Abstract
Polhillia, Wiborgia and Wiborgiella species are shrub legumes endemic to the Cape fynbos of South Africa. They have the ability to fix atmospheric N2 when in symbiosis with soil bacteria called ‘rhizobia’. The aim of this study was to assess the morpho-physiological and phylogenetic characteristics of rhizobia associated with the nodulation of Polhillia, Wiborgia and Wiborgiella species growing in the Cape fynbos. The bacterial isolates from root nodules consisted of a mixture of fast and intermediate growers that differed in colony shape and size. The isolates exhibited tolerance to salinity (0.5–3% NaCl) and pH (pH 5–10) and different antibiotic concentrations, and could produce 0.51 to 51.23 µg mL−1 of indole-3-acetic acid (IAA), as well as solubilize tri-calcium phosphate. The ERIC-PCR results showed high genomic diversity in the rhizobial population and grouped them into two major clusters. Phylogenetic analysis based on 16S rRNA, atpD, glnII, gyrB, nifH and nodC gene sequences revealed distinct and novel evolutionary lineages related to the genus Rhizobium and Mesorhizobium, with some of them being very close to Mesorhizobium australicum. However, the phylogenetic analysis of glnII and nifH genes of some isolates showed incongruency.
Similar content being viewed by others
Introduction
Polhillia, Wiborgia and Wiborgiella species belong to the family Leguminosae and tribes Genisteae and Crotalarieae1,2,3. They are endemic to the Cape fynbos biome, recognized as one of the richest areas of flowering plants in the world4,5,6. These legumes have bright yellow and/or white flowers, which are a major attraction for tourists3,7. They also contribute to the fertility of fynbos soil through N2 fixation with native soil rhizobia. In fact, Polhillia brevicalyx, Polhillia pallens, Wiborgia sericea, Wiborgia tetraptera, Wiborgia obcordata and Wiborgiella sessilifolia are reported to derive between 61 and 91% of their N nutrition from symbiotic N2 fixation8.
Some rhizobial bacteria are capable of tolerating acidic conditions, often characterised by high H+ concentration and the increased solubility of heavy metals and trace elements9,10, as well as tolerance to high soil salinity which can inhibit bacterial survival, growth and persistence11. Some bacteria can also solubilize P from unavailable soil P complexes for plant uptake as well as produce IAA, a hormone that is involved in root formation and root elongation for increased uptake of water and nutrients12,13. The identification of acid, salinity and antibiotic tolerant rhizobia with the ability to produce IAA and solubilize P is a first step to selecting rhizobia for inoculant production.
Rhizobia nodulating various Cape fynbos shrub legumes have been reported14,15,16,17. However, information on the microsymbionts nodulating Polhillia, Wiborgia and Wiborgiella species endemic to the Cape is lacking. Therefore, the aim of this study was to evaluate the morpho-physiological diversity and phylogeny of bacterial symbionts associated with the nodulation of Polhillia, Wiborgia and Wiborgiella species. We hypothesized that the rhizobial strains nodulating these legumes have genomic stability and were same type of rhizobial species due to the restricted habitat of these wild fynbos legumes. To test these hypotheses, the following questions were addressed (1) Which rhizobial species nodulate these wild shrub legumes? (2) What are the phylogenetic behaviours of the isolates?
Materials and methods
Nodule sampling and description of study sites
Root nodules were collected from Wiborgiella sessilifolia and Wiborgia sericea at Bredasdorp and Travellers Rest farm, respectively (Table 1). Due to the limited number of Polhillia pallens plants in the Witkoppies farm, as well as the difficulties in uprooting Wiborgia obcordata plants in their natural stands, mature seeds and rhizosphere soil samples were collected from their respective sites (Table 1) and used to trap rhizobia in the glasshouse. All the methods were performed in accordance with the relevant regulations and guidelines. Collecting root nodules, seeds and rhizosphere soil samples was done randomly according to plant availability at each site during the wet (July to September 2018) season.
Trapping rhizobia and their isolation
Seeds of P. pallens and W. obcordata were pre-germinated using the acid scarification method18,19 and transplanted into sterile sand in pots20. Seedlings were inoculated with their respective rhizosphere soil suspension21. Five replicate pots were used for each treatment. All seedlings were supplied with N-free fahraeus solution as a source of nutrients22. After 42 days of growth in the glasshouse, effective root nodules with a red or pinkish colour were harvested from the glasshouse-grown plants for rhizobial isolation. The root nodules obtained from the field and those from the glasshouse were surface sterilized and used to isolate rhizobia, following standard procedures20.
Rhizobial authentication
Surface sterilised pre-germinated seeds of P. pallens, and W. sessilifolia, were transplanted in sterilized plastic pots (1.2 dm3) containing autoclaved sand. Each seedling was inoculated with 1 mL (107 to 108 rhizobial cells ml−1) of the test bacterial culture under axenic conditions. The pots with seedlings were then transported to the glasshouse and left to grow under glasshouse conditions. Three replicate pots were used for each isolate and the plants were watered twice a week with N-free ferrous nutrient solution22. Similarly, all the test isolates were used to also inoculate cowpea seedlings in a host-range test.
DNA extraction and ERIC-PCR genomic fingerprinting
Genomic DNA of the rhizobial isolates grown in YMB (~1 × 109 rhizobia cells ml−1) was extracted using a GenElute Bacterial Genomic DNA kit, according to the manufacturer’s instructions (Sigma Aldrich, USA).
Fingerprints of the rhizobial isolates were evaluated by the ERIC-PCR method performed in a 15 μl reaction mixture containing 1 μl DNA (50–80 ng μl−1), 7 μl 2 × My Taq PCR master mix, 1 μl each ERIC forward and reverse primers and 5 μl double distilled sterilised water.
Amplification was performed in a Thermal cycle (T100 BIORAD, USA). The primers used and the amplification conditions are shown in Table S1. The PCR-amplified products were analysed by horizontal gel electrophoresis at 100 V for 3.0 h in a 1.2% agarose gel stained with ethidium bromide (1 µg ml–1) in 1X TAE buffer. A standard molecular marker (GeneDirex 1 kb ladder) was included to estimate the size of the fragments. The gels were photographed under UV illumination using a gel documentation system (GeldocTm XR + , Bio-RAD, USA).
Cluster analysis
The banding patterns were scored directly from the gel photographs and the isolates were grouped through visual inspection of the banding. The DNA fingerprints (bands) obtained from the ERIC-PCR products was used for cluster analysis. Only distinct, well-resolved, and unambiguous bands were scored faint bands and ≤ 50 bp band sizes were excluded in the cluster analysis. A binary scoring system (1 for presence and 0 for the absence of a band) was used to generate an input matrix. This was analyzed by means of the Unweighted Paired-Group Method with arithmetic mean (UPGMA)23. A dendrogram was then generated from the matrix using NTSYS-Pc software24.
