Abstract
A molecular potential model is proposed and the solutions of the radial Schrӧdinger equation in the presence of the proposed potential is obtained. The energy equation and its corresponding radial wave function are calculated using the powerful parametric Nikiforov–Uvarov method. The energies of cesium dimer for different quantum states were numerically obtained for both negative and positive values of the deformed and adjustable parameters. The results for sodium dimer and lithium dimer were calculated numerically using their respective spectroscopic parameters. The calculated values for the three molecules are in excellent agreement with the observed values. Finally, we calculated different expectation values and examined the effects of the deformed and adjustable parameters on the expectation values.
Similar content being viewed by others
Introduction
In the recent time, exponential-type potential has been the subject of interest in the quantum mechanics which greatly popularized the relativistic and non-relativistic wave equations such as the Schrӧdinger equation, Klein–Gordon equation, Dirac equation and others1,2,3,4,5,6,7,8,9,10,11,12,13,14,15. The approximate solutions of these wave equations have been obtained mostly for one-dimensional system with various exponential-type potentials using different approximation methods developed by different authors. The frequently used methods are Nikiforov–Uvarov method16,17, asymptotic iteration method18, supersymmetric approach19,20, factorization method21, exact and proper quantization rule22,23. Recently, Ikot et al.24,25 have used a new approach called NU Functional analysis method. The different methods have different approach for the solutions of the wave equations but give results that are approximately the same. For instance, the solutions of the radial Schrӧdinger equation under the Deng-Fan potential model has been studied by Dong and Gu26 using factorization method. Zhang et al.27 and Onate et al.28 respectively, also studied the potential via supersymmetry quantum mechanics and parametric Nikiforov–Uvarov method. The results of these authors agreed with one another.
The solutions of the wave equations studied for different potentials, have been applied to the study of several systems such as Theoretic quantities29,30,31,32 and Thermal properties (mean energy, heat capacity, free energy and entropy)33,34,35,36,37,38. In ref.27, the result was used to study the rotation transition frequency for HF. In ref.28, the wave function was used to study some theoretic quantities such Shannon entropy and Rényi entropy. In ref.39, the problem of \(so(2,2)\) was studied under the Pӧschl-Teller potential. Several authors have also studied the energy eigenvalues for many diatomic molecules on molecular dynamics and spectroscopy in the field of chemistry and molecular physics40,41. This provides explanations about the dynamics and physical properties of some molecules. The potential energy function involved are used to study the bonding between atoms, hence the predictions to the behaviour of some class of molecules42. Some of these potentials can be used to describe some experimental values. Generally, a good empirical internuclear potential function should reproduce the experimental potential energy curves as determined by the RKR method. Considering this, the present study wants to examine an approximate solutions of the Schrӧdinger equation with a new modified and deformed exponential-type molecular potential model confined on a cesium dimer, sodium dimer and lithium dimer. The study also aims to investigate the potential with two different values for each of the deformed parameter and adjustable parameter under the same cesium dimer. This potential has not been reported for any study yet to the best of our understanding.
The cesium dimer is an important molecule that has many applications, e.g. vibrational cooling of molecules, population dynamics, and even coherent control43,44,45,46,47. The cesium molecule is an attractive system for examining a possible variation of the electron-to-proton mass ratio and of the fine-structure constant48. It is noted that \(3^{3} \sum_{g}^{ + }\) state of cesium dimer has a strong Fermi contact interaction with the nuclei, and possesses a large hyperfine splitting49. The potential energy curve of the cesium dimer for \(3^{3} \sum_{g}^{ + }\) and \(a^{3} \sum_{u}^{ + }\) states has been reported in ref.49,50. The modified and deformed exponential-type molecular potential model under consideration, is given as
where \(C\) is a modified parameter, \(q_{0}\) is a deformed parameter and \(q_{1}\) is an adjustable parameters whose value can be taken as \(\pm 1.\) When the value of the adjustable parameter equals the value of the deformed parameter within \(\pm 1,\) the results of potential (1) gives other useful results. \(D_{e}\) is the dissociation energy \(r_{e}\) is the equilibrium bond separation and \(\alpha\) is the screening parameter. Its numerical value can be obtain using the formula
where \(W\) is the Lambert function, \(\mu\) is the reduced mass, \(c\) is the speed of light and \(\omega_{e}\) is the vibrational frequency.
