIC cycle length of ≥ 15 min, using one or two limbs, employing barbiturates anesthetics and in male animals.
The majority of papers in this review used rodents, predominately rats. Despite being the most applicable animal models for research related to stroke, the demand for larger models, such as rabbits and even nonhuman primates, is increasing to better understand the disease and RIC mechanism of action64.
Most RIC studies used transient focal cerebral ischemia with intraluminal suture stroke model because it closely mimics the human ischemic stroke65. The optimal conditioning protocol for RIC to elicit organ protection remains unknown. Less than three cycles or more than 15 min of treatment intensity can have a significant role in ischemic neuroprotection. However, more than three ischemic cycles or cycles < 5 min did not have such a neuroprotective effect. The present evidence suggests that there may be a minimum threshold value for the neuroprotective effect of RIC. RIC was beneficial in all three temporal variants after its initial application: RIPreC, RIPerC and RIPostC. Despite this, RIC was found most effective when delivered after stroke injury (RIPostC) followed by the application during stroke (RIPerC). Both approaches are suitable to be translated to patients, where RIC would be applied during ambulance transportation once admission at the emergency room is done or during the first 24 h after the stroke. The preclinical evidence supported the current clinical trials on-going on RIPerC and RIPostC. Interestingly, the reduce in infarct size is related to neurological functional improvement.
Most studies performed RIC as a single application. A single bout of RIC activates at least 2 distinct time frames of neuroprotection against I/R injury of the brain. The initial neuroprotection is short-lasting (2 h) and occurs immediately after RIC66. The delayed form of neuroprotection reappears after 12–24 h and lasts 48–72 h67. In addition to the short-lasting benefits of a single bout of RIC, long-term benefits may be induced with repeated daily conditioning54. A limited number of studies have explored the effect of repeated RIC in an animal model for brain ischemia28,30,68,69. RIC reduced infarct volume in both male and female animals but provided significantly more protection in males. It must be pointed out that only four studies examined the effect of RIC in female animals, so more experimental research on female animals should be done to determine the RIC effects on female animals.
Both rat and mice studies demonstrated significant statistical reduction in infarct volume in RIC groups compared to controls. Subgroup analysis shown that in mice experiments, there was a significant interaction with RIPerC. Subspecies analysis showed no significant interaction with duration of ischemia and number of RIC cycles. However, our analysis demonstrated > 100 min of ischemia to be more effective than < 90 min in rats. Similarly, 60 min of ischemia was more powerful than < 60 min in mice. We found 3 and > 3 cycles to be equally effective in rats, being < 3 the most beneficial. Conversely, > 3 cycles in mice provided a greater neuroprotection. These differences might be related with the total ischaemic dose (cycle number and duration). Interestingly, in rats, doses above 15 min were more effective, while in mice the opposite occurs. The shorter the length of each RIC cycle, the better reduction of the infarct size.
In all species, significant sex-differences were observed in experiments performed on rats but not on mice, showing a significant effect on males. This observation would be explained by the interaction of female’s hormones with the RIC’s molecular cascade and that most of the studies were performed in male mice. Taking in consideration the sex differences is particularly important because of the translational goal, and it could lead to better treatments for cerebrovascular diseases if RIC might have a differential sex-effect.
Our analysis supports the previous findings of no significant differences in RIC effect when it was applied on one or two limbs70. We also noticed a reduction of efficacy if isoflurane is used during surgical procedure36,71. Signaling protective pathways associated with the induction of brain ischemic tolerance are known for the inhalational anesthetics, however very little is known about the infused ones. Clinical and experimental studies of the anesthesia effect on ischemic preconditioning should be conducted in the future to determine its effect.
Although the exact mechanisms by which RIC reduces ischemic injury in the brain remain unclear, the currently accepted hypothesis is that preconditioning, perconditioning and postconditioning are all involve in both humoral and neural mechanisms12. RIC has been successfully reproduced by dozens of experimental laboratories but translation to the human clinical setting is still a challenge6. Despite many clinical trials shown protection to the heart, large randomized controlled trials found no improvement in clinical outcome and mortality in patients undergoing coronary bypass grafting72. Several trials are currently ongoing to explore the effects of RIC in ischemic stroke patients73. Data from these trials will help to better understand the effectiveness of RIC in AIS patients and will guide potential future implementation of RIC in the clinical practice.
