Abstract
Complete recycling of Nd2Fe14B sludge by chemical methods has gained significance in recent years, however, it is not easy to recycle highly contaminant sludge and obtain product with good magnetic properties. Herein we report a simple four-step process to recycle the Nd2Fe14B sludge containing ~ 10% of contaminants. Sludge was leached in H2SO4 and selectively co-precipitated in two steps. In the first co-precipitation, Al3+ and Cu2+ were removed at pH 6. Thereafter, in the second co-precipitation Fe2+ and RE3+ sulfates were converted to the Fe and RE hydroxides. By annealing at 800 °C RE and Fe hydroxides precipitates were converted to the oxides and residual carbon was oxidized to CO2. After the addition of boric acid, Fe and RE oxides were reduced and diffused to the (Nd-RE)2Fe14B by calciothermic reduction diffusion. Removal of CaO by washing with D.I. water in glove box reduced the oxygen content (~ 0.7%), improved crystallinity and enhanced the magnetic properties significantly. Coercivity increased more than three times (from 242.71 to 800.55 kA/m) and Mr value was also enhanced up to more than 20% (from 0.481 to 0.605 T). In this green process Na2SO4 and Ca(OH)2 were produced as by-product those are non-hazardous and were removed conveniently.
Similar content being viewed by others
Introduction
Among permanent magnets, Nd2Fe14B type hard magnets exhibit the highest recorded BHmax1,2,3,4,5. They have drawn attention due to their applications in modern appliances which lead to large market demand and a rapid increase in their production6,7,8,9,10,11. A huge amount (21%) of rare earth elements (RE) are being consumed for the synthesis of permanent magnets. RE resources are depleting and the cost of RE extraction from the ores is continuously soaring, hence recycling of the Nd2Fe14B sludge is becoming an important area of modern research.
A large quantity (~ 30%) of the Nd2Fe14B sludge is produced in the cutting and grinding process and more than 95 wt% of it, is recyclable12. However, because of costly physical recycling processes and high level of contamination, usually, recycling magnet sludge is not economically feasible in most part of the world. Nd2Fe14B sludge mainly consists of oxidized particles of Nd2Fe14B with different RE, C, Al, and d-block transition metals (e.g. Cu, Co, Zn, Mn, Cr, Ni). Al, Zn, Mn, Cr, and Ni come from the protective coatings, those are applied to avoid the corrosion of bulk magnet surface. Cu is added to the sintered Nd2Fe14B magnets to decouple the magnetic grains to stop the fast flip-over of the magnetic domains and enhance the coercivity11. Co addition to enhances the Mr value and curie temperature. Sludge can have a high quantity of carbon because of the mixing of machine oil and lubricant during the cutting process. All these contaminants reduce the value of sludge.
Commonly used physical method for recycling of Nd2Fe14B sludge is a multi-step process which requires a huge amount of chemicals, energy, and produces hazardous wastes as byproduct13. These wastes include oxides (of carbon, sulfur, and nitrogen), dangerous metals (e.g. As), organic solvents, RE vapors and electrolytes. Several chemical methods have been introduced for the recovery of RE from Nd2Fe14B magnets scrap/sludge12,13,14,15,16,17,18,19,20,21,22,23,24,25,26 but complete recycling of Nd2Fe14B, via chemical route is relatively new field. Haider et al.12 and Yin et al.13 have recently introduced chemical methods. These methods are very innovative and useful, but they deal with sludge with a low level of contamination. It is very difficult to control the contamination in the sludge, hence it was required to introduce a new method to recycle this kind of sludge with good magnetic properties.
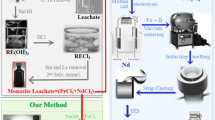
Herein we propose a time and energy efficient-recycling method for recycling of Nd2Fe14B sludge which is equally useful for sludge with high contamination and variable composition. Na2SO4 and Ca(OH)2 are produced as byproducts, those are non-hazardous and easy to remove. Magnetic properties are further enhanced by the removal of contamination and reduction of oxygen content. A comparison of between our method and the common physico-chemical12 method used for the recycling of Nd2Fe14B sludge is given in the Fig. 1a, b.
(a) Common physico-chemical method for the recycling of Nd2Fe14B sludge used these days12. (b) Our experimental process. Waste products produced in both methods are shown in red color. (c) Chemistry of the process. (d) Composition of the sludge and hydroxides precipitates produced after regular and selective co-precipitation.
Experimental section
Materials
All chemicals used in this work, including neodymium (III) chloride hexahydrate (Nd2(SO4)3·6H2O), sodium hydroxide (NaOH), boric acid (H3BO3), calcium hydride (CaH2), sulfuric acid (H2SO4), ethyl alcohol (C2H5OH) and acetone (CH3COCH3) were analytical grade and obtained from Sigma-Aldrich Co. Magnet sludge was obtained during the cutting process of Nd2Fe14B.
