Abstract
In this paper, laser welding-brazing of TC4 Titanium (Ti) alloy and Al2O3 ceramic dissimilar material was carried. The results showed that the Ti alloy and Al2O3 were joined by melting filler metal when the laser was concentrated in the Ti alloy side of the joint. The joint with one fusion weld and one brazed weld separated by remaining unmelted Ti alloy. Laser beam offset the Ti alloy 1.5 mm, Ti alloy would not be completely melted in joint. Through heat conduction, the filler metal melted occurred at the Ti-ceramic interface. A brazed weld was formed at the Ti-ceramic interface with the main microstructure of β-CuZn + Ti2Zn3, β-CuZn and Al2Cu + β-CuZn. The joint fractured at the brazed weld with the maximum tensile strength of 169 MPa.
Similar content being viewed by others
Introduction
Alumina (Al2O3) ceramics have excellent high temperature strength and corrosion and wear resistance, and are widely used in electronics, aerospace, nuclear, automotive and other industries1,2,3. Ti811 Ti alloy is an important part of the compressor blade of aviation engine, but its application is limited by its low surface hardness and poor wear resistance. A laser cladding composite coating was prepared on the Ti811 surface using the coaxial powder feeding method to improve the micro-hardness and wear resistance of the Ti811 alloy4. However, the inherent brittleness associated with Al2O3 ceramics limits their structural applications, particularly in the fabrication of complex geometries5,6. Therefore, the jointing of Al2O3 ceramics to themselves or to metals is usually preferred. Titanium-based (Ti) alloys have excellent high temperature strength, good creep properties and oxidation resistance and can be used in high temperature environments. The successful combination of Al2O3 ceramics and Ti alloys to prepare ceramic metal components can give full play to the advantages of the two materials and is widely used in vacuum pipes, energy converters, semiconductor devices, missiles, rockets and satellites. However, the main difficulty to realize the effective jointing between Al2O3 ceramics and Ti alloy lies in the great difference of their physical and chemical properties. The difference of physical properties is manifested in the mismatch of elastic modulus and linear expansion coefficient, which will lead to large residual stress at the interface after welding7. The difference in chemical properties is mainly due to the fact that the bonding form of the Al2O3 ceramic is mainly a covalent bond, and the Ti alloy is a metal bond.
Many jointing methods in the research of ceramic/metal connection technology has been reported, including brazing, diffusion welding, friction welding, local transition liquid phase jointing, ultrasonic welding, microwave welding, explosive welding, fusion welding, self propagating high temperature synthesis welding and hot pressing reaction sintering connection8,9,10,11,12,13. At present, brazing is one of the most effective ways to joint metal and ceramic materials. However, brazing of ceramics presents difficulties because conventional filler metals do not easily wet alumina. Recently, several welding techniques have been documented14, such as vacuum brazing15, diffusion bonding16, and partial transient liquid phase (PTLP) brazing17. Among these methods, active brazing is a relatively simple and reliable technology for bonding Al2O3 ceramics due to the improved wettability and joining quality by active elements in braze alloys18. In the brazing joint of metal and ceramic, active elements can be added to the brazing filler metal to improve the wetting ability of the brazing filler metal to the ceramic joint interface. The most commonly used active element in the solder is Ti, followed by Zr, V, Cr, etc.19. However, the conditions of use may make a particular process inappropriate. The vacuum brazing process generally takes a long time to implement. In addition, the required joint geometry may make brazing difficult to apply. In fact, the butt welding of Al2O3 and Ti alloy is technically convenient20. As an efficient and flexible non-contact welding technology, laser welding has made great achievements in the jointing of welding refractory materials and dissimilar metals.
Based on the above analysis, two welding mechanisms (fusion welding and brazing) are combined to avoid melting and liquid mixing of the base metals during welding. Using laser as welding heat source and Cu-based fillers as interlayer material. The welding process was set up to ensure that the Ti alloy was partly melted as expected from previous work21. The melted Ti alloy formed a fusion weld. Meanwhile, a brazed weld was formed at the interface between unmelted Ti alloy and Al2O3. In this way, a peculiar joint was acquired. So far, this kind of joint has been rarely reported.