The current systematic review and meta-analysis is the most recent revision of the literature on preclinical studies of RIC. It has considered three RIC strategies individually to define its effects independently, by contrast two recent systematic reviews and meta-analyses74,75 considered only two RIC strategies (RIPreC vs RIPerC + RIPostC; RIPreC vs RIPostC). We have considered a subgroup analysis by species (mice, rats, monkeys) because of the vascular hemodynamics of each specie. A detailed summary of the three systematic reviews is provided on Table 3.
Some potential limitations should be stated. First, a large proportion of studies included in the meta-analysis use young male rodents with an absence of animals with co-morbidities which may inhibit the effects of RIC and a lack of adults/aged animals. In clinical studies, RIC would be used to treat aged persons with hypertension, diabetes and dyslipidaemia, which are not represented in preclinical models at this time. Second, considering that the incidence of stroke is higher among women compared to men, with women experiencing poorer outcomes, it is imperative to include more females in future studies. Third, anesthesia during RIC delivery is another concern because it is reportedly neuroprotective in preclinical models of stroke. Fourth, apart from infarct volume, we did not perform the meta-analysis of neurological function outcome because it was reported in a wide variety of different tests which make the analysis weak and deficient, and the high variability might be complicated to understand.
Conclusion
This article, to our knowledge, is the first meta-analysis of RIC in preclinical stroke models that includes data on lesion volume, neurological impairment and mechanisms involved in RIC. This study demonstrated that RIC is a feasible and safe strategy and supported the ability of RIC to reduce infarct size and improve neurological function. However, the present study detected moderate statistical heterogeneity across studies influenced by species. Precise knowledge of RIC optimal dosage, the effects of comorbidities, sex and anesthesia is yet to be found. Further investigation in pre-clinical characterization of the RIC protocol obeying animal research guidelines is needed so that it can be successfully translated to humans.
References
Roth, G. A. et al. Demographic and epidemiologic drivers of global cardiovascular mortality. N. Engl. J. Med. 372(14), 1333–1341 (2015).
Leng, T. & Xiong, Z.-G. Treatment for ischemic stroke: From thrombolysis to thrombectomy and remaining challenges. Brain Circ. 5(1), 8 (2019).
Zhao, W. et al. Multiphase adjuvant neuroprotection: A novel paradigm for improving acute ischemic stroke outcomes. Brain Circ. 6(1), 11 (2020).
Mohammad Seyedsaadat, S., Kallmes, D. & Brinjikji, W. Remote ischemic conditioning approach for the treatment of ischemic stroke. Neural Regen. Res. 15(6), 1033–1034 (2020).
O’Collins, V. E. et al. 1026 experimental treatments in acute stroke. Ann. Neurol. 59(3), 467–477 (2006).
Hess, D. C. et al. Remote ischaemic conditioning-a new paradigm of self-protection in the brain. Nat. Rev. Neurol. 11(12), 698–710 (2015).
Murry, C. E., Jennings, R. B. & Reimer, K. A. Preconditioning with ischemia: A delay of lethal cell injury in ischemic myocardium. Circulation 74(5), 1124–1136 (1986).
Liu, X. et al. Attenuation of reperfusion injury by renal ischemic postconditioning: The role of NO. Biochem. Biophys. Res. Commun. 359(3), 628–634 (2007).
Ateş, E. et al. Renal protection by brief liver ischemia in rats. Transplantation 74(9), 1247–1251 (2002).
Dezfulian, C., Garrett, M. & Gonzalez, N. R. Clinical application of preconditioning and postconditioning to achieve neuroprotection. Transl. Stroke Res. 4(1), 19–24 (2013).