Experimental process
Magnet sludge obtained during the cutting of Nd2Fe14B magnet was dissolved in 2M H2SO4 by leaching process. ICP analysis (by inductively coupled plasma atomic emission spectrometer) of the leachate showed that it consisted of Nd, Pr, Tb, Fe, Cu, Al, and C (Fig. 1a). Total RE:Fe molar ratio in the leachate was calculated as 15.8:83.8. In order to compensate for the lower RE concentration in the leachate and to make up the RE:Fe molar ratio as 15:77, extra Nd2(SO4)3·6H2O was added to the leachate solution.
Co-precipitation of leachate was performed with a 3.5M NaOH solution which was added drop-by-drop, to raise the pH of the leachate solution up to 6. At pH 6, Al, Zn, and Cu chlorides were converted to their respective hydroxide precipitates. These precipitates were removed from the reaction mixture by centrifugation. The left-over reaction mixture was co-precipitated by further addition of NaOH solution. By maintaining the pH at 13, the solution was stirred for 30 min, which lead to the formation of RE and Fe hydroxides precipitates. The precipitates were washed thrice with de-ionized water to remove the Na2SO4 and NaOH and then annealed at 800 °C to convert all Fe and RE hydroxides to oxides. The annealing was done for 30 min while the air was flowing in the furnace. Produced oxide particles were mixed with boric acid and CaH2 in glove box and the mixture was pressed to the pellet. Pellet increases the physical contact between the constituents e.g. calcium hydride, boric acid, oxides of RE and Fe, which is useful for the efficient R-D (reduction-diffusion). Boric acid was added in such a way that the molar ratio of RE:Fe:B was kept as 15:77:8. Oxides (+ boric acid):CaH2 weight ratio was fixed as 1:1.
RE and Fe oxides (mixed with CaH2) were reduced and diffused by annealing at 1000 °C for 3 h. Products obtained after R-D were washed with water in the glove box repeatedly to remove CaO and rinsed with acetone twice. Finally, vacuum drying was done and recycled (Nd-RE)2Fe14B powder was stored in inert conditions. Flow diagram of experimental process and summary of chemical reactions during the process are provided as Fig. 1b, c. Advantages (energy efficient and cost effective) of the chemical method over the commonly used method are described in the Figs. S1 and S2, in the supporting information.
Characterization
The concentration of the elements in the leachate was determined by the inductively coupled plasma atomic emission spectrometer (ICP-AES, Shimadzu). Crystal structure and phases were determined by X-ray diffraction (XRD) patterns using a Rigaku Diffractometer (XRD, Rigaku). The morphology, size, and elemental distribution were observed with field emission scanning electron microscope (FE-SEM, Merlin), conventional transmission electron microscopy (TEM, JEM-2100F), and aberration-corrected TEM (ARM-200F) with energy- dispersive X-ray spectroscopy (EDS). TEM was operated at the accelerating voltage of 200 kV. Magnetic properties (M-H curves) of final product were measured by Physical Property Measurement System (PPMS, Evercool II–9T) in the vibrating sample magnetometer mode. LECO ON-736 analyzer was used to determine the oxygen content in (Nd-RE)2Fe14B.
Specimens for TEM were prepared by focused ion beam (FIB- NX2000, Hitachi) using the lift-out technique. For TEM measurement, the sample was treated as the same process reported by Kim et al.27 and orientation of the sample along the required zone axis was confirmed by using electron backscatter diffraction (EBSD) by TEAM™ Pegasus, Ametek Co. Ltd. USA.
Results and discussion
Magnet sludge produced during the cutting of Nd2Fe14B was dissolved in the H2SO4. Overall composition of the sludge before and after the precipitation is provided in Fig. 1d. Sludge mainly consisted of RE (Nd, Pr, Tb, Dy) Fe, Cu, Al, and C. Traces of other elements (e.g. Ho, Zr, Ga, Ni, Co) were also detected in the ICP analysis but their total concentration was ~ 0.4%. Effect of these small impurities on the magnetic properties of the final product was studied and provided in the supporting information. Cu, Al and C were ~ 10% those could significantly reduce the magnetic properties of the (Nd-RE)2Fe14B produced from them. After leaching of sludge in the H2SO4, next step was the selective precipitation to remove Al3+ and Cu2+. Precipitation of multivalent (e.g. Al3+ and Cu2+) ions from the solution depends on many factors e.g. oxidation state, Ksp value, the concentration of other ions, precipitating agent, and temperature. Precipitation of Fe3+, Al3+, and Cu2+ from their chloride solution occurred at pH values of 3.5, 5.0, and 6.028. However, different results were observed, when the solution of acid mine drainage containing Fe3+, Al3+, and Cu2+ was co-precipitated28. In the acid mine drainage experiment, Fe3+, Al3+, and Cu2+ chlorides were precipitated at pH 3.5, 4.5, and 5.5, respectively28. Precipitation of Al3+ and Cu2+ at pH value of 5.5 was also observed29. Different pH of the precipitation for Al3+ and Cu2+ in the previous studies indicated is a complicated process. It is commonly observed that Fe3+ precipitates completely at pH value ~ 3, however, Fe2+ precipitates at pH value of ~ 730. In our study, when pH approached 4, Al (Ksp constant = 1.9 × 10−33) started to precipitate.