Amplification of the 16S rRNA, housekeeping and symbiotic genes
The genomic DNA of representative rhizobial strains of different clusters in the ERIC-PCR dendrogram were amplified with primers for 16S rRNA, housekeeping (atpD, gyrB and glnII) and symbiotic (nifH and nodC) genes. Amplification was performed in a 25 μl reaction mixture containing 1 μl (50–80 ng) of genomic DNA template, 3 μl 5 × My Taq Buffer, 1 μl (10 μM) forward primer, 1 μl (10 μM) reverse primer, 0.1 μl (5U) Taq polymerase (Bioline, USA) and 18.9 μl sterile distilled water. Amplifications were performed in a Thermal cycle (T100 BIORAD, USA). The primers used, and amplification conditions are indicated in Table S1. The amplified products were separated by electrophoresis at 80 V for 1 h in a 1.2% agarose gel stained with ethidium bromide (1 µg ml–1) in 1X TAE buffer. Standard molecular markers (GeneDirex 100 bp and 1 kb ladders) were included to estimate the length of the fragments.
Sequencing of the 16S rRNA, atpD, gyrB, glnII, nifH and nodC genes and their processing
The amplified PCR products were purified using PCR clean up kit (NEB, USA) and sent to Macrogen company, The Netherlands, for sequencing. The quality of the sequences were assessed using BioEdit 7.0.0 software25. Closely related species were identified using the BLASTn (Basic Local Alignment Search Tool) program in the NCBI (National Centre for Biotechnology Information) server. The 16S rRNA, atpD, gyrB and glnII, nodC, and nifH gene sequences of the reference or type strains used in this study were retrieved from the NCBI-GenBank database. Close reference type strain sequences from the NCBI GenBank database were selected and aligned with sequences of the test strains using MUSCLE26, and used to construct phylogenetic trees using the MEGA 6.0 program27. Phylogenetic trees were generated using the P-distance method to calculate evolutionary distance28, and evolutional history was inferred using the Maximum likelihood method29 algorithm with 1000 bootstraps to allow for a strong support30. The MEGA 6 program was used to calculate transition-transversion-ratio to know the content of homoplasy.
Biochemical and physiological characterization of isolates
The rhizobial isolates were grown in YMB which was adjusted to different pH levels (pH 3, 5, 9 and 10). The YMB media at pH 7 was used as a control. To screen for pH tolerance, 10 µL (≈108 cells/mL) of freshly prepared broth culture of each isolate was dropped into 4 mL of freshly made broth previously adjusted to the different pH levels20. Thereafter, they were incubated at 28 °C for seven days with constant agitation (200 rpm) on a shaker. The pH levels of 5 and 6 were maintained with a buffer using 40 mM MES, while 30 mM HEPES was used for pH 7 and 9, and 30 mM CHES for pH 1031,32. After seven days of incubation, the optical density of the broth cultures was measured at 660 nm using vis spectrophotometer (7300 Jenway UK).
The phosphate solubilization test was done using double agar layer plates containing B3 media (basal layer) and tri-calcium phosphate [Ca3(PO4)2] (top layer), as described by Dabo et al.33. The diameter of the halo zone produced around each bacterial colony was measured and taken as indicative of P-solubilizing activity. The phosphate-solubilizing index (PSI) of each isolate was derived as the ratio of the diameter of the halo zone (R) and colony diameter (r).
A colorimetric method was used to test for IAA production by isolates in tryptophan-supplemented YMA broth, as described by Ibny et al.13.
To test for salt (NaCl) tolerance of the rhizobial isolates, a 20 µl volume of each matured bacterial isolate was dropped on a YMA plate containing different concentrations (0.5%, 1%, 2% and 3%) of NaCl, with 0.01% NaCl as the control13.
Intrinsic antibiotic resistance
Rhizobial growth was tested in YM agar media supplemented with different concentrations of each antibiotic: streptomycin (50, 100, and 200 µg ml−1), kanamycin, chloramphenicol and ampicillin (25, 50 and 75 µg ml−1) as well as neomycin (1, 5 and 10 µg ml−1) with 0 µg ml−1 antibiotic as a control34. All assays were done in triplicates. Colony growth was assessed after incubation at 28 °C. Isolates showing growth in all triplicate plates were considered tolerant, and isolates which did not grow, were considered susceptible to that antibiotic concentration.
Results
Rhizobia isolated
The original host plants (Polhillia pallens and Wiborgia obcordata) were able to nodulate with rhizosphere soil suspensions from their respective sites of collection. (Table S2). After isolation, a total of 35 isolates were obtained, five obtained from Wiborgiella sessilifolia, ten from Polhillia pallens, five from Wiborgia sericea, and 15 from Wiborgia obcordata, (Table S2).
Morpho-physiological characterization of rhizobial isolates
About 36% of the isolates were fast-growers which took 2 to 4 days to appear on yeast mannitol agar (YMA) plates, while the remaining isolates exhibited intermediate growth rate (Table S2). Furthermore, 94% of the isolates showed small colony size (≤ 1–2 mm diameter), 77% were non-elastic in texture and cream white in colour, while 83% showed a flat-round shape.
Authentication and host range test of rhizobial isolates
The 35 test isolates were tested for host range under glasshouse conditions. Two isolates from Wiborgiella sessilifolia (TUTFWB17 and TUTFWB31) and three (TUTPP4, TUTPP8 and TUTPP10) from P. Pallens could nodulate their original host, due to the unavailability of Wiborgia sericea seeds and the very poor germination of Wiborgia obcordata seeds, authentication of the isolates with their original hosts was not possible. Cowpea was tested as host plant for all 35 isolates, and 86% of the isolates effectively nodulated cowpea (Table S3).
Salinity tolerance
The rhizobial isolates differed in their response to sodium chloride concentrations. All the 35 isolates could grow in medium supplemented with 0.01% NaCl (control) as well as 0.5% and 1% NaCl. 66% and 25% of isolates tolerated 2 and 3% NaCl concentrations, respectively (Table S2). Isolates TUTPP1, TUTPP4 and TUTPP5 from P. pallens were susceptible to 2% NaCl, while isolates TUTFWB17 and TUTFWB31 from W. sessilifolia, TUTPP1, TUTPP4, TUTPP5 and TUTPP10 from P. pallens, TUTGWO1, TUTGWO3, TUTGWO5, TUTGWO6 TUTGWO7 and TUTGWO12 from W. obcordata could tolerate up to 2% NaCl, susceptible at 3% NaCl (Table S2). All isolates from Wiborgia sericea, tolerated up to 3% NaCl concentration.
Acidity tolerance
The rhizobial isolates differed in their response to varying pH levels. All the isolates tested grew in YMA medium pH 7 (control), while, 51% grew well at pH 5 (Table S2). In contrast, isolates TUTGWO9, TUTGWO11 and TUTGWO15 from Wiborgia obcordata grew at alkaline pH 9–10 (Table S2), while 14% of the isolates tolerated a wide range of pH conditions ranging from pH 5 to pH 9, and these included isolates TUTPP3 from Polhillia pallens, TUTGWS2 and TUTGWS3 from Wiborgia sericea, TUTGWO12 and TUTGWO13 from Wiborgiella obcordata.