Woodruff, T. M. et al. Pathophysiology, treatment, and animal and cellular models of human ischemic stroke. Mol. Neurodegener. [Internet.] 6(1), 11 (2011).
Chen, G., Mrugesh, T., Robinson, C. & Doré, S. Limb remote ischemic conditioning: Mechanisms, anesthetics, and the potential for expanding therapeutic options. Front. Neurol. [Internet.] 9(6), 40. https://doi.org/10.3389/fneur.2018.00040 (2018).
Pico, F. et al. A multicenter, randomized trial on neuroprotection with remote ischemic per-conditioning during acute ischemic stroke: The REmote iSchemic Conditioning in acUtE BRAin INfarction study protocol. Int. J. Stroke. 11(8), 938–943 (2016).
Hougaard, K. D. et al. Remote ischemic perconditioning as an adjunct therapy to thrombolysis in patients with acute ischemic stroke: A randomized trial. Stroke 45(1), 159–167 (2014).
An, J. et al. Safety and efficacy of remote ischemic postconditioning after thrombolysis in patients with stroke. Neurology. 95(24), e3355–e3363 (2020).
Purroy, F. et al. Induced neuroprotection by remote ischemic perconditioning as a new paradigm in ischemic stroke at the acute phase, a systematic review. BMC Neurol. 20(1), 266 (2020).
Shamseer, L. et al. Preferred reporting items for systematic review and meta-analysis protocols (prisma-p): Elaboration and explanation. BMJ [Internet] 349(January), 1–25. https://doi.org/10.1136/bmj.g7647(2015) (2015).
Moher, D. et al. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 6(7) (2009).
Yensen, J. PICO search strategies. Online J. Nurs. Informatics. 17(3), 1–6 (2013).
Team RC. R: A language and environment for statistical computing. R Foundation for Statistical Computing [Internet]. Vienna, Austria (2020). https://www.r-project.org/. Accessed September 2021.
Liu, X. et al. Remote ischemic postconditioning alleviates cerebral ischemic injury by attenuating endoplasmic reticulum stress-mediated apoptosis. Transl. Stroke Res. 5(6), 692–700 (2014).
Li, H. et al. The role of p38MAPK signal pathway in the neuroprotective mechanism of limb postconditioning against rat cerebral ischemia/reperfusion injury. J. Neurol. Sci. [Internet.] 357(1–2), 270–275. https://doi.org/10.1016/j.jns.2015.08.004 (2015).
Guo, L. et al. Short-term remote ischemic conditioning may protect monkeys after ischemic stroke. Ann. Clin. Transl. Neurol. 6(2), 310–323 (2019).
He, J. T., Li, H., Yang, L. & Cheng, K. L. Involvement of endothelin-1, H2S and Nrf2 in beneficial effects of remote ischemic preconditioning in global cerebral ischemia-induced vascular dementia in mice. Cell Mol. Neurobiol. [Internet]. 39(5), 671–686. https://doi.org/10.1007/s10571-019-00670-y (2019).
Li, Y. et al. Role of exosomes induced by remote ischemic preconditioning in neuroprotection against cerebral ischemia. NeuroReport 30(12), 834–841 (2019).
Hu, S. et al. Noninvasive limb remote ischemic preconditioning contributes neuroprotective effects via activation of adenosine A1 receptor and redox status after transient focal cerebral ischemia in rats. Brain Res. [Internet]. 1459, 81–90. https://doi.org/10.1016/j.brainres.2012.04.017 (2012).
Jachova, J. et al. Neuroprotection mediated by remote preconditioning is associated with a decrease in systemic oxidative stress and changes in brain and blood glutamate concentration. Neurochem. Int. [Internet]. 129(March), 104461. https://doi.org/10.1016/j.neuint.2019.05.005 (2019).
Chandra, A., Li, W., Stone, C., Geng, X., Ding, Y. Enhanced oxidative stress response and neuroprotection of combined limb remote ischemic conditioning and atorvastatin after transient ischemic stroke in rats. Brain Circ. 35–40 (2017).