Ksp constants of Cu(OH)2 is 1.6 × 10−19 and it was next to be precipitated hence precipitated out between the pH values of 5–6. Almost ~ 90% hydroxides of Al and Cu were separated in the form of precipitates at pH 6, they were removed from the leachate solution by centrifugation. Separated Al and Cu hydroxides were analyzed, and analysis details are provided in the supporting information (Figs. S3, S4).
Fe in the leachate exists as Fe2+ which does not precipitate below pH 6 but slightly (~ 2%) precipitates at pH 6 (Fig. S5). However, when pH value exceeded 6, Fe2+ (Ksp constant = 7.9 × 10−15) started to precipitate as Fe(OH)2. Soe et al.31 reported that Nd3+, Pr3+, Dy3+, and Tb3+ also start to precipitate at pH value of ~ 7 and similar was observed in our study.
Co-precipitation was stopped at pH values of 10, 11, 12 and 13 in four different experiments. The maximum percentage yield was obtained at pH value of 13 (Fig. S6). At pH 13, a mixture of hydroxides of RE, and Fe, was obtained with traces of some impurities e.g. Cu, Al, Ho, Zr, Ga, Ni, Co (less than 1%). In a separate experiment, selective precipitation was not performed and all the elements in the leachate solution were co-precipitated, hence, a mixture of RE, Fe, Al, and Cu hydroxide was obtained.
Hydroxides obtained by co-precipitation were aqua complexes of RE and other metals. Being amorphous, these hydroxides could not be detected by XRD (Fig. 2e) analysis. SEM image of the hydroxide precipitates produced by regular and selective co-precipitation (Fig. 2a, b) confirmed that hydroxide particles were of irregular morphology and size (Fig. 2c, d). TEM analysis revealed that the average size of the hydroxide precipitates was ~ 25 nm. TEM-EDS images (Figs. S7, S8) confirmed that oxides of all the metals are homogeneously mixed.
Most of the carbon was removed during the precipitation and centrifugation but still, noticeable quantity was detected in the hydroxide precipitates (Fig. 1d). Leftover carbon was removed by the annealing in air at 800 °C. Oxidation at 800 °C converted all the C to CO2. Meanwhile, annealing also converted hydroxide precipitates to the oxides. SEM analysis revealed that the average particle size of the oxide particles was ~ 150 nm (Fig. 3a, b).
XRD confirmed the presence of Fe2O3 and REFeO3 phases in the oxide mixture (Fig. 3m). The mechanism of formation of these oxides is given in Fig. 1c. Peaks of Cu and Al oxides were also observed in the XRD patterns of oxides produced from Nd, RE, Fe, Al, Cu hydroxide precipitates. TEM-EDS images reveal that all RE, Fe, Cu, and Al are distributed evenly throughout the oxide intermediates (Fig. 3c–l). Co-precipitation brought the Fe2O3 and REFeO3 particles very close. This homogeneous distribution is very effective for the efficient reduction diffusion process. Oxides produced from both the selective and regular co-precipitation were mixed with the boric acid and CaH2 in two separate experiments. These mixtures were reduced and diffused at 1000 °C to obtain (Nd-RE)2Fe14(AlCu)B and (Nd-RE)2Fe14B. Both of these products contained CaO byproduct, and were washed with water to remove it. (Nd-RE)2Fe14(AlCu)B was washed in the air while (Nd-RE)2Fe14B was divided into two parts. One part was washed in the air and other part was washed in the glove box to minimize the exposure to oxygen. In this way three different products (Nd-RE)2Fe14(AlCu)B, (Nd-RE)2Fe14B and (Nd-RE)2Fe14B (low oxygen), were obtained. To determine the detailed structure of the products XRD, SEM, SEM–EDS, TEM, TEM-EDS, and HRTEM analysis were performed. Nd2Fe14B, Dy2Fe14B, Tb2Fe14B, and Pr2Fe14B have similar crystal structures, hence their XRD patterns are also very similar. It was very hard to distinguish between all four RE2Fe14B phases with the help of XRD. Hence all these phases are marked as Nd2Fe14B (JCPSD #36-1296) in the XRD patterns shown in Fig. 4e. NdCu (JCPSD #337-1037) phase was also detected in the XRD analysis of (Nd-RE)2Fe14(AlCu)B. To evaluate the crystallinity of the of (Nd-RE)2Fe14(AlCu)B, SAED patterns (Fig. 3b) of the Nd2Fe14B and ZnCu were obtained. With d-spacing value of 0.239 nm, [214] facet of (Nd-RE)2Fe14B was detected. Any peak of Al or Al alloy was not detected in the XRD, however, in TEM-EDS image, Al was detectable (Fig. 4i). This may refer to that Al or Al alloy was not crystallized well during reduction-diffusion or maybe oxidized during the washing with water and became amorphous. However, [121] facet of NdCu was identified in the HRTEM with the d-spacing value of 0.256 nm (Fig. 4d).