Parametric Nikiforov–Uvarov method
The parametric Nikiforov–Uvarov method is one of the shortest and accurate traditional techniques to solve bound state problems. This method was derived from the conventional Nikiforov–Uvarov method by Tezcan and Sever17. According to the authors, the reference equation for the parametric Nikiforov–Uvarov is given as
Following the work of these authors, the condition for eigenvalues equation and wave function are respectively given by17,51
The parametric constants in Eqs. (3) and (4) are deduced as follows
The radial Schrӧdinger equation and the interacting potential
To obtain the energy eigenvalues of the Schrödinger equation with potential (1), we consider the original Schrödinger equation given by
Setting the wave function \(\psi (r) = U_{n,\ell } (r)Y_{m,\ell } (\theta ,\phi )r^{ - 1} ,\) and consider the radial part of the Schrӧdinger equation, Eq. (7) becomes
where \(V(r)\) is the interacting potential given in Eq. (1), \(E_{n\ell }\) is the non-relativistic energy of the system, \(\hbar\) is the reduced Planck’s constant, \(\mu\) is the reduced mass, \(n\) is the quantum number, \(U_{n\ell } (r)\) is the wave function. Substituting Eq. (1) into (8), and by defining \(y = \frac{1}{{e^{ - \alpha r} }},\) the radial Schrӧdinger equation with the deformed exponential-type potential turns to be
where
Comparing Eq. (9) with Eq. (3), the parametric constants in Eq. (6) are obtain as follows
Substituting the parameters in Eq. (13) into Eq. (4), we have the energy equation for the system as
and the corresponding wave function is obtain when the values of \(\alpha_{10}\) to \(\alpha_{13}\) in Eq. (6) are substituted into Eq. (5),
Expectation values
In this section, we calculated some expectation values using Hellmann-Faynman Theorey (HFT)52,53,54,55,56. When a Hamiltonian \(H\) for a given quantum system is a function of some parameter \(v,\) the energy-eigenvalue \(E_{n}\) and the eigenfunction \(U_{n} (v)\) of \(H\) are given by
with the effective Hamiltonian as
Setting \(v = \mu\) and \(v = D_{e,} ,\) we have the expectation values of \(p^{2}\) and \(V\) respectively are
The average deviation of the calculated results from the experimental results is obtained using the formula
where \(E_{ER}\) is the experimental data, \(E_{CR}\) is the calculated values and \(N\) is the total number of the experimental data.
Discussion of result
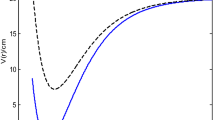
The comparison of the observed values of RKR and calculated values for \(3^{3} \Sigma_{g}^{ + }\) state of cesium dimer with \(q_{0} = q_{1} = 1,\) \(q_{0} = q_{1} = - 1,\) \(D_{e} = 2722.28\;{\text{cm}}^{ - 1} ,\) \(r_{e} = 5.3474208\) Å, and \(\omega_{e} = 28.891\;{\text{cm}}^{ - 1}\) is reported in Table 1. The results for two values for each of the deformed parameter and adjustable parameter agreed with the observed values of the cesium dimer. However, the results obtained with \(q_{0} = q_{1} = - 1\) are higher than their counterpart obtained with \(q_{0} = q_{1} = 1.\) In Table 2, the comparison of vibrational energies of sodium dimer and lithium dimer respectively are reported. When the deformed parameter and the adjustable parameter are taken as one with \(D_{e} = 79885\;{\text{cm}}^{ - 1} ,\) \(r_{e} = 1.097\) Å, \(\omega_{e} = 2358.6\;{\text{cm}}^{ - 1} ,\) the results agreed with the observed values of \(5^{1} \Delta_{g}\) state of sodium dimer. Taken the deformed parameter and adjustable parameter respectively as minus one, with \(D_{e} = 2722.28\;{\text{cm}}^{ - 1} ,\) \(r_{e} = 4.173\) Å and \(\omega_{e} = 65.130\;{\text{cm}}^{ - 1} ,\) the results obtained correspond to the observed values of lithium dimer.