Du, X. et al. Hypoxia-inducible factor 1α and 2α have beneficial effects in remote ischemic preconditioning against stroke by modulating inflammatory responses in aged rats. Front. Aging Neurosci. 12(March), 1–11 (2020).
Liang, W. et al. Preactivation of Notch1 in remote ischemic preconditioning reduces cerebral ischemia-reperfusion injury through crosstalk with the NF-κB pathway. J. Neuroinflam. 16(1), 181 (2019).
Zhao, Y. et al. Role of the Janus kinase 2/signal transducers and activators of transcription 3 pathway in the protective effect of remote ischemia preconditioning against cerebral ischemia-reperfusion injury in rats. NeuroReport 30(9), 664–670 (2019).
Vlasov, T. D., Korzhevskii, D. É. & Polyakova, E. A. Ischemic preconditioning of the rat brain as a method of endothelial protection from ischemic/repercussion injury. Neurosci. Behav. Physiol. 35(6), 567–572 (2005).
Shan, L. Y. et al. Platelet-derived microparticles are implicated in remote ischemia conditioning in a rat model of cerebral infarction. CNS Neurosci. Ther. 19(12), 917–925 (2013).
Garcia-Bonilla, L. et al. Endogenous protection from ischemic brain injury by preconditioned monocytes. J. Neurosci. 38(30), 6722–6736 (2018).
Liu, C., Yang, J., Zhang, C., Geng, X. & Zhao, H. Remote ischemic conditioning reduced cerebral ischemic injury by modulating inflammatory responses and ERK activity in type 2 diabetic mice. Neurochem. Int. [Internet]. 135(1), 104690. https://doi.org/10.1016/j.neuint.2020.104690 (2020).
Chen, C. et al. Splenic responses play an important role in remote ischemic preconditioning-mediated neuroprotection against stroke. J. Neuroinflam. 15(1), 1–14 (2018).
Qi, Z. et al. Bcl-2 phosphorylation triggers autophagy switch and reduces mitochondrial damage in limb remote ischemic conditioned rats after ischemic stroke. Transl. Stroke Res. 6(3), 198–206 (2015).
Su, J., Zhang, T., Wang, K., Zhu, T. & Li, X. Autophagy activation contributes to the neuroprotection of remote ischemic perconditioning against focal cerebral ischemia in rats. Neurochem. Res. 39(11), 2068–2077 (2014).
Wang, J., Han, D., Sun, M., Feng, J. A combination of remote ischemic perconditioning and cerebral ischemic postconditioning inhibits autophagy to attenuate plasma HMGB1 and induce neuroprotection against stroke in rat. J Mol Neurosci. 58(4), 424–431 (2016).
Ren, C. et al. Limb remote ischemic conditioning increases Notch signaling activity and promotes arteriogenesis in the ischemic rat brain. Behav. Brain Res. [Internet]. 340, 87–93. https://doi.org/10.1016/j.bbr.2016.10.036 (2018).
Ren, C. et al. Limb ischemic perconditioning attenuates blood–brain barrier disruption by inhibiting activity of MMP-9 and occludin degradation after focal cerebral ischemia. Aging Dis. 6(6), 406–417 (2015).
Hoda, M. N. et al. Remote ischemic perconditioning is effective after embolic stroke in ovariectomized female mice. Transl. Stroke Res. 5(4), 484–490 (2014).
Ren, C. et al. Limb ischemic conditioning improved cognitive deficits via eNOS-dependent augmentation of angiogenesis after chronic cerebral hypoperfusion in rats. Aging Dis. 9(5), 869–879 (2018).
Ma, J. et al. Prevention of the collapse of pial collaterals by remote ischemic perconditioning during acute ischemic stroke. J. Cereb. Blood Flow Metab. 37(8), 3001–3014 (2017).
Chen, G., Yang, J., Lu, G., Guo, J. & Dou, Y. Limb remote ischemic post-conditioning reduces brain reperfusion injury by reversing eNOS uncoupling. Indian J. Exp. Biol. 52(6), 597–605 (2014).