Experimental
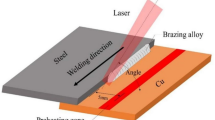
The TC4 alloy plates (88 wt.% Ti, 6.06 wt.% Al and 4.03 wt.% V) and Al2O3 ceramic plates (Al2O3 96%, SiO2 2%, B2O3 0.5% and C3H5NO 0.8%) were 1 mm thick, the sample plates size were 40 mm × 20 mm × 1 mm. The filler metal was 0.2 mm Cu-based filler (61.2 wt.% Cu, 37.2 wt.% Zn, 0.28 wt.% Si, and 0.89 wt.% Sn). The specimen was mechanically and chemically cleaned before welding. The gap between the Ti alloy and the ceramic was important for adequate heat transfer and prevention of pore formation. In order to minimize the gap between the edges, the test pieces were clamped to each other. A continuous laser with an average power of 1.20 KW, a wavelength of 1080 nm and a spot diameter of 0.1 mm was used. The welding process is shown in the Fig. 1a,b. In order to ensure that the Ti alloy was not completely melted, the laser beam was focused on the Ti alloy plate with a distance of 1.5 mm from the Ti-filler metal interface. The welding parameters were: laser beam power of 420 W, defocusing distance of + 5 mm, welding speed of 550 mm/min. After welding, the surface of the joint was polished to a smooth state to perform corrosion and optical microscope tests. The specimens and tensile specimens were machined by wire electrical discharge machining. Tensile strength of the joints was measured by using universal testing machine (MTS Insight 10 kN) with cross head speed of 0.2 mm/min. Argon gas with the purity of 99.99% was applied as a shielding gas with total flow of 20 L/min at top of the joint.
Results and discussion
The optical microscope (OM) image of the cross section of the joint is shown in the Fig. 1c. It could be seen that the joint was divided into three parts: the fusion weld formed at the Ti alloy side, unmelted Ti alloy and the brazed weld formed at the Ti-Al2O3 interface. The average width of fusion weld, unmelted Ti alloy and brazed weld was 1.2 mm, 0.55 mm and 0.25 mm, respectively. Local heating on the Ti alloy side produced an uneven volume expansion and thermal stress, which helped to obtain a close joining between the Ti alloy, filler metal and Al2O3 interface. The high temperature and the close contact at the Ti-Al2O3 interface provided favorable conditions for atomic diffusion, which leads the brazing process at the Ti-Al2O3 interface very well. The microstructure of fusion weld was quite different from that of braze weld, and the brazed weld became black after corrosion. Figure 1d shows the OM image of the brazed weld before corrosion. It was not found the pores, macro-cracks and other defects. In addition, the eutectic joining between Ti and filler was realized by heat conduction, and the Cu-based filler has good ductility, it was beneficial to alleviate and adjust the thermal stress of the Ti-Al2O3 joint, which was beneficial to improve the mechanical properties of the joint.
In order to obtain a good joint, it was especially important to precisely control the position of the laser spot. When the laser spot was away from the Ti alloy interface, it could not be brazed at the Ti-Al2O3 interface. When the laser spot was close to the Ti alloy interface, the Ti alloy and Cu filler began to melt in the braze weld, and the content of the brittle intermetallic compound was greatly increased, and the joining of the Ti/Al2O3 dissimilar material could not be achieved.
Figure 2 shows the physical model formed of the joint. When the laser beam was fixed to the side of the Ti alloy plate at a certain distance from the interface of the Ti alloy, a molten pool with small holes was generated inside the Ti alloy plate, thereby absorbing a large amount of heat, as seen in Fig. 2a,b. Due to the high absorption of the laser in the small holes, the Ti alloy at the boundary of the small holes was sharply melted, but the Ti alloy side was not completely melted22. The temperature of the unmelted Ti alloy increased rapidly with the increase of the heat of the small hole boundary, and was transmitted to the Al2O3 side, as seen in Fig. 2c. Therefore, the unmelted Ti alloy had a sufficiently high temperature to promote melting of the filler metal at the Ti-Al2O3 interface, as seen in Fig. 2d. The solidification of the weld pool and the liquid filler metal in the joint could start from the Ti alloy side, resulting in a composite joint between the fusion weld and the braze weld, as seen in Fig. 2e,f.
(a) Laser beam was focused on the Ti alloy plate; (b) formation of welding pool on Ti alloy side; (c) heat was transferred from welding pool to the Ti alloy-Al2O3 interface; (d) atomic interdiffusion at Ti alloy-Al2O3 interface; (e) formation of fusion zone on Ti alloy side; (f) formation of brazed weld on Ti alloy-Al2O3 interface.