Screening for phosphate-solubilizing bacteria (PSB)
Phosphate-solubilizing bacteria are characteristically identified by the formation of a clear halo around their colonies due to phosphate solubilization on double agar-layered plates. Out of the 35 isolates tested, 34 were able to solubilize tri-calcium phosphate, though the phosphate-solubilizing ability differed as measured by the phosphate-solubilizing index (PSI) (Table S2). Isolate TUTFWB17 from Wiborgiella sessilifolia recorded the largest PSI index (5.0) while isolates TUTGWO9 and TUTGWO11 from Wiborgia obcordata showed the least Index (Table S2). Isolate TUTGWO1 from W. obcordata was incapable of solubilizing P.
Indole acetic acid production
The isolates showed marked differences in their ability to produce IAA in tryptophan supplemented YMB media. Of the 35 isolates tested, 31% (11 isolates) produced a detectable amount of IAA, which ranged from 0.51 µg ml−1 by TUTGWO14 from W. obcordata to 51.23 µg ml−1 by TUTPP5 from P. pallens (Table S2).
Intrinsic antibiotic resistance
A number of isolates were tolerant to a wide range of antibiotics tested, namely streptomycin, kanamycin, chloramphenicol, ampicillin and neomycin (Table S2). The results showed that 31, 3 and 3% of the 35 test isolates tolerated 50, 100 and 200 µg ml−1 streptomycin respectively. Isolate TUTPP9 from P. pallens was tolerant to 200 µg ml−1 streptomycin. However, all the isolates from Wiborgia obcordata were susceptible to streptomycin even at its lowest concentration of 50 µg ml−1. For kanamycin, 89% of the test isolates were tolerant to 25 µg ml−1, and 11% susceptible. Only 63 and 29% at 50 and 75 µg mL−1, respectively were tolerant to those concentrations of kanamycin. The results also showed that 83, 80 and 63% of the 35 test isolates were tolerant to 25, 50 and 75 µg mL−1 chloramphenicol, respectively. All isolates from Wiborgia sericea, were tolerant to 75 µg ml−1, while isolates TUTFWB31 from W. sessilifolia, TUTPP5 and TUTPP10 from P. pallens, TUTGWO1, TUTGWO2, TUTGWO3 and TUTGWO7 from W. obcordata, were susceptible to 25 µg ml−1 chloramphenicol. Moreover, a total of 29, 40 and 54% of the test isolates could not tolerate ampicillin at 25, 50 and 75 µg ml−1 concentrations respectively. All W. sericea isolates were tolerant to 75 µg ml−1 ampicillin except for isolates TUTGWS3 which was susceptible to 75 µg mL−1. The majority of W obcordata (75%) isolates were susceptible to 75 µg mL−1 ampicillin. However, all test isolates (100%) were resistant to 1 and 5 µg mL−1 concentrations of neomycin, with 43% being unable to grow at 10 µg mL−1 concentration.
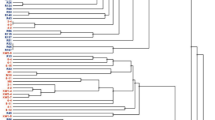
ERIC-PCR amplification
PCR amplification of the ERIC region of the genomic DNA from each isolate yielded distinctive banding patterns. The dendrogram generated from the DNA fingerprints placed the 35 isolates into two major clusters (Fig. 1). Cluster I consisted of 23 isolates obtained from all the host plants with a similarity coefficient of 0.10. Isolates TUTGWO10, TUTGWO13 and TUTGWO14 from W. obcordata showed the highest similarity coefficients of 1.00 in Cluster I. Twelve mixed isolates from all host plants were grouped in Cluster II. (Fig. 1).
Phylogenetic analysis of the 16S-rRNA gene
The maximum likelihood phylogeny of the 16S-rRNA gene revealed very close sequence similarities of test isolates to the genus Mesorhizobium.
Such that, isolates from Wiborgia obcordata, Wiborgia sericea and Polhillia pallens showed close relationship with Mesorhizobium spp. In cluster I, isolate TUTPP2 from P. pallens was closely related to with M. erdmanii strains and shared 99.4% sequence identity, while P. pallens isolates TUTPP4, TUTPP5 and TUTPP10 shared 99.1% sequence identity with M. sangaii group as their closest relative in Cluster II. Isolates TUTGWO7, TUTGWO6, TUTGWO14 and TUTGWO2 from W. obcordata and TUTGWS2 from W. sericea revealed 95.0 to 100% sequence identity with M. australicum as the closest relative in Cluster III. W. sessilifolia isolate TUTFWB31 aligned closely with P. pallens isolates and together had M. sangaii as the closest relative with 100% sequence identity in Cluster II (Fig. 2).
The maximum likelihood phylogenetic relationships of root nodule rhizobial isolates isolated from Polhillia pallens (red), Wiborgia obcordata (blue), Wiborgia sericea (black) and Wiborgiella sessilifolia (green), based on Mesorhizobium-16S rRNA sequence analysis. Test isolates are shown in bold. The significance of each branch is indicated by a bootstrap value = > 50 for each node (1000 replicates). The scale bar represents the number of changes per nucleotide position.
Sequence and phylogenetic analyses of housekeeping genes (atpD, glnII and gyrB)
In addition to 16S rRNA, three conserved housekeeping genes (atpD, glnII and gyrB) were selected for phylogenetic analysis. Based on BLASTn, the isolates were placed within the Rhizobium and Mesorhizobium groups. For a clear view of the isolate groupings with reference type strains, separate phylogenies of Rhizobium and Mesorhizobium were constructed (Figs. 3, 4, 5, 6). Due to incompatibility of the primer pairs some isolates did not constantly appear in all phylogenies. Isolates from Wiborgia obcordata, P. pallens, W. sericea, and Wiborgiella sessilifolia occupied space in the Mesorhizobium trees with some discrepancies.
The maximum likelihood phylogenetic relationships of root nodule rhizobial isolates associated with Polhillia pallens (red), Wiborgia obcordata (blue) and Wiborgiella sessilifolia (green), based on Mesorhizobium-atpD sequence analysis. Test isolates are shown in bold. The significance of each branch is indicated by a bootstrap value = > 50 for each node (1000 replicates). The scale bar represents the number of changes per nucleotide position.
The maximum likelihood phylogenetic relationships of root nodule rhizobial isolates associated with Polhillia pallens (red), Wiborgia obcordata (blue), and Wiborgiella sessilifolia (green), based on Mesorhizobium-glnII sequence analysis. Test isolates are shown in bold. The significance of each branch is indicated by a bootstrap value = > 50 for each node (1000 replicates). The scale bar represents the number of changes per nucleotide position.
The maximum likelihood phylogenetic relationships of root nodule rhizobial isolates associated with Polhillia pallens (red), Wiborgia obcordata (blue), Wiborgia sericea (black), and Wiborgiella sessilifolia (green), based on Rhizobium-glnII sequence analysis. Test isolates are shown in bold. The significance of each branch is indicated by a bootstrap value = > 50 for each node (1000 replicates). The scale bar represents the number of changes per nucleotide position.