To deduce the effect of the deformed and adjustable parameters on the numerical values and discrepancy of the calculated results from the experimental data, we used the formula given in Eq. (28). For cesium dimer, the average percentage deviation for \(q_{0} = q_{1} = 1\) is 0.0038% while the average percentage deviation for \(q_{0} = q_{1} = - 1\) is 0.0002%. For sodium dimer with \(q_{0} = q_{1} = 1,\) the average percentage deviation is 0.0342% while the average percentage deviation for lithium dimer with \(q_{0} = q_{1} = - 1,\) is 0.0016%. In Table 3, we presented the numerical results for the two different expectation values calculated in Eq. (20) and Eq. (21). The effect of the deformed and adjustable parameters on the expectation values can be seen in Table 3. For \(\left\langle {p^{2} } \right\rangle ,\) the values obtained with \(q_{0} = q_{1} = 1\) are higher than their counterpart obtained with \(q_{0} = q_{1} = - 1.\) However, for \(\left\langle V \right\rangle_{n}\) the values obtained with \(q_{0} = q_{1} = - 1\) are higher than their counterpart obtained with \(q_{0} = q_{1} = 1.\)
The effect of the screening parameter on the energy eigenvalues with two values each of the deformed parameter and adjustable parameter are shown in Fig. 1. In each case, the energy of the system varies inversely with the screening parameter. The energy of the system for \(q_{0} = q_{1} = 1\) are lower than the energy of the system for \(q_{0} = q_{1} = - 1.\)
Conclusion
The solutions of a one-dimensional Schrӧdinger equation is obtained for a molecular potential model using parametric Nikiforov–Uvarov method. By changing the numerical values of the deformed parameter and adjustable parameter, the results obtained for different molecules agreed with experimental values. However, the results obtained with \(q_{0} = q_{1} = - 1\) are closer to the experimental values compared with the results obtained with \(q_{0} = q_{1} = 1.\) The results for lithium dimer are more closer to the experimental values followed by the results for cesium dimer obtained with \(q_{0} = q_{1} = - 1.\)
References
Hu, X. T., Liu, J. Y. & Jia, C. S. The 33Σ+g state of Cs2 molecule. Comput. Theor. Chem. 1019, 137 (2013).
Lino da Silva, M., Guerra, V., Loureiro, J. & Sá, P. A. Vibrational distributions in N2 with an improved calculation of energy levels using the RKR method. Chem. Phys. 348, 187 (2008).
Egrifes, H., Demirhan, D. & Buyukkılıc, F. Exact solutions of the Schrödinger equation for the deformed hyperbolic potential well and the deformed four-parameter exponential type potential. Phys. Lett. A 275, 229 (2000).
Horchani, R., Al-Kindi, N. & Jelassi, H. Ro-vibrational energies of caesium molecules with the Tietz-Hua oscillator. Mol. Phys. https://doi.org/10.1080/00268976.2020.1812746 (2020).
Whang, T. & Cheng, C.-P. Observation of L uncoupling in the 51Δg Rydberg state of Na2. J. Chem. Phys. 123, 224303 (2005).
Farout, M., Bassalat, A. & Ikhdair, S. M. Exact quantized momentum eigenvalues and eigenstates of a general potential model. J. Appl. Math. Phys. 8, 1434 (2020).
Farout, M., Bassalat, A. & Ikhdair, S. M. Feinberg-Horodecki exact momentum states of improved deformed exponential-type potential. J. Appl. Math. Phys. 8, 1496 (2020).
Jia, C.-S. et al. Equivalence of the Wei potential model and Tietz potential model for diatomic molecules. J. Chem. Phys. 137, 014101 (2012).
Liu, J.-Y., Hu, X.-T. & Jia, C.-S. Molecular energies of the improved Rosen−Morse potential energy model. Can. J. Chem. 92, 40 (2014).
Song, X.-Q., Wang, C.-W. & Jia, C.-S. Thermodynamics properties of sodium dimer. Chem. Phys. Lett. 673, 50 (2017).
Hamzavi, M., Rajabi, A. A. & Thylwe, K.-E. The rotation-vibration spectrum of diatomic molecules with the Tietz-Hua rotating oscillator. Int. J. Quant. Chem. 112, 2701 (2012).
Falaye, B. J., Oyewumi, K. J., Ikhdair, S. M. & Hamzavi, M. Eigensolution techniques, their applications and Fisherʼs information entropy of the Tietz-Wei diatomic molecular model. Phys. Scr. 89, 115204 (2014).
Onyeaju, M. C. & Onate, C. A. Vibrational entropy and complexity measures in modified Pöschl-Teller plus Woods-Saxon potential. Few-Body Syst. 61, 21 (2020).
Okorie, U. S., Ibekwe, E. E., Onyeaju, M. C. & Ikot, A. N. Solutions of the Dirac and Schrödinger equations with shifted Tietz-Wei potential. Eur. Phys. J. Plus 133, 433 (2018).