Peng, B. et al. Remote ischemic postconditioning protects the brain from global cerebral ischemia/reperfusion injury by up-regulating endothelial nitric oxide synthase through the PI3K/Akt pathway. Brain Res. [Internet]. 1445, 92–102. https://doi.org/10.1016/j.brainres.2012.01.033 (2012).
Pignataro, G. et al. NNOS and p-ERK involvement in the neuroprotection exerted by remote postconditioning in rats subjected to transient middle cerebral artery occlusion. Neurobiol. Dis. [Internet]. 54, 105–114. https://doi.org/10.1016/j.nbd.2013.02.008 (2013).
Li, S. et al. Remote ischemic post-conditioning improves neurological function by AQP4 down-regulation in astrocytes. Behav. Brain Res. [Internet]. 289, 1–8. https://doi.org/10.1016/j.bbr.2015.04.024 (2015).
Zhang, Y. et al. Immediate remote ischemic postconditioning reduces cerebral damage in ischemic stroke mice by enhancing leptomeningeal collateral circulation. J. Cell Physiol. 2018, 12637–12645 (2018).
Li, P. et al. Remote limb ischemic postconditioning protects mouse brain against cerebral ischemia/reperfusion injury via upregulating expression of Nrf2, HO-1 and NQO-1 in mice. Int. J. Neurosci. 126(6), 552–559 (2016).
Wang, Q. et al. Limb remote postconditioning alleviates cerebral reperfusion injury through reactive oxygen species-mediated inhibition of delta protein kinase C in rats. Anesth. Analg. 113(5), 1180–1187 (2011).
Chen, G. et al. Limb remote ischemic postconditioning reduces ischemia-reperfusion injury by inhibiting NADPH oxidase activation and MYD88-TRAF6-P38MAP-kinase pathway of neutrophils. Int. J. Mol. Sci. 17(12), 1971 (2016).
Zhou, M., Xia, Z. Y., Lei, S. Q., Leng, Y. & Xue, R. Role of mitophagy regulated by Parkin/DJ-1 in remote ischemic postconditioning-induced mitigation of focal cerebral ischemia-reperfusion injury in rats. Eur. Rev. Med. Pharmacol. Sci. 19(24), 4866–4871 (2015).
Liang, D. et al. Remote limb ischemic postconditioning promotes motor function recovery in a rat model of ischemic stroke via the up-regulation of endogenous tissue kallikrein. CNS Neurosci. Ther. 24(6), 519–527 (2018).
Khan, M. B. et al. Remote ischemic postconditioning: harnessing endogenous protection in a murine model of vascular cognitive impairment. Transl. Stroke Res. 6(1), 69–77 (2014).
Kong, Y., Rogers, M. R. & Qin, X. Effective neuroprotection by ischemic postconditioning is associated with a decreased expression of RGMa and inflammation mediators in ischemic rats. Neurochem. Res. 38(4), 815–825 (2013).
Qi, W. et al. Remote ischemic postconditioning protects ischemic brain from injury in rats with focal cerebral ischemia/reperfusion associated with suppression of TLR4 and NF-κB expression. NeuroReport 27(7), 469–475 (2016).
Zong, Y. et al. Limb remote ischemic postconditioning protects cerebral ischemia from injury associated with expression of HIF-1aα in rats. BMC Neurosci. 16(1), 1–8 (2015).
Liu, Z. J. et al. Remote ischemic preconditioning-mediated neuroprotection against stroke is associated with significant alterations in peripheral immune responses. CNS Neurosci. Ther. 22(1), 43–52 (2016).
Qi, Z. F. et al. AKT/GSK3β-dependent autophagy contributes to the neuroprotection of limb remote ischemic postconditioning in the transient cerebral ischemic rat model. CNS Neurosci. Ther. 18(12), 965–973 (2012).
Cheng, Z. et al. Non-invasive remote limb ischemic postconditioning protects rats against focal cerebral ischemia by upregulating STAT3 and reducing apoptosis. Int. J. Mol. Med. 34(4), 957–966 (2014).