The maximum likelihood phylogenetic relationships of root nodule rhizobial isolates from Wiborgia obcordata (blue), Wiborgia sericea (blue) and Wiborgiella sessilifolia (green) based on Mesorhizobium-gyrB sequence analysis. Test isolates are shown in bold. The significance of each branch is indicated by a bootstrap value = > 50 for each node (1000 replicates). The scale bar represents the number of changes per nucleotide position.
For example, isolates TUTGWO5, TUTGWO6, TUTGWO7, TUTGWO11 and TUTGWO14 from Wiborgia obcordata were aligned with M. australicum as the closest relative with sequence identity ranging from 97.8 (TUTGWO6) to 99.5% (TUTGWO11) in the atpD phylogram (Fig. 3), 98.1 (TUTGWO7) to 99.6% (TUTGWO5, TUTGWO6 and TUTGWO11 in the glnII phylogeny (Fig. 4), as well as 95.1 (TUTGWO11) to 95.9% (TUTWGO6, TUTGWO7 and TUTGWO14) in the gyrB tree (Fig. 6). Surprisingly, some isolates from W. obcordata aligned with Rhizobium in the glnII phylogeny. Isolates TUTGWO8 and TUTGWO9 aligned with R. esperanzae and respectively shared 93.2 and 97.8% sequence identity, TUTGWO1 aligned with R. skierniewicense and shared 90.4% sequence identity, while isolate TUTGWO2 aligned closely with R. leucaenae and shared 97.0% sequence identity in Clusters III, II and I respectively (Fig. 5).
Similarly, isolates from Wiborgiella sessilifolia which aligned with M. australicum appeared to be closest relative to isolate TUTFWB31 in the atpD, glnII and gyrB phylogenies with sequence identity of 72.8, 94.2 and 92.7% respectively. Also, isolates TUTFWB26 and TUTFWB22 had 99.5 and 93.9% sequence identity with M. australicum as the closest relative in the atpD and gyrB phylogenies, respectively (Figs. 3, 6). Interestingly, glnII sequences of isolate TUTFWB15 and TUTFWB26 aligned with Rhizobium spp. and recorded 99.5 and 83.4% sequence identity respectively with R. leucaenae as their closest relative (Fig. 5).
Isolates from Polhillia pallens aligned with Mesorhizobium in the atpD and glnII phylogenies. As found with W. obcordata and W. sessilifolia isolates, some isolates from P. pallens also aligned with Rhizobium in the glnII phylogeny. For instance, isolates TUTPP5 and TUTPP10 aligned together in Cluster I with M. australicum as their closest relative species with sequence identity of 96.0 and 95.15% in the atpD phylogeny (Fig. 3). Isolates TUTPP4 and TUTPP10 shared a low 84.4% sequence identity with M. australicum as their closest relative in the glnII phylogeny (Fig. 4). In contrast, isolate TUTPP9 aligned closely with TUTGWO1 from W. obcordata and shared 97.0% sequence identity with R. skierniewicense as the closest by with relative in Cluster II, while isolates TUTPP2 and TUTPP1 showed sequence identities of 92.2 and 99.5% respectively with R. leucaenae in Cluster I (Fig. 5).
Furthermore, the isolates from Wiborgia sericea aligned with Mesorhizobium and Rhizobium in the gyrB and glnII phylogenies respectively. With the Rhizobium phylogenies, isolates TUTGWS1, TUTGWS4 and TUTGWS5 were identical and had R. leucaenae as a close relative with 99.5% sequence identity in the glnII phylogeny (Fig. 5). Moreover, isolate TUTGWS2 had R. esperanzae as a closer relative species and together they shared 86.6% sequence identity in cluster III of the glnII phylogeny (Fig. 5). However, the sequences of isolates TUTGWS2 and TUTGWS4 aligned with M. australicum as their closest relative with 93.0 and 90.7% sequence identity respectively in the gyrB phylogeny (Fig. 6).
Isolates’ phylogenetic position based on nifH and nodC genes
Phylogenetic analyses of nifH and nodC genes placed the test isolates closer to the Rhizobium and Mesorhizobium genera in various clusters, similar to the housekeeping gene phylograms (Figs. 7, 8), although some sequence inconsistencies between the phylogenies were observed. Wiborgia obcordata isolates occupied space mainly in the Mesorhizobium phylogeny, though some were found with Rhizobium. Isolates TUTGWO5 aligned closely with some P. pallens isolates and had 92.8% sequence identity with M. chacoence as the closest relative in Cluster II, while isolates TUTGWO6, TUTGWO14, TUTGWO7, TUTGWO11, TUTGWO2 and TUTGWO3 form W. obcordata assembled together in Cluster I and shared a low 90.5% sequence identity with M. chacoense as their closest relative in the nifH phylogeny (Fig. 7). Similarly, in the nodC phylogeny, W. obcordata isolates TUTGWO13, TUTGWO5, TUTGWO1, TUTGWO9, TUTGWO3 and TUTGWO11 aligned together and had a low relationship with the Mesorhizobium reference type strains as they shared between 82.5 and 85.8% sequence identity with M. chacoense, their closest relative in Cluster II (Fig. 8). In contrast to the results from the 16S rRNA, atpD, glnII, and gyrB phylogenies, isolates TUTGWO14 and TUTGWO9 aligned with Rhizobium in the nodC and nifH phylogenies respectively, where they shared 99.7% sequence identity with R. tropici as the closest relatives (data not shown).
The maximum likelihood phylogenetic relationships of root nodule rhizobial isolates obtained from Polhillia pallens (red) and Wiborgia obcordata (blue) based on Mesorhizobium-nifH sequence analysis. Test isolates are shown in bold. The significance of each branch is indicated by a bootstrap value = > 50 for each node (1000 replicates). The scale bar represents the number of changes per nucleotide position.
The maximum likelihood phylogenetic relationships of root nodule rhizobial isolate obtained from Polhillia pallens (red), Wiborgia obcordata (blue) and Wiborgiella sessilifolia (green) based on Mesorhizobium-nodC sequence analysis. Test isolates are shown in bold. The significance of each branch is indicated by a bootstrap value = > 50 for each node (1000 replicates). The scale bar represents the number of changes per nucleotide position.
But, similar to the results obtained from the 16S rRNA and housekeeping phylogenies, isolate TUTFWB31 from W. obcordata aligned with Mesorhizobium in the nodC phylogeny and shared 82.2% sequence identity with M. chacoense (Fig. 8). Similarly, Polhillia pallens isolates aligned with Mesorhizobium, and isolates TUTPP4 and TUTPP5 which aligned together in the atpD and glnII phylogenies had M. chacoense (Figs. 3, 4) as their closest relative and shared 90.4 and 92.8%, as well as 83.4 and 82.5% sequence identity the nifH and nodC phylogenies respectively (Figs. 7, 8).