Onate, C. A., Adebiyi, L. S. & Bankole, D. T. Eigensolutions and theoretic quantities under the nonrelativistic wave equation. J. Theor. Comput. Chem. 19, 2050007 (2020).
Nikiforov, A. F. & Uvarov, V. B. Special Functions of Mathematical Physics (Birkhäuser, 1988).
Tezcan, C. & Sever, R. A general approach for the exact solution of the Schrödinger equation. Int. J. Theor. Phys. 48, 337 (2009).
Bayrak, O. & Boztosun, I. Bound state solutions of the Hulthén potential by using the asymptotic iteration method. Phys. Scr. 76, 92 (2007).
Witten, E. Dynamical breaking of supersymmetry. Nucl. Phys. B 188, 513 (1981).
Cooper, F., Khare, A. & Sukhatme, U. Supersymmetry and quantum mechanics. Phys. Rep. 251, 267 (1995).
Dong, S.-H. Factorization Method in Quantum Mechanics (Springer, 2007).
Ma, Z.-Q. & Xu, B.-W. Quantum correction in exact quantization rules. Europhys. Lett. 69, 685 (2005).
Qiang, W.-C. & Dong, S.-H. Proper quantization rule. EPL 89, 10003 (2010).
Ikot, A. N., Okorie, U. S., Rampho, G. J. & Amadi, P. O. Approximate analytical solutions of the Klein-Gordon equation with generalized Morse potential. Int. J. Thermophys. 42, 10 (2021).
Ikot, A. N. et al. The Nikiforov–Uvarov-functional analysis (NUFA) method: A new approach for solving exponential-type potential. Few Body Syst. 62, 1 (2021).
Dong, S.-H. & Gu, X. Y. Arbitrary l state solutions of the Schrödinger equation with the Deng-Fan molecular potential. J. Phys. Conf. Ser. 96, 012109 (2008).
Zhang, L. H., Li, X. P. & Jia, C.-S. Approximate solutions of the Schrödinger equation with the generalized Morse potential model including the centrifugal term. Int. J. Quant. Chem. 111, 1870 (2011).
Onate, C. A., Ikot, A. N., Onyeaju, M. C., Ebomwonyi, O. & Idiodi, J. O. A. Effect of dissociation energy on Shannon and Rẻnyi entropies. Karbala Int. J. Mod. Scien. 4, 134–142 (2018).
Najafizade, S. A., Hassanabadi, H. & Zarrinkamar, S. Nonrelativistic Shannon information entropy for Kratzer potential. Chin. Phys. B 25, 040301 (2016).
Ghafourian, M. & Hassanabadi, H. Shannon information entropies for the three-dimensional Klein-Gordon problem with the Poschl-Teller potential. J. Korean Phys. Soc. 68, 1267 (2016).
Boumali, A. & Labidi, M. Shannon entropy and Fisher information of the one-dimensional Klein-Gordon oscillator with energy-dependent potential. Mod. Phys. Lett. A 33, 1850033 (2018).
Idiodi, J. O. A. & Onate, C. A. Entropy, Fisher information and variance with Frost-Musulin potential. Commum. Theor. Phys. 66, 269 (2016).
Okorie, U. S., Ikot, A. N., Chukwuocha, E. O. & Rampho, G. J. Thermodynamic properties of improved deformed exponential-type potential (IDEP) for some diatomic molecules. Results Phys. 17, 103078 (2020).
Khordad, R. & Ghanbari, A. Theoretical prediction of thermodynamic functions of TiC: Morse ring-shaped potential. Low Temp. Phys. 199, 1198 (2020).
Wang, J. et al. Thermodynamic properties for carbon dioxide. ACS Omega 4, 19193 (2019).
Onate, C. A. & Ojonubah, J. O. Relativistic and nonrelativistic solutions of the generalized Pὅschl-Teller and hyperbolical potentials with some thermodynamic properties. Int. J. Mod. Phys. E 24, 1550020 (2015).
Yahya, W. A. & Oyewumi, K. J. Thermodynamic properties and approximate solutions of the ℓ-state Pöschl-Teller-type potential. J. Ass. Arab. Univ. Basic Appl. Sci. 21, 53 (2016).
Oyewumi, K. J., Falaye, B. J., Onate, C. A., Oluwadare, O. J. & Yahya, W. A. Thermodynamic properties and the approximate solutions of the Schrödinger equation with the shifted Deng-Fan potential model. Mol. Phys. 112, 127 (2014).