SEM images in Fig. 4a–c revealed that the magnetic particles had irregular morphology and the size varied from 0.3 to 10 μm. It was determined that the average particle size of all three particles was ~ 1.8 µm (Fig. 4a–c). Different studies have revealed that Dy, Pr Tb, and other heavy RE are substituted inside the Nd2Fe14B crystal lattice32,33,34,35,36,37,38,39,40,41,42,43. In our study HRTEM and SEM–EDS images (Fig. 4l–n) also confirmed it. HRTEM shows that the crystal lattice d-spacing at [214] facet is 0.239 nm, which is slightly smaller (241 nm) than NdFe14B [214] facet. Slight reduction in the d-spacing may also indicate the substitution of Pr, Tb, or Dy in the crystal lattice. TEM-EDS images of (Nd-RE)2Fe14(AlCu)B confirmed the homogeneous mixing of all RE, indicate the substitution of Dy, Pr, and Tb in the Nd2Fe14B crystal lattice (Fig. 4f–n). TEM and TEM-EDS images of (Nd-RE)2Fe14B and (Nd-RE)2Fe14B (low oxygen) are provided in supporting information as Figs. S9, S10 and S11.
Oxygen content in the commercial (Nd-RE)2Fe14B powders is ~ 0.4%. This one of the reasons that commercial (Nd-RE)2Fe14B powders exhibit excellent magnetic properties. In the rare earth based magnetic particles produced by the R-D, washing with the water is employed to remove the CaO, byproduct of the R-D process44. On average, (Nd-RE)2Fe14B magnetic particles produced by the reduction diffusion process contains ~ 2% of oxygen. It was concluded that oxygen content reduces the crystallinity Nd2Fe14B because no oxide is detected in the XRD patterns. To solve the oxidation problem, (Nd-RE)2Fe14B was washed in the glove box. Before washing the (Nd-RE)2Fe14B with the water in the glove box, nitrogen gas was blown in the water, which further removed the dissolved oxygen in the water. N2 was simply purged into the water at the rate of 25 ml/s for 40 min. This condition was taken from the work by Butler et al.45 as they have reported that more than 60% of the oxygen can be removed at this optimum condition. The glove box was filled with the Ar, with the oxygen level reduced to the ~ 50 ppm. By taking these preventive measures, the oxygen content of (Nd-RE)2Fe14B was reduced to ~ 0.7%. Figure 4e shows that the Nd peak is absent when the washed (Nd-RE)2Fe14B was washed in air. This further confirms that washing with the water reduces the crystallinity of the Nd too. It is well-known fact that the addition of the Nd phase (up to a certain limit) enhances the magnetic properties especially, coercivity12.
LAADF-STEM images (Fig. 5b–d) were zoomed in to investigate the micro-structure of the magnetic particles. Line EDS mapping from the HAADF-STEM image was taken and studies to determine the degree of oxidation near the grain boundary (Fig. 5e, f). Pr, Dy, and Tb are substituted inside the (Nd-RE)2Fe14B, hence their EDS mapping was not studied to avoid the complexity of data. It was found that oxygen content suddenly increased near the grain boundary because these parts of the grain are directly exposed to the water during the washing process. Pb and Sn detected in TEM-EDS come from the solder.