Discussion
Ecological adaptation of native rhizobia to the Cape fynbos
The N2-fixing effectiveness of rhizobia is important for their ability to contribute N to cropping soil systems and/or the natural environment. However, this can be compromised by various biotic and abiotic factors. Thus, their adaptation to various stress factors is crucial for their survival in the rhizosphere35,36. In this study, 35 native rhizobial isolates from the Cape fynbos were tested for their tolerance to different levels of salinity, acidity and antibiotics commonly produced by antagonistic soil-borne microbes. The results revealed strong variations in their tolerance to these environmental factors. The Cape fynbos is generally characterized by sandy acidic soils. The rhizosphere soils from our study sites (except Bredasdorp) were quiet acidic (pH 4.3 and 5.5), which implies adaption of these isolates to the low pH soils of the fynbos37. It was therefore not surprising that 51% of the isolates in this study showed tolerance to low pH (pH 5), a finding consistent with the report for Mesorhizobium38 in the Cape fynbos.
It was also important to note that Wiborgiella sessilifolia isolates from the alkaline soils of the Bredasdorp site grew better at neutral and acidic pH 5, suggesting their ability to naturally maintain an intracellular pH of between 7.2 and 7.5 even with an external unfavourable pH39,40,41. The 19% of test isolates that tolerated both acidic and alkaline conditions (pH 5 to pH 9) closely mirrored the rhizobia reported to nodulate wild Cajanas cajan at pH 3 and 11 and Acacia species at pH 4.8 and 8.841. Although alkalinity is less harmful to the survival of bacteria than acidity, it can lead to unavailability of certain essential minerals such as iron and manganese42,43, and thus affect plant growth and rhizobial survival. However, three isolates from Wiborgia obcordata which had M. australicum as their closest relative in the 16S rRNA, housekeeping and symbiotic gene analysis, could increase their cell division and grow well under alkaline conditions at pH 9 (TUTGWO11 and TUTGWO15) and pH 10 (TUTGWO9).
Furthermore, 72% of the 35 test isolates were tolerant to 3% NaCl concentrations, a finding consistent with an earlier report that isolates from wild legumes can tolerate high NaCl (3.5%) concentrations44. High pH and salinity are also a feature of deserts, such as the Thar desert of India45,46,47, and low pH is determinant for rhizobial selection by native legumes in central Brazil48. With climate change and the potential for an increase in irrigated crop production, soil salinity is likely to become a problem. Therefore, identifying rhizobial isolates with high salinity tolerance would be a solution for increased grain legume production. Additionally, in this study, 35% of the isolates could produce IAA at high concentrations, even higher than those reported for Mesorhizobium species49. IAA is a common by-product of L-tryptophane metabolism in several microorganisms, including rhizobia50, and secretion can promote plant root growth and increase nitrogen fixation via upregulation of the genes involved in carbon transport to N2-fixing bacteroids. Thus, N2-fixing rhizobia native to the sandy nutrient-poor soils of the Cape fynbos would have IAA production as an adaptation to supporting root growth of their homologous host legumes. This argument is re-enforced by the fact that the biosynthesis of IAA has been reported in species of Burkholderia, Rhizobium, Mesorhizobium and Bradyrhizobium in the Cape fynbos16,51.
Antibiosis or microbial warfare is common in resource-limited soils such as the low nutrients reported for the Cape fynbos. Under those conditions, soil microbes produce antibiotics that can inhibit cell growth and/or kill susceptible bacteria52,53. These antibiotics act by inhibiting protein synthesis and are therefore translational inhibitors to the target microbes. In this study, the antibiotic resistance of rhizobial isolates to streptomycin, kanamycin, ampicillin, chloramphenicol and neomycin was evaluated and found to differ markedly among isolates. About 37% of the isolates were susceptible to 10 µg ml−1 concentration of neomycin, an indication that this antibiotic was the least in limiting bacterial growth. Furthermore, 57% of the isolates in this study were susceptible to streptomycin, contrary to reports that fast-growing isolates from wild legumes are more tolerant of streptomycin54,55. More specifically, 15 isolates from Wiborgia obcordata, which were mostly related to Mesorhizobium australicum in the phylogenies, were susceptible to 25 µg ml−1 streptomycin. This indicates some vulnerability in their survival in soils that are rich in this antibiotic through inhibition of protein synthesis and translational errors in bacterial cells56.
Phylogenetic analysis of microsymbionts nodulating Polhillia, Wiborgia and Wiborgiella in the Cape fynbos
In this study,similarities in isolate alignments and positions were observed in the glnII, gyrB and atpD phylogenies. For example, in the Mesorhizobium trees, the four isolates TUTGWO5, TUTGWO6, TUTGWO7 and TUTGWO11 from W. obcordata consistently aligned closer to M. australicum reference strain with sequence identity of up to 99.6%, a clear indication that W. obcordata is nodulated by M. australicum strain. Furthermore, isolates from P. pallens (TUTPP4 and TUTPP10), W. sericea (TUTGWS2, TUTGWS4) and W. sessilifolia (TUTFWB31 and TUTFWB22) also showed consistency in their alignment with Mesorhizobium reference type strains, with low sequence similarity values (≤ 97%), possibly suggesting novel species within Mesorhizobium genus. These results support the reports by Lemaire et al15 and Dludlu et al.17, that Mesorhizobium is a common and underestimated nodulator of most legumes in the Cape region, capable of competing effectively with Burkholderia. Further evidence is provided by earlier studies which reported Mesorhizobium species to be compatible with a variety of shrub legumes endemic to fynbos region15,57,58,59.
Some isolates in this study showed incongruency in phylogenies. For example, the phylogenetic analyses of glnII for isolates TUTGWS1, TUTGWS2, TUTGWS4, TUTGWS5 from W. sericea, TUTGWO9, TUTGWO8, TUTGWO1, TUTGWO2 from W. obcordata and TUTPP9, TUTPP1 and TUTPP2 from P. pallens, as well as isolates TUTFWB26 and TUTFWB15 from W. sessilifolia, suggest that this gene was probably transferred from Mesorhizobium to Rhizobium as it showed incongruency with 16S rRNA, gyrB, atpD, nodC and nifH phylogenies. Our results therefore agree with reports from Lemaire et al.60 who revealed events of horizontal gene transfer between Rhizobium and Mesorhizobium genera in the Cape fynbos region. Furthermore, our results supports the suggestion by Gogarten et al.61 who reported that the evidence for potential gene transfer events generally fall into two classes: (1) identification of genes with an unduly high level of similarity to genes found in otherwise unrelated taxa, and (2) genes whose phylogenetic relationships are not congruent with the relationships inferred from other genes in their respective genomes. Reports from Andrew et al.62 confirms HGT as a common and unrestricted process which can happen within and between bacterial genera. The disagreement of glnII with 16S rRNA phylogeny in this study was also reported by Turner and Young63. Phylogenetic analysis of the glutamine synthase gene of rhizobia can also provide strong evidence for horizontal or lateral gene transfer between different genera of rhizobia63. Because of possible horizontal gene transfer (or recombination) and variable mutations, single gene-based phylogenetic trees do not always reflect organismal phylogeny64.