The OM image of the fusion weld is shown in Fig. 3a,b, and there were no defects in it. The fusion weld mainly consists of acicular α' martensite. The SEM image of the brazed weld is shown in the Fig. 3c. The results showed that the brazed weld was a layered structure, which could be divided into three zones I, II and III according to the shape and color, as shown in the Fig. 3c. Figure 3d–f correspond to the three zones in Fig. 3c, respectively. The composition of each reaction zone (indicated by the letters A-C in Fig. 3) was investigated by SEM–EDS, and the composition of the reaction product was measured. The results were shown in in Table 1. According to the previous analysis, the microstructure of the brazed weld was mainly composed of molten filler metal. The composition of A point in the Zone I was consistent with the filler metal. According to the results of the energy spectrum analysis and the Cu–Zn phase diagram, the main microstructure of the zone I was determined to be β-CuZn phase. When the laser beam was focused on one side of the Ti alloy, elemental diffusion between the matrix material and the filler occurred immediately, resulting into their composition to deviate from the original composition. Therefore, liquid phase formation and elemental diffusion occurred simultaneously. As the solid phase dissolved into the liquid phase, impurities in the liquid phase diffused into the Al2O3 and Ti alloy to form a solid phase reaction layer, which existed only in a small area of the interface. As shown in the Fig. 3e,f, zones II and III were reaction layers formed by elemental diffusion. According to the Cu–Zn–Ti phase diagram17,23, it was determined that the microstructure of the zone II and the zone III was β-CuZn + Ti2Zn3 phase, Al2Cu + β-CuZn phase, respectively. Therefore, the main microstructures of brazed weld were β-CuZn + Ti2Zn3, β-CuZn and Al2Cu + β-CuZn. Oxygen was mainly distributed in the reaction layer on the ceramic side, and no TiOx-type phase was detected. Titanium was not detected in this compound, and the ceramic is melted and wetted by thermally conductive solder, but the filler itself does not contain titanium, and the titanium in the brazing weld comes from the Ti alloy.
Prepared three tensile specimens for each weld. The maximum tensile strength of the joint was about 169 MPa (Fig. 4a). There are minor differences in the processing of each sample, resulting in slightly different tensile strength. So the average tensile strength of the joint was about 147 MPa. The joint fractured in zone I of the brazed weld during tensile tests (Fig. 4b), which indicates that filler has good wettability on base materials. Figure 4c shows fracture surface of the joint exhibiting typical brittle characteristics. Moreover, as shown in Fig. 4d, XRD analyses of fracture surface detected β-CuZn phase. This confirmed the presence of β-CuZn phase at fracture surfaces. It should be noted that there was no Ti-Fe intermetallics in the brazed weld. The brazed weld became the weak zone of the joint, which led to the failure in the tensile test.
Conclusions
Due to the presence of unmelted Ti alloy, the formation of intermetallic compounds was not found in the fusion weld and brazed weld, which improved the mechanical properties of the joints. A brazed weld was formed at the Ti alloy-Al2O3 interface with the microstructure of β-CuZn + Ti2Zn3, β-CuZn and Al2Cu + β-CuZn. A great amount of atomic diffusion occurs at the Ti alloy-Al2O3 interface during welding, and the thickness of diffusion weld can reach tens of micrometres. The tensile resistance of the joint was determined by brazed weld. The maximum tensile strength of joint was 169 MPa.
References
Bai, J. G., Yang, X. H., Xu, S. C., Shi, Y. G. & Yang, J. F. Fabrication of highly dense Al2O3 ceramics. Scr. Mater. 68, 393–395 (2013).
Ning, H. L. et al. Joining of sapphire and hot pressed Al2O3 using Ag70.5Cu27.5Ti2 brazing filler metal. Ceram. Int. 29, 689–694 (2003).
Rohde, M., Südmeyer, I., Urbanek, A. & Torge, M. Joining of alumina and steel by a laser supported brazing process. Ceram. Int. 35, 333–337 (2009).
Liu, Y. N. et al. Microstructure and properties of ln-situ TiC/TiNi composite coating prepared via laser cladding on titanium alloy. Chin. J. Lasers 48, 115–125 (2021).
Liu, C. T., Schneibel, J. H., Maziasz, P. J., Wright, J. L. & Easton, D. S. Tensile properties and fracture toughness of TiAl alloys with controlled microstructures. Intermetallics 4, 429–440 (1996).
Wu, X. H. Review of alloy and process development of TiAl alloys. Intermetallics 14, 1114–1122 (2006).
Fernie, J. A., Drew, R. A. L. & Knowles, K. M. Joining of engineering ceramics. Int. Mater. Rev. 54, 283–331 (2009).