It is evident from the XRD patterns (Fig. 4e) that Cu and Al reduce the crystallinity of the final product, as well as few crystal facets (e.g. [216],[324]), are also missing. The exact mechanism that how Cu and Al affects the (Nd-RE)2Fe14B crystal is yet unknown but most probably Cu and Al interfere during the diffusion of RE, Fe, and B. Poor crystallization after reduction-diffusion is the major factor that reduced the magnetic properties of (Nd-RE)2Fe14(AlCu)B. The second reason for the poor magnetic properties of (Nd-RE)2Fe14(AlCu)B is the non-magnetic behavior of Cu. Cu has an electronic configuration of [Ar] 3d10 4s1 which indicates the absence of unpaired electrons. Hence Cu and its alloys tend to be non-magnetic. In the bulk sintered (Nd-RE)2Fe14B magnets, Cu is used to decouple the large magnetic grains. Nd-Cu reduces the effect of the surge of domain wall removal in bulk magnets and enhances the coercivity. But in the case of magnetic particles, the grain decoupling phenomenon does not work efficiently. Hence non-magnetic Nd-Cu phase reduces the overall magnetic properties of the (Nd-RE)2Fe14(Cu-Al)B. Al is detectable in the final product but it is in the amorphous form. Effect of amorphous Al or its possible amorphous alloy on the magnetic properties is not clear.
Decreasing order of the Ms value in all the products is given as (Nd-RE)2Fe14(AlCu)B > (Nd-RE)2Fe14B-(low oxygen) > (Nd-RE)2Fe14B. (Nd-RE)2Fe14(AlCu)B has highest Ms value, which indicates the presence of amorphous Fe or low anisotropy field. (Nd-RE)2Fe14B-(low oxygen) has slightly higher Ms value as compared to the (Nd-RE)2Fe14B that refers to the enhanced Mr value of (Nd-RE)2Fe14B-(low oxygen), which slightly affected the Ms. However, this enhancement in the Ms value is much higher as compared to the Ms value, which is also evident in the squareness ratio graph (Fig. 5a) showing the enhanced squareness ratio of (Nd-RE)2Fe14B-(low oxygen).
(Nd-RE)2Fe14(AlCu)B exhibit the highest magnetic moment (21.6 μB) and Ms (1.057 T) value among all three products. Higher magnetic moment and low Mr value of (Nd-RE)2Fe14(AlCu)B refer to the low anisotropic field or presence of amorphous soft magnetic phase (e.g. Fe). Higher Mr value of (Nd-RE)2Fe14B-(low oxygen) contributes to its highest squareness ratio among all three products (Fig. 5a). Individual values of magnetic moments of (Nd-RE)2Fe14(AlCu)B, (Nd-RE)2Fe14B, and (Nd-RE)2Fe14B-(low oxygen) were determined as 21.06, 20.5, and 18.4 μB, respectively. These values of magnetic moments were determined by the Ms values from hysteresis loops (Fig. 5a). Complete hysteresis loops with applied magnetic field range of − 9.5 to 9.5 Tesla are provided in supporting information as Fig. S12.
Reduction in magnetic moment enhanced the coercivity. From the hysteresis loops, coercivity values of (Nd-RE)2Fe14(AlCu)B, (Nd-RE)2Fe14B, and (Nd-RE)2Fe14B-(low oxygen) were determined as 242.71, 568.13 and 800.55 kA/m, respectively. (Nd-RE)2Fe14B-(low oxygen) showed the highest coercivity due to low oxygen content, better crystallinity, and least magnetic moment. Oxygen content values of (Nd-RE)2Fe14(AlCu)B, (Nd-RE)2Fe14B and (Nd-RE)2Fe14B-(low oxygen) are recorded as 2.2, 2.1 and 0.7%. The increasing order of coercivity is (Nd-RE)2Fe14(AlCu)B < (Nd-RE)2Fe14B < (Nd-RE)2Fe14B-(low oxygen) and the decreasing order of magnetic moment is (Nd-RE)2Fe14(AlCu)B > (Nd-RE)2Fe14B-(low oxygen) > (Nd-RE)2Fe14B. Individual Mr values of (Nd-RE)2Fe14(AlCu)B, (Nd-RE)2Fe14B, and (Nd-RE)2Fe14B-(low oxygen) were determined as 0.481, 0.489, 0.605 T, respectively. Mr (emu/g), Ms (emu/g), squareness ratio (Sq), magnetic moment (μB), and coercivity (Hc), for the all the products are shown in Fig. 5a and Table S2, comparatively.
Conclusion
Contaminated Nd2Fe14B sludge was recycled by four-step chemical process. The process consisted of leaching of sludge in H2SO4, removal of impurities by selective co-precipitation, annealing, and calciothermic reduction diffusion. Al3+ and Cu3+ were removed by co-precipitation at pH 6 and residual carbon was removed by annealing at 800 °C. CaO byproduct was separated by washing in the glove box, in presence of very low level of oxygen that reduced the oxidation of (Nd-RE)2Fe14B produced. Removal of impurities and low oxygen content (~ 50 ppm) tripled the coercivity (over 800 kA/m) and enhanced the Mr value up to 0.605 T. The Method reported in this study is simple, eco-friendly, and energy efficient.