The identification of Rhizobium glnII gene in isolates TUTGWS2, TUTGWS4, TUTGWS1, TUTGWS5, TUTGWO9, TUTGWO8, TUTGWO1, TUTGWO2, TUTPP9, TUTPP1, TUTPP2, TUTFWB26 and TUTFWB15 strongly supports the view that horizontal transfer of this gene occurred in fynbos soil. Some studies have reported that wild species of Phaseolus such as Phaseolus parvulus, and Phaseolus pauciflorus are nodulated by Bradyrhizobium species65,66. A few years ago, Bradyrhizobium paxllaeri and Bradyrhizobium icense were identified in Peru as novel bradyrhizobial species from root nodules of Phaseolus lunatus67. Even in Angola within Sub-Saharan Africa, bradyrhizobia were also isolated from common bean nodules68.
Isolate TUTGWO14 from W. obcordata grouped with Rhizobium in the nodC phylogeny, but with Mesorhizobium australicum in the 16S rRNA, atpD, glnII and gyrB phylogenies. This again suggests a transfer of symbiotic nodC gene from Mesorhizobium to Rhizobium, and thus mirrored the previous reports of the transfer of symbiotic genes between different groups of bacterial species65,69,70,71,72. Incongruency between the phylogenies of symbiotic (nod and nif) genes and those of chromosomal genes have been reported in a number of studies on rhizobia and has been confirmed as an indication of horizontal inheritance of the symbiosis genes73,74,75. Furthermore, a previous report from the Cape fynbos region has indicated that species within the Crotalarieae are capable of horizontal transfer of symbiosis genes between different genera of rhizobia17. Another study indicated has suggested that Sphaerophysa salsula isolates identified as Rhizobium using 16S rRNA gene sequences showed similar nifH sequences to those of the Mesorhizobium isolates, while a Bradyrhizobium isolate (16S rRNA) from Caragana intermedia had similar nodC sequence to the Mesorhizobium isolates76.
In this study, the phylogenetic incongruency found between glnII and the 16S rRNA, gyrB, atpD, nodC and nifH trees of our isolates indicates their genome plasticity and the lack of clarity in species boundaries, which together support horizontal gene transfer in the test isolates. Ochman et al.77 suggested that inter-specific recombination is responsible for the blurring of species boundaries, while phylogenetic incongruency documents gene transfer-mediated organismal diversification. The transfer of core and symbiotic genes between rhizobial genera adapted to local soil conditions can be the consequences of broad mutualistic relationships between test wild legumes and rhizobial genera.
Conclusion
The morpho-genetically diverse rhizobia isolated from Polhillia, Wiborgia, and Wiborgiella species from the Cape fynbos region of South Africa were found to tolerate exposure to factors such as acidity, alkalinity, salinity and antibiotics. These isolates also differed in their varying abilities to solubilize P and/or produce IAA, thus suggesting varying ability to promote plant growth. In this study, Mesorhizobium australicum is the microsymbiont nodulating Wiborgia obcordata, while Polhillia pallens, Wiborgia sericea and Wiborgiella sessilifolia are nodulated by some possible novel Mesorhizobium spp.. The genomes arrangement of the test isolates indicate genetic plasticity which suggests the need to evaluate the symbiotic functioning and competitive advantage of these isolates using their homologous host plants.
Data availability
Data used in this study are available under following accession numbers. 16SrRNA (MW158788-MW158799), atpD (MW159787- MW159799), glnII (MW159804-MW159813), gyrB (MW159814- MW159823); nifH (MW159830-MW159846, MW161258); nodC (MW159847- MW159861).
References
Stirton, C. H. Polhillia, a new genus of papilionoid legumes endemic to South Africa. South African J. Bot. 52, 167–180 (1986).
Boatwright, J. S., Tilney, P. M. & Van Wyk, B.-E. Taxonomy of Wiborgiella (Crotalarieae, Fabaceae), a genus endemic to the greater Cape Region of South Africa. Syst. Bot. 35, 325–340 (2010).
Moiloa, N. A., Chimphango, S. B. M. & Muasya, A. M. A phylogenetic study of the genus Wiborgia (Crotalarieae, Fabaceae). South African J. Bot. 115, 179–193 (2018).
Myers, N., Mittermeier, R. A., Mittermeier, C. G., Da Fonseca, G. A. B. & Kent, J. Biodiversity hotspots for conservation priorities. Nature 403, 853 (2000).
Goldblatt, P. & Manning, J. C. Plant diversity of the Cape region of southern Africa. Ann. Missouri Bot. Gard. 281–302 (2002).
Forest, F., Colville, J. F. & Cowling, R. M. Evolutionary diversity patterns in the Cape flora of South Africa. in Phylogenetic Diversity 167–187 (Springer, 2018).
Boatwright, J. S. & Cupido, C. N. Aspalathus crewiana sp. Nov. (Crotalarieae, Fabaceae) from the Western Cape Province, South Africa. Nord. J. Bot. 29, 513–517 (2011).
Mpai, T., Jaiswal, S. K. & Dakora, F. D. Accumulation of phosphorus and carbon and the dependency on biological N-2 fixation for nitrogen nutrition in Polhillia, Wiborgia and Wiborgiella species growing in natural stands in cape fynbos, South Africa. SYMBIOSIS (2020).
Van Zwieten, L. et al. Enhanced biological N 2 fixation and yield of faba bean (Vicia faba L.) in an acid soil following biochar addition: Dissection of causal mechanisms. Plant Soil 395, 7–20 (2015).
Jaiswal, S. K., Naamala, J. & Dakora, F. D. Nature and mechanisms of aluminium toxicity, tolerance and amelioration in symbiotic legumes and rhizobia. Biol. Fertil. Soils https://doi.org/10.1007/s00374-018-1262-0 (2018).
Araújo, S. S. et al. Abiotic stress responses in legumes: Strategies used to cope with environmental challenges. CRC. Crit. Rev. Plant Sci. 34, 237–280 (2015).
Etesami, H., Alikhani, H. & Akbari, A. Evaluation of plant growth hormones production (IAA) ability by Iranian soils rhizobial strains and effects of superior strains application on wheat growth. World Appl. Sci. J. 6, 1576–1584 (2009).
Ibny, F. Y. I., Jaiswal, S. K., Mohammed, M. & Dakora, F. D. Symbiotic effectiveness and ecologically adaptive traits of native rhizobial symbionts of Bambara groundnut (Vigna subterranea L. Verdc.) in Africa and their relationship with phylogeny. Sci. Rep. 9, 1–17 (2019).
Kanu, S. A. & Dakora, F. D. Symbiotic nitrogen contribution and biodiversity of root-nodule bacteria nodulating Psoralea species in the Cape Fynbos, South Africa. Soil Biol. Biochem. 54, 68–76 (2012).
Lemaire, B. et al. Symbiotic diversity, specificity and distribution of rhizobia in native legumes of the Core Cape Subregion (South Africa). FEMS Microbiol. Ecol. 91, 2–17 (2015).
Brink, C., Postma, A. & Jacobs, K. Rhizobial diversity and function in rooibos (Aspalathus linearis) and honeybush (Cyclopia spp.) plants: A review. South African J. Bot. 110, 80–86 (2017).
Dludlu, M. N., Chimphango, S. B. M., Walker, G., Stirton, C. H. & Muasya, A. M. Horizontal gene transfer among rhizobia of the Core Cape Subregion of southern Africa. South African J. Bot. 118, 342–352 (2018).