Kobashi, M., Ninomiya, T., Kanetake, N. & Choh, T. Effect of alloying elements in the brazing sheet on the bonding strength between Al2O3 and aluminum. Scr. Mater. 34, 415–420 (1996).
Barrena, M. I., Matesanz, L. & Salazar, J. M. G. D. Al2O3/Ti6Al4V diffusion bonding joints using Ag-Cu interlayer. Mater Charact. 60, 1263–1267 (2009).
Börne, F. D., Lippmann, W. & Hurtado, A. Laser-joined Al2O3 and ZrO2 ceramics for high-temperature applications. J. Nucl. Mater. 405, 1–8 (2010).
Yang, M. X., Lin, T. S., He, P. & Huang, Y. D. In situ synthesis of TiB whisker reinforcements in the joints of Al2O3/TC4 during brazing. Mater Sci. Eng. A. 528, 3520–3525 (2011).
Tetsui, T. Effects of brazing filler on properties of brazed joints between TiAl and metallic materials. Intermetallics 9, 253–260 (2001).
Kelkar, G. P., Spear, K. E. & Carim, A. H. Thermodynamic evaluation of reaction products and layering in brazed alumina joints. J. Mater. Res. 9, 2244–2250 (1994).
Sun, Z., Zhang, L. X., Zhang, Z. H., Hao, T. D. & Feng, J. C. Microstructure andmechanical response of the SiO2f/SiO2 composite and Invar alloy joints brazed with an AgCuTi alloy. Mater Des. 111, 239–247 (2016).
Qiao, G. J., Zhang, C. G. & Jin, Z. H. Thermal cyclic test of alumina/kovar joint brazed by Ni-Ti active filler. Ceram Int. 29, 7–11 (2003).
Hwang, H. R. & Lee, R. Y. The effects of metal coating on the diffusion bonding in Al2O3/Inconel 600 and the modulus of rupture strength of alumina. J. Mater. Sci. 31, 2429–2435 (1996).
Zhang, J. X., Chandel, R. S., Chen, Y. Z. & Seow, H. P. Effect of residual stress on the strength of an alumina-steel joint by partial transient liquid phase (PTLP) brazing. J. Mater. Process. Technol. 122, 220–225 (2002).
Liu, H. B., Zhang, L. X., Wu, L. Z., Liu, D. & Feng, J. C. Vacuum brazing of SiO2 glass ceramic and Ti-6Al-4V alloy using AgCuTi filler foil. Mater. Sci. Eng. A. 498, 321–326 (2008).
Loehman, R. E., Hosking, F. M., Gauntt, B. & Kotula, P. G. Reactions of Hf-Ag and Zr-Ag alloys with Al2O3 at elevated temperatures. J. Mater. Sci. 40, 2319–2324 (2005).
Ismail, M. I. S., Okamoto, Y., Okada, A., Uno, Y. & Ueoka, K. Direct micro-joining of flexible printed circuit and metal electrode by pulsed Nd:YAG laser. Int. J. Precis. Eng. Manuf. 13, 321–329 (2012).
Zhang, Y., Bi, Y. B., Zhou, J. P., Sun, D. Q. & Li, H. M. Microstructure and mechanical property improvement of Ti alloy and stainless steel joint based on a hybrid connection mechanism. Mater. Lett. 2020, 274 (2020).
Zhang, Y., Sun, D. Q., Gu, X. Y. & Li, H. M. A hybrid joint based on two kinds of bonding mechanisms for titanium alloy and stainless steel by pulsed laser welding. Mater. Lett. 185, 152–155 (2016).
Sun, Z. X., Fang, S. Y., Lin, Y. & Hu, Y. H. Photo-assisted methanol steam reforming on solid solution of Cu–Zn–Ti oxide. iScience. 375, 121909 (2019).
Acknowledgements
This project is supported by the National Natural Science Foundation of China (Grant No.5210040951). This research also was supported by Autonomous Region Natural Science Foundation (2020D01C061).
Author information
Authors and Affiliations
Contributions
Y.Z. contributed to the conception of the study; Y.C. performed the experiment; J.Z. contributed significantly to analysis and manuscript preparation; D.S. performed the data analyses and wrote the manuscript; H.L. helped perform the analysis with constructive discussions.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, Y., Chen, Y., Zhou, J. et al. Compound connection mechanism of Al2O3 ceramic and TC4 Ti alloy with different joining modes. Sci Rep 11, 21251 (2021). https://doi.org/10.1038/s41598-021-00815-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-00815-4
This article is cited by
-
Laser Applications in Ceramic and Metal Joining: A Review
Metals and Materials International (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.