References
Muljadia, M. & Sardjonoa, P. Preparation and characterization of 5 wt% epoxy resin bonded magnet NdFeB for micro generator application. Energy Procedia 68, 282–287. https://doi.org/10.1016/j.egypro.2015.03.257 (2015).
Honshima, M. & Ohashi, K. High-Energy NdFeB agnets and their applications. J. Mat. Eng. Perform. 3(2), 218–222. https://doi.org/10.1007/BF02645846 (1994).
Ma, X. H. et al. Preparation of Nd–Fe–B by nitrate–citrate auto-combustion followed by the reduction–diffusion process. Nanoscale 7, 8016–8022. https://doi.org/10.1039/C5NR01195G (2015).
Jeong, H. et al. Chemical synthesis of Nd2Fe14B hard phase magnetic nanoparticles with an enhanced coercivity value: effect of CaH2 amount on the magnetic properties. New J. Chem. 40, 10181. https://doi.org/10.1039/C6NJ02436J (2016).
Kim, C. W., Kim, Y. H., Pal, U. & Kang, Y. S. Facile synthesis and magnetic phase transformation of Nd–Fe–B nanoclusters by oxygen bridging. J. Mater. Chem. C 1, 27. https://doi.org/10.1039/C2TC00083K (2013).
Amirouche, F., Zhou, Y. & Johnson, T. Current micro pump technologies and their biomedical applications. Microsyst. Technol. 15, 647–666. https://doi.org/10.1007/s00542-009-0804-7 (2009).
Chen, Z., Miller, D. & Herchenroeder, J. High performance nanostructured Nd–Fe–B fine powder prepared by melt spinning and jet milling. J. Appl. Phys. 107, 09A730.1–0A7303.4. https://doi.org/10.1063/1.3348544 (2010).
Riaño, S. & Binnemans, K. Extraction and separation of neodymium and dysprosium from used NdFeB magnets: an application of ionic liquids in solvent extraction towards the recycling of magnets. Green Chem. 17, 2931–2942. https://doi.org/10.1039/C5GC00230C (2015).
Reimer, M. V., Schenk-Mathes, H. Y., Hoffmann, M. F. & Elwert, F. Recycling decisions in 2020, 2030, and 2040, when can substantial NdFeB extraction be expected in the EU?. Metals 8, 867.1-867.5. https://doi.org/10.3390/met8110867 (2018).
Cha, H. G., Kim, Y. H., Kim, C. W. & Kang, Y. S. Preparation for exchange-coupled permanent magnetic composite between α-Fe (soft) and Nd2Fe14B (hard). Curr. Appl. Phys. 7(4), 400–403. https://doi.org/10.1016/j.cap.2006.09.010 (2007).
Akiya, T., Kato, H., Sagawa, M. & Koyama, K. Enhancement of coercivity in Al and Cu added Nd-Fe-B sintered magnets by high field annealing. IOP Conf. Ser. Mat. Sci. Eng. https://doi.org/10.1088/1757-8981/1/1/012034 (2009).
Syed, K. H., Jin-Young, L., Dongsoo, K. & Kang, Y. S. Eco-friendly facile three-step recycling method of (Nd-RE)2Fe14B magnet sludge and enhancement of (BH)max by ball milling in ethanol. ACS Sustain. Chem. Eng. 8, 8156–8163. https://doi.org/10.1021/acssuschemeng.0c00584 (2020).
Yin, X. et al. Recycled Nd-Fe-B sintered magnets prepared from sludges by calcium reduction-diffusion process. J. Rare Earth 36, 1284–1291. https://doi.org/10.1016/j.jre.2018.03.028 (2018).
Saguchi, A., Asabe, K., Takahashi, W., Suzuki, R. O. & Ono, K. Recycling of rare earth magnet scrap, Part 3 carbon removal from Nd magnet grinding sludge under vacuum heating. Mater. Trans. 43, 256–260. https://doi.org/10.2320/matertrans.43.256 (2002).
Asabe, K., Saguchi, A., Takahashi, W., Suzuki, R. O. & Ono, K. Recycling of rare earth magnet scrap: part 1 carbon removal by high temperature oxidation. Mater. Trans. 42, 2487–3249. https://doi.org/10.2320/matertrans.42.2487 (2001).
Saito, T., Sato, H., Ozawa, S., Yu, J. & Motegi, T. The extraction of Nd from waste Nd–Fe–B alloys by the glass slag method. J. Alloys Compd. 353, 189–193. https://doi.org/10.1016/S0925-8388(02)01202-1 (2003).