Sun, J. et al. Protective effect of delayed remote limb ischemic postconditioning: Role of mitochondrial K ATP channels in a rat model of focal cerebral ischemic reperfusion injury. J. Cereb. Blood Flow Metab. 32(5), 851–859 (2012).
Meng, X., Zhang, D. & Sui, S. Acute remote ischemic preconditioning alleviates free radical injury and inflammatory response in cerebral ischemia/reperfusion rats. Exp. Ther. Med. 1953–1960 (2019).
Cook, D. J. & Tymianski, M. Nonhuman primate models of stroke for translational neuroprotection research. Neurotherapeutics 9(2), 371–379 (2012).
Hermann, D. M., Popa-Wagner, A., Kleinschnitz, C. & Doeppner, T. R. Animal models of ischemic stroke and their impact on drug discovery. Expert Opin. Drug Discov. [Internet]. 14(3), 315–326. https://doi.org/10.1080/17460441.2019.1573984 (2019).
Ren, C., Gao, X., Steinberg, G. K., Zhao, H. Limb remote-preconditioning protects against focal ischemia in rats and contradicts the dogma of therapeutic time windows for preconditioning. Neuroscience 151(4), 1099–1103 (2008).
Hausenloy, D. J. & Yellon, D. M. The Second Window of Preconditioning (SWOP) where are we now?. Cardiovasc Drugs Ther. 24(3), 235–254 (2010).
Zhang, Y. et al. Protective effects of remote ischemic preconditioning in rat hindlimb on ischemia-reperfusion injury. Neural Regen. Res. 7(8), 583–587 (2012).
Chen, G., Kamat, P. K., Ahmad, A. S. & Doré, S. Distinctive effect of anesthetics on the effect of limb remote ischemic postconditioning following ischemic stroke. PLoS ONE 15(1), e0227624 (2020).
Wever, K. E. et al. Determinants of the efficacy of cardiac ischemic preconditioning: A systematic review and meta-analysis of animal studies. PLoS ONE 10(11), 1–17 (2015).
Chen, Q. et al. Limb remote ischemic preconditioning protects against cerebral ischemia through down-regulation of aquaporin-4. Int. J. Clin. Exp. Med. 9(7), 13878–13889 (2016).
Hausenloy, D. J. & Yellon, D. M. Ischaemic conditioning and reperfusion injury. Nat. Rev. Cardiol. [Internet]. 13(4), 193–209. https://doi.org/10.1038/nrcardio.2016.5 (2016).
Zhao, W. et al. Remote ischemic conditioning for stroke: Clinical data, challenges, and future directions. Ann. Clin. Transl. Neurol. 6(1), 186–196 (2019).
Ripley, A. J. et al. Neuroprotection by remote ischemic conditioning in rodent models of focal ischemia: A systematic review and meta-analysis. Transl. Stroke Res. https://doi.org/10.1007/s12975-020-00882-1 (2021).
Weir, P., Maguire, R., O’Sullivan, S. E. & England, T. J. A meta-analysis of remote ischaemic conditioning in experimental stroke. J. Cereb. Blood Flow Metab. https://doi.org/10.1177/0271678X20924077 (2019).
Liu, Q. et al. A feasible strategy for focal cerebral ischemiareperfusion injury: Remote ischemic postconditioning. Neural Regen. Res. 9(15), 1460–1463 (2014).
Yang, J. et al. Hypoxia inducible factor 1α plays a key role in remote ischemic preconditioning against stroke by modulating inflammatory responses in rats. J. Am. Heart Assoc. 7(5), 1–10 (2018).
Hahn, C. D., Manlhiot, C., Schmidt, M. R., Nielsen, T. T. & Redington, A. N. Remote ischemic per-conditioning: A novel therapy for acute stroke?. Stroke 42(10), 2960–2962 (2011).
Silachev, D. N. et al. Effect of anesthetics on efficiency of remote ischemic preconditioning. Biochemistry 82(9), 1006–1016 (2017).