Aliero, B. L. Effects of sulphuric acid, mechanical scarification and wet heat treatments on germination of seeds of African locust bean tree, Parkia biglobosa. African J. Biotechnol. 3, 179–181 (2004).
Hematifar, M., Tehranifar, A. & Abedi, B. Facilitating Seed Germination of Eight Species of Hawthorn (Crataegus spp.) Native of Iran, Using Chemical Scarification and Cold Stratification. Iran. J. Seed Res. 4, 13–22 (2018).
Vincent, J. M. A Manual for the Practical Study of Root-Nodule Bacteria: A Manual for the Practical Study of Root-Nodule Bacteria Vol. 15 (Blackwell Scientific, 1970).
Unkovich, M. & Baldock, J. Measurement of asymbiotic N2 fixation in Australian agriculture. Soil Biol. Biochem. 40, 2915–2921 (2008).
Somasegaran, P. & Hoben, H. J. Handbook for Rhizobia: Methods in Legume-Rhizobium Technology (Springer, 2012).
Sneath, P. H. A., Sokal, R. R. Numerical taxonomy. The principles and practice of numerical classification. (1973).
Rohlf, F. J., Applied Biostatistics, I. & Exeter Software (Firm). NTSYS-pc : Numerical taxonomy and multivariate analysis system. (Applied Biostatistics, Inc., 2009).
Hall, T. BioEdit version 7.0. 0. Distributed by the author, website: www.mbio.ncsu.edu/BioEdit/bioedit.html. (2004).
Edgar, R. C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 32, 1792–1797 (2004).
Tamura, K., Stecher, G., Peterson, D., Filipski, A. & Kumar, S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 30, 2725–2729 (2013).
Nei, M. & Kumar, S. Molecular Evolution and Phylogenetics (Oxford University Press, 2000).
Saitou, N. & Nei, M. The neighbor-joining method : A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4, 406–425 (1987).
Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 39, 783–791 (1985).
Morón, B. et al. Low pH changes the profile of nodulation factors produced by Rhizobium tropici CIAT899. Chem. Biol. 12, 1029–1040 (2005).
Moroenyane, I., Chimphango, S. B. M., Wang, J., Kim, H. K. & Adams, J. M. Deterministic assembly processes govern bacterial community structure in the Fynbos, South Africa. Microb. Ecol. 72, 313–323 (2016).
Dabo, M., Jaiswal, S. K. & Dakora, F. D. Phylogenetic evidence of allopatric speciation of bradyrhizobia nodulating cowpea ( Vigna unguiculata L. walp ) in South African and Mozambican soils Department of Crop Sciences, Tshwane University of Technology, Private Bag Chemistry Department. Tshw. FEMS Microbiol. Ecol. 19, 1–14 (2019).
Singh, S. K., Jaiswal, S. K., Vaishampayan, A. & Dhar, B. Physiological behavior and antibiotic response of soybean (Glycine max L.) nodulating rhizobia isolated from Indian soils. African J. Microbiol. Res. 7, 2093–2102 (2013).
Hayat, R., Ali, S., Amara, U., Khalid, R. & Ahmed, I. Soil beneficial bacteria and their role in plant growth promotion: A review. Ann. Microbiol. 60, 579–598 (2010).
Berendsen, R. L., Pieterse, C. M. J. & Bakker, P. A. H. M. The rhizosphere microbiome and plant health. Trends Plant Sci. 17, 478–486 (2012).
Maseko, S. T. & Dakora, F. D. Rhizosphere acid and alkaline phosphatase activity as a marker of P nutrition in nodulated Cyclopia and Aspalathus species in the Cape fynbos of South Africa. South African J. Bot. 89, 289–295 (2013).
Dludlu, M. N., Chimphango, S., Stirton, C. H. & Muasya, A. M. Differential preference of burkholderia and mesorhizobium to pH and soil types in the core cape subregion, South Africa. Genes 9, 2 (2017).
Graham, P. H. et al. Acid pH tolerance in strains of Rhizobium and Bradyrhizobium, and initial studies on the basis for acid tolerance of Rhizobium tropici UMR1899. Can. J. Microbiol. 40, 198–207 (1994).
Fikri-Benbrahim, K., Chraibi, M., Lebrazi, S., Moumni, M. & Ismaili, M. Phenotypic and Genotypic Diversity and Symbiotic Effectiveness of Rhizobia Isolated from Acacia sp. Grown in Morocco. J. Agric. Sci. Technol. 19, (2017).
Moumni, M., Fikri-Benbrahim, K., Ismaili, M., Lebrazi, S. & Chraibi, M. Phenotypic and G enotypic D iversity and S ymbiotic E ffectiveness of R hizobia I solated from Acacia sp. G rown in Morocco. JKUAT (2018). http://hdl.handle.net/123456789/3738
Farissi, M. et al. Growth, nutrients concentrations, and enzymes involved in plants nutrition of alfalfa populations under saline conditions. (2014).
Lebrazi, S. & Benbrahim, K. F. Environmental stress conditions affecting the N2 fixing Rhizobium-legume symbiosis and adaptation mechanisms. African J. Microbiol. Res. 8, 4053–4061 (2014).
Bhargava, Y., Murthy, J. S. R., Kumar, T. V. R. & Rao, M. N. Phenotypic, stress tolerance and plant growth promoting characteristics of rhizobial isolates from selected wild legumes of semiarid region, Tirupati, India. Adv. Microbiol. 6, 1 (2016).
Sankhla, I. S. et al. Molecular characterization of nitrogen fixing microsymbionts from root nodules of Vachellia (Acacia) jacquemontii, a native legume from the Thar Desert of India. Plant Soil 410, 21–40 (2017).
Rathi, S. et al. Selection of Bradyrhizobium or Ensifer symbionts by the native Indian caesalpinioid legume Chamaecrista pumila depends on soil pH and other edaphic and climatic factors. FEMS Microbiol. Ecol. 94, 1–17 (2018).
Choudhary, D., Rai, M. K., Shekhawat, N. S. & Kataria, V. In vitro propagation of Farsetia macrantha Blatt. \& Hallb.: An endemic and threatened plant of Indian Thar Desert. Plant Cell, Tissue Organ Cult. 142, 519–526 (2020).
de Castro Pires, R. et al. Soil characteristics determine the rhizobia in association with different species of Mimosa in central Brazil. Plant Soil 423, 411–428 (2018).
Verma, J. P., Yadav, J., Tiwari, K. N. & Kumar, A. Effect of indigenous Mesorhizobium spp. and plant growth promoting rhizobacteria on yields and nutrients uptake of chickpea (Cicer arietinum L.) under sustainable agriculture. Ecol. Eng. 51, 282–286 (2013).
Datta, C. & Basu, P. S. Indole acetic acid production by a Rhizobium species from root nodules of a leguminous shrub, Cajanus cajan. Microbiol. Res. 155, 123–127 (2000).
Brink, C. J. Plant Growth-Promoting Properties of Fynbos Rhizobia and Their Diversity (Stellenbosch University, 2018).