Takeda, O., Okabe, T. H. & Umetsu, Y. Phase equilibria of the system Fe–Mg–Nd at 1076K. J. Alloys Compd. 392, 206–213. https://doi.org/10.1016/j.jallcom.2004.09.020 (2005).
Rabatho, J. P., Tongamp, W., Takasaki, Y., Haga, K. & Shibayama, A. Recovery of Nd and Dy from rare earth magnetic waste sludge by hydrometallurgical process. J. Mater. Cycles Waste Manag. 15, 171–178. https://doi.org/10.1007/s10163-012-0105-6 (2013).
Vander Hoogerstraete, T., Blanpain, B., Van Gerven, T. & Binnemans, K. From NdFeB magnets towards the rare-earth oxides: a recycling process consuming only oxalic acid. RSC Adv. 4, 64099–64111. https://doi.org/10.1039/C4RA13787F (2014).
Abrahami, S. T., Xiao, Y. & Yang, Y. Rare-earth elements recovery from post-consumer hard-disc drives. Trans. Inst. Min. Metall. Sect. C 124, 106–115. https://doi.org/10.1179/1743285514Y.0000000084 (2015).
Lai, W., Liu, M., Li, C., Suo, H. & Yue, M. Recovery of a composite powder from NdFeB slurry by co-precipitation. Hydrometallurgy 150, 27–33. https://doi.org/10.1016/j.hydromet.2014.08.014 (2014).
Itakura, T., Sasai, R. & Itoh, H. Resource recovery from Nd–Fe–B sintered magnet by hydrothermal treatment. Bull. Chem. Soc. Jpn. 79, 1303–1307. https://doi.org/10.1246/bcsj.79.1303 (2006).
Önal, M. A. R., Borra, C. R., Guo, M., Blanpain, B. & Van, G. T. Hydrometallurgical recycling of NdFeB magnets: Complete leaching, iron removal and electrolysis. J. Rare Earths 35, 574–584. https://doi.org/10.1016/S1002-0721(17)60950-5 (2017).
Adachi, G., Shinozaki, K., Hirashima, Y. & Machida, K. Rare earth separation using chemical vapor transport with LnCl,-AlCl, gas phase complexes. J. Less-Common Met. 169, L1–L4. https://doi.org/10.1016/0022-5088(91)90225-S (1991).
Gergoric, M., Ravaux, C., Steenari, B. M., Espegren, F. & Retegan, T. Leaching and recovery of rare-earth elements from neodymium magnet waste using organic acids. Metals 8, 721–738. https://doi.org/10.3390/met8090721 (2018).
Baba, Y., Kubota, F., Kamiya, N. & Goto, M. Selective recovery of dysprosium and neodymium ions by a supported liquid membrane based on ionic liquids. Solvent Extr. Res. Dev. Jpn. 18, 193–198. https://doi.org/10.15261/serdj.18.193 (2011).
Kim, T. A., Kang, M. C., Jung, G. B., Kim, D. S. & Yang, C. W. Novel method for preparing transmission electron microscopy samples of micrometer-sized powder particles by using focused ion beam. Microsc. Microanal. 23, 1055–1060. https://doi.org/10.1017/S1431927617012557 (2017).
Park, S. M., Yoo, J. C., Ji, S. W., Yang, J. & Baek, K. Selective recovery of dissolved Fe, Al, Cu, and Zn in acid mine drainage based on modeling to predict precipitation pH. Environ. Sci. Pollut. Res. 22, 3013–3022. https://doi.org/10.1007/s11356-014-3536-x (2015).
Paula A., Mónica C., Adriana S., Jorge T. & Denise E. Precipitation of Metals from Synthetic Laterite Nickel Liquor by Naoh. Hydroprocess, in 8th International Seminar on process Hydrometallergy (2016).
Olena, O. & Serge, S. Investigation of FeCl3 induced coagulation processes using electrophoretic measurement, nanoparticle tracking analysis and dynamic light scattering: importance of pH and colloid surface charge. Colloids Surf. A Physicochem. Eng. Asp. 461, 212–219. https://doi.org/10.1016/j.colsurfa.2014.07.049 (2014).
Nwe, S., Lwin, T. S. & Kay, T. L. Study on extraction of lanthanum oxide from monazite concentrate. Int. Sch. Sci. Res. Innov. 2(10), 226–229 (2008).
Liu, X. B. & Altounian, Z. The partitioning of Dy and Tb in NdFeB magnets: a first-principles study. J. Appl. Phys. 111, 07A701.1-07A701.3. https://doi.org/10.1063/1.3670054 (2012).
Yu, N. J., Pan, N. J., Zhang, P. Y. & Ge, H. L. The origin of coercivity enhancement of sintered NdFeB magnets prepared by Dy addition. J. Magn. 18(3), 235–239. https://doi.org/10.4283/JMAG.2013.18.3.235 (2013).