Mesa, A. D. S., Quesne, C. & Smirnov, Y. F. Generalized Morse potential: Symmetry and satellite potentials. J. Phys. A Math. Theor. 31, 321 (1998).
Rong, Z., Kjaergaard, H. G. & Sage, M. L. Comparison of the Morse and Deng-Fan potentials for X-H bonds in small molecules. Mol. Phys. 101, 2285 (2003).
Gordillo-Vazquez, F. J. & Kunc, J. A. Comparison of fluorescence-based temperature sensor schemes: Theoretical analysis and experimental validation. J. Appl. Phys. 84, 4649 (1998).
Chackerian, C. & Tipping, R. H. Vibration-rotational and rotational intensities for CO isotopes. J. Mol. Spectrosc. 99, 431 (1983).
Manai, I., Horchani, R., Lignier, H., Pillet, P. & Comparat, D. Rovibrational cooling of molecules by optical pumping. Phys. Rev. Lett. 109, 183001 (2012).
Viteau, M. et al. Optical pumping and vibrational cooling of molecules. Science 321, 232 (2008).
Vala, J., Dulieu, O., Masnou-Seeuws, F., Pillet, P. & Kosloff, R. Coherent control of cold-molecule formation through photoassociation using a chirped-pulsed-laser field. Phys. Rev. A 63, 013412 (2000).
Fioretti, A. et al. Cold cesium molecules: From formation to cooling. J. Mod. Opt. 56, 2089 (2009).
Vatasescu, M. Preparing isolated vibrational wave packets with light-induced molecular potentials by chirped laser pulses. Nucl. Instrum. Methods B 279, 8 (2012).
Beloy, K., Borschevsky, A., Flambaum, V. V. & Schwerdtfeger, P. Effect of α variation on a prospective experiment to detect variation of me/mp in diatomic molecules. Phys. Rev. A 84, 042117 (2011).
Li, D., Xie, F. & Li, L. Observation of the Cs2(33Σ+g)state by infrared–infrared double resonance. Chem. Phys. Lett. 458, 267 (2008).
Li, D. et al. The 33Σ+g and a 3Σ+u states of Cs2: Observation and calculation. Chem. Phys. Lett. 441, 39 (2007).
Onate, C. A. & Idiodi, J. O. A. Fisher information and complexity measure of generalized morse potential model. Commun. Theor. Phys. 66, 275 (2016).
Jia, C.-S., Liu, J.-Y., Wang, P.-Q. & Lin, X. Approximate analytical solutions of the Dirac equation with the hyperbolic potential in the presence of the spin symmetry and pseudo-spin symmetry. Int. J. Theor. Phys. 48, 2633 (2009).
Ikot, A. N. et al. Klein-Gordon equation and nonrelativistic thermodynamic properties with improved screened Kratzer potential. J. Low Temp. Phys. https://doi.org/10.1007/S10909-020-02544-w (2021).
Dong, S.-H., Lozada-Cassou, M., Yu, J., Jiménez-Ấngeles, F. & Rivera, A. L. Hidden symmetries and thermodynamic properties for a harmonic oscillator plus an inverse square potential. Int. J. Quant. Chem. 107, 366 (2007).
Eshghi, M., Mehraban, H. & Ikhdair, S. M. Approximate energies and thermal properties of a position-dependent mass charged particle under external magnetic fields. Chin. Phys. B 26, 060302 (2017).
Okorie, U. S., Edet, C. O., Ikot, A. N., Rampho, G. J. & Sever, R. Thermodynamic functions for diatomic molecules with modified Kratzer plus screened Coulomb potential. Indian J. Phys. 95, 411 (2021).
Yanar, H., Aydoǧdu, O. & Salti, M. Modelling of diatomic molecules. Mol. Phys. 114, 3134–3142 (2016).
Author information
Authors and Affiliations
Contributions
C.A. Onate; Formulate the work, solved the calculations I.B. Okon; Wrote the introduction M.C. Onyeaju; Makes all the plots and discussed the results O. Ebomwonyi; Solved the calculations and typeset the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Onate, C.A., Okon, I.B., Onyeaju, M.C. et al. Vibrational energies of some diatomic molecules for a modified and deformed potential. Sci Rep 11, 22498 (2021). https://doi.org/10.1038/s41598-021-01998-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-01998-6
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.