Naamala, J., Jaiswal, S. K. & Dakora, F. D. Antibiotics resistance in Rhizobium: Type, process, mechanism and benefit for agriculture. Curr. Microbiol. 72, 804–816 (2016).
Baba, T. & Schneewind, O. Instruments of microbial warfare: Bacteriocin synthesis, toxicity and immunity. Trends Microbiol. 6, 66–71 (1998).
Menezes, K. A. S., Nunes, G. F. O. & Sampaio, A. A. Diversity of new root nodule bacteria from Erythrina velutina Willd., a native legume from the Caatinga dry forest (Northeastern Brazil). Rev Cienc Agrárias 39, 222–233 (2016).
Pagano, M. C. Rhizobia associated with neotropical tree Centrolobium tomentosum used in riparian restoration. Plant, Soil Environ. 54, 498–508 (2008).
Hong, W., Zeng, J. & Xie, J. Antibiotic drugs targeting bacterial RNAs. Acta Pharm. Sin. B 4, 258–265 (2014).
Elliott, G. N. et al. Nodulation of Cyclopia spp. (Leguminosae, Papilionoideae) by Burkholderia tuberum. Ann. Bot. 100, 1403–1411 (2007).
Hassen, A. I., Bopape, F. L., Habig, J. & Lamprecht, S. C. Nodulation of rooibos (Aspalathus linearis Burm. f.), an indigenous South African legume, by members of both the α-proteobacteria and β-proteobacteria. Biol. Fertil. Soils 48, 295–303 (2012).
Gerding, M., O’Hara, G. W., Bräu, L., Nandasena, K. & Howieson, J. G. Diverse Mesorhizobium spp. with unique nodA nodulating the South African legume species of the genus Lessertia. Plant Soil 358, 385–401 (2012).
Lemaire, B. et al. Recombination and horizontal transfer of nodulation and ACC deaminase (acdS) genes within Alpha-and Beta-proteobacteria nodulating legumes of the Cape Fynbos biome. FEMS Microbiol. Ecol. 91, (2015).
Gogarten, J. P., Doolittle, W. F. & Lawrence, J. G. Prokaryotic evolution in light of gene transfer. Mol. Biol. Evol. 19, 2226–2238 (2002).
Andrews, M. et al. Horizontal transfer of symbiosis genes within and between rhizobial genera: Occurrence and importance. Genes 9, 321 (2018).
Turner, S. L. & Young, J. P. W. The glutamine synthetases of rhizobia : Phylogenetics and evolutionary implications. 17, 309–319 (2000).
Gevers, D. et al. Re-evaluating prokaryotic species. Nat. Rev. Microbiol. 3, 733 (2005).
Ormeño-Orrillo, E. et al. Phylogenetic evidence of the transfer of nodZ and nolL genes from Bradyrhizobium to other rhizobia. Mol. Phylogenet. Evol. 67, 626–630 (2013).
Parker, M. A., Lafay, B., Burdon, J. J. & Van Berkum, P. Conflicting phylogeographic patterns in rRNA and nifD indicate regionally restricted gene transfer in Bradyrhizobiumaa. Microbiology 148, 2557–2565 (2002).
Duran, D. et al. Bradyrhizobium paxllaeri sp. Nov. and Bradyrhizobium icense sp. Nov., nitrogen-fixing rhizobial symbionts of Lima bean (Phaseolus lunatus L.) in Peru. Int. J. Syst. Evol. Microbiol. 64, 2072–2078 (2014).
Grönemeyer, J. L., Kulkarni, A., Berkelmann, D., Hurek, T. & Reinhold-Hurek, B. Identification and characterization of rhizobia indigenous to the Okavango region in Sub-Saharan Africa. Appl. Environ. Microbiol. https://doi.org/10.1128/AEM.02417-14 (2014).
Rogel, M. A., Ormeno-Orrillo, E. & Romero, E. M. Symbiovars in rhizobia reflect bacterial adaptation to legumes. Syst. Appl. Microbiol. 34, 96–104 (2011).
Lindstrom, K., Murwira, M., Willems, A. & Altier, N. The biodiversity of beneficial microbe-host mutualism : The case of rhizobia. Res. Microbiol. 161, 453–463 (2010).
Barcellos, F. G., Menna, P., da Silva Batista, J. S. & Hungria, M. Evidence of horizontal transfer of symbiotic genes from a Bradyrhizobium japonicum inoculant strain to indigenous diazotrophs Sinorhizobium (Ensifer) fredii and Bradyrhizobium elkanii in a Brazilian Savannah soil. Appl. Environ. Microbiol. 73, 2635–2643 (2007).
Jourand, P., Mateille, T., Fargette, M. & Rapior, S. Nematostatic activity of aqueous extracts of West African Crotalaria species. Nematology 6, 765–771 (2004).
Chen, W.-M. et al. Legume symbiotic nitrogen fixation by β-proteobacteria is widespread in nature. J. Bacteriol. 185, 7266–7272 (2003).
Aoki, S., Ito, M. & Iwasaki, W. From β-to α-proteobacteria: The origin and evolution of rhizobial nodulation genes nodIJ. Mol. Biol. Evol. 30, 2494–2508 (2013).
Moulin, L., Béna, G., Boivin-Masson, C. & Stkepkowski, T. Phylogenetic analyses of symbiotic nodulation genes support vertical and lateral gene co-transfer within the Bradyrhizobium genus. Mol. Phylogenet. Evol. 30, 720–732 (2004).
Lu, Y. L. et al. Genetic diversity and biogeography of rhizobia associated with Caragana species in three ecological regions of China. Syst. Appl. Microbiol. 32, 351–361 (2009).
Ochman, H., Lawrence, J. G. & Groisman, E. A. Lateral gene transfer and the nature of bacterial innovation. Nature 405, 299–304 (2000).
Acknowledgements
This study was supported with grants from the National Research Foundation, the Department of Science and Technology, Tshwane University of Technology, and the South African Research Chair in Agrochemurgy and Plant Symbioses. TM is grateful to GreenMatter for providing a partial Scholarship. We thank Mr Matthias Streicher and his family for granting us permission to collect seeds of Polhillia species on their farm, as well as to sample rhizosphere soils for use as bacterial inoculum for plant nodulation in the glasshouse. We are also grateful to the Cape Nature Conservation for granting us a Permit to sample plant material, rhizosphere soils, and where possible, root nodules from Wiborgia and Wiborgiella species from various locations in the Cape fynbos.
Author information
Authors and Affiliations
Contributions
T.M. performed the experiments. T.M. and S.K.J. analysed data and drafted the manuscript. C.N.C. helped in samples collection from fynbos region. F.D.D. edited the manuscript and provided funding for the study.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mpai, T., Jaiswal, S.K., Cupido, C.N. et al. Ecological adaptation and phylogenetic analysis of microsymbionts nodulating Polhillia, Wiborgia and Wiborgiella species in the Cape fynbos, South Africa. Sci Rep 11, 23614 (2021). https://doi.org/10.1038/s41598-021-02766-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-02766-2
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.