Li, W. F., Sepehri-Amin, H., Ohkubo, T., Hase, N. & Hono, K. Distribution of Dy in high-coercivity (Nd, Dy)–Fe–B sintered magnet. Acta Mater. 59, 3061–3069. https://doi.org/10.1016/j.actamat.2011.01.046 (2011).
Tan, X., Parmar, X., Zhong, Y., Chaudhary, V. & Ramanujan, R. V. Effect of Dy substitution on the microstructure and magnetic properties of high (BH)max Nd-Dy-Fe-Co-B nanoparticles prepared by microwave processing. J. Magn. Magn. Mater. 471, 278–285. https://doi.org/10.1016/j.jmmm.2018.09.017 (2019).
Wenlong, Y. et al. Influence of gadolinium on microstructure and magnetic properties of sintered NdGdFeB magnets. J. Rare Earth 30, 133–136. https://doi.org/10.1016/S1002-0721(12)60009-X (2012).
Zhong, Y., Chaudhary, V., Tan, X., Parmar, H. & Ramanujan, R. V. High coercivity Dy substituted Nd-Fe-Co-B magnetic nanoparticles produced by mechanochemical processing. Magn. Magn. Mater. 475, 554–562. https://doi.org/10.1016/j.jmmm.2018.08.061 (2019).
Rahimi, H., Ghasemi, A., Mozaffarinia, R. & Tavoosi, M. Coercivity enhancement mechanism in Dy-substituted Nd–Fe–B nanoparticles synthesized by sol–gel base method followed by a reduction diffusion process. J. Magn. Magn. Mater. 429, 182–191. https://doi.org/10.1016/j.jmmm.2017.01.041 (2017).
Khan, I. & Hong, J. Electronic structure and magnetic properties of Nd2Fe14B. J. Kor. Phys. Soc. 68, 1409–1414. https://doi.org/10.3938/jkps.68.1409 (2016).
Alam, A., Khan, M., McCallum, R. W. & Johnson, D. D. Site-preference and valency for rare-earth sites in (R-Ce)2Fe14B magnets. Appl. Phys. Lett. 102, 0424021–0424025. https://doi.org/10.1063/1.4789527 (2013).
Kitagawa, I. & Asari, Y. Magnetic anisotropy of R2Fe14B (R=Nd, Gd, Y): Density functional calculation by using the linear combination of pseudo-atomic-orbital method. Phys. Rev. B 81, 214408.1-214408.7. https://doi.org/10.1103/PhysRevB.81.214408 (2010).
Haider, S. K. et al. Determination of Dy substitution site in Nd2−xDyxFe14B by HAADF-STEM and illustration of magnetic anisotropy of “g” and “f” sites, before and after substitution. Sci. Rep. 11, 6347. https://doi.org/10.1038/s41598-021-85713-5 (2021).
Haider, S. K., Lee, J. Y., Pawar, A. U. et al. Novel eco-friendly low cost and energy efficient synthesis of (Nd–Pr–Dy)2Fe14B magnetic powder from monazite concentrate. Sci Rep. 11, 20594. https://doi.org/10.1038/s41598-021-99464-w (2021).
Haider, S. K., Ngo, H. M., Kim, D. et al. Enhancement of anisotropy energy of SmCo5 by ceasing the coupling at 2c sites in the crystal lattice with Cu substitution. Sci Rep. 11, 10063. https://doi.org/10.1038/s41598-021-89331-z (2021).
Butler, I. B., Schoonen, M. A. & Rickard, D. T. Removal of dissolved oxygen from water: a comparison of four common techniques. Talanta 41, 211–215. https://doi.org/10.1016/0039-9140(94)80110-X (1994).
Acknowledgements
This work was supported by Leader Project at the Sogang University funded by the Ministry of Science and ICT through the National Research Foundation of Korea (No. 2020R1A3B3079715). Syed Kamran Haider appreciates the support of the National Institute of Science and Technology Human Resources Development grant by the Korea government (NST) (No. 202139031.01). Young Soo kang also appreciates the support of the National Research Council of Science and Technology (NST) grant by the Korea government (MSIT) (No.CRC-15-06-KIGAM).
Author information
Authors and Affiliations
Contributions
S.K.H. performed experiment did characterization and wrote manuscript. Y.S.K. helped in data interpretation and manuscript writing. D.K. helped in experiment and characterization.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Haider, S.K., Kim, D. & Kang, Y.S. Four-step eco-friendly energy efficient recycling of contaminated Nd2Fe14B sludge and coercivity enhancement by reducing oxygen content. Sci Rep 11, 22255 (2021). https://doi.org/10.1038/s41598-021-01382-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-01382-4
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.