Hoda, M. N. et al. Remote ischemic perconditioning is effective alone and in combination with intravenous tissue-type plasminogen activator in murine model of embolic stroke. Stroke 43(10), 2794–2799 (2012).
Liu, C., Yang, J., Zhang, C., Geng, X. & Zhao, H. The changes of systemic immune responses during the neuroprotection induced by remote ischemic postconditioning against focal cerebral ischemia in mice. Neurol. Res. [Internet]. 41(1), 26–36 (2019).
Li, J. et al. Limb remote ischemic postconditioning protects integrity of the blood-brain barrier after stroke. Neural Regen. Res. 13(9), 1585–1593 (2018).
Xiao, Y. et al. Neuroprotection by peripheral nerve electrical stimulation and remote postconditioning against acute experimental ischaemic stroke. Neurol. Res. 37(5), 447–453 (2015).
Ren, C. et al. Limb remote ischemic postconditioning protects against focal ischemia in rats. Brain Res [Internet]. 1288, 88–94. https://doi.org/10.1016/j.brainres.2009.07.029 (2009).
Xu, C. et al. Limb remote ischemic postconditioning is effective but also time-course-limited in protecting the brain from I/R injury. Turkish J. Med. Sci. 42(5), 918–929 (2012).
Zhong, C. G., Yun, S. X., Sheng, L. X. & Miao, T. H. Remote ischemic postconditioning protects the brain from focal ischemia/reperfusion injury by inhibiting autophagy through the mTOR/p70S6K pathway. Neurol Res. [Internet]. 40(3), 182–188. https://doi.org/10.1080/01616412.2018.1424696 (2018).
Huang, D., Liu, H., Qu, Y. & Wang, P. Non-invasive remote ischemic postconditioning stimulates neurogenesis during the recovery phase after cerebral ischemia. Metab. Brain Dis. 32(6), 1805–1818 (2017).
Meng, X., Li, Y., Zhang, J., Jiang, X. & ZhaO, J. Effects of Limb Remote Postconditioning on Apoptosis and Long-Term Neurological Outcomes of Focal Cerebral Ischemia/Reperfusion Injury in Rats. J Diabetes Metab. 06(09), 6–11 (2015).
Zhang, W., Wang, Y. & Bi, G. Limb remote ischemic postconditioning-induced elevation of fibulin-5 confers neuroprotection to rats with cerebral ischemia/reperfusion injury: Activation of the AKT pathway. Clin. Exp. 44(6), 656–663 (2017).
Acknowledgements
We are grateful to all members of Clinical Neuroscience group at IRBLleida for scientific discussions.
Funding
This study was supported by the Government of Catalonia-Agència de Gestió d'Ajuts Universitaris i de Recerca (FP: 2017 SGR 1628), Instituto de Salud Carlos III and co-funded by European Union (ERDF/ESF, “Investing in your future”) (FP: Project PI17-01725) and the INVICTUS plus Research Network (Carlos III Health Institute). C.T-Q. was supported by a Grant from Contratos predoctorales de formación en investigación en salud (PFIS; FI18/00319).
Author information
Authors and Affiliations
Contributions
C.T.-Q., G.A., F.P. conceived the study and designed experiments. C.T.-Q., M.Q. performed data analysis. C.T.-Q., G.A., F.P. interpreted data and draft the manuscript. F.P. procured funding. All authors critically revised and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Torres-Querol, C., Quintana-Luque, M., Arque, G. et al. Preclinical evidence of remote ischemic conditioning in ischemic stroke, a metanalysis update. Sci Rep 11, 23706 (2021). https://doi.org/10.1038/s41598-021-03003-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-03003-6
This article is cited by
-
Long-term inorganic nitrate administration protects against myocardial ischemia-reperfusion injury in female rats
BMC Cardiovascular Disorders (2023)
-
Effects of different remote ischemia perconditioning methods on cerebral infarct volume and neurological impairment in rats
Scientific Reports (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.