Abstract
Transcutaneous spinal direct current stimulation (tsDCS) is a safe and convenient method of neuromodulation. It has been proven to alter sensory processing at cervicomedullary level by amplitude changes of the P30 response of tibial nerve somatosensory evoked potentials (TN SEPs). With knowledge that tsDCS affects cortical circuits, we hypothesized that tsDCS may also affect intracortical excitability of the somatosensory cortex assessed by paired stimulation suppression (PSS). Fourteen healthy men were included in this prospective, single-blinded, placebo-controlled crossover study. Single (SS) and paired stimulation (PS) TN SEPs were recorded over the scalp before, immediately as well as 30 and 60 min after applying 15 min of tsDCS over the twelfth thoracic vertebra. Each volunteer underwent three independent and randomized sessions of either cathodal, anodal or sham stimulation. tsDCS showed no effect on peak-to-peak amplitudes or latencies of cortical P40-N50 response after SS. Furthermore, tsDCS failed to induce significant changes on amplitude ratios of PSS, thus showing no impact on intracortical excitability of the somatosensory cortex in healthy subjects. Further research is required to reveal the different mechanisms and to strengthen clinical use of this promising technique.
Similar content being viewed by others
Introduction
Modulation of cortical excitability and plasticity using non-invasive and weak electric currents to stimulate neural tissue is a recent topic of neuroscience1,2,3. Transcranial direct current stimulation has been shown to induce lasting changes in cortical neural activity4,5,6,7 and is established as a clinical tool by now8,9,10 resulting in level B evidence for treating fibromyalgia, depression and craving1. A similar approach is the application of homologous direct currents to the spinal cord: transcutaneous spinal direct current stimulation (tsDCS).
Invasive pulsed spinal cord stimulation (SCS) induces cortical changes described as alterations of SEP amplitudes or pain perception in humans11,12,13. In animal studies, invasive spinal direct current stimulation showed polarity-specific and state-dependent supraspinal effects on the activity of the gracile nucleus and the primary somatosensory cortex14. Analogously, non-invasive tsDCS has been proven to influence ascending pathways including the nociceptive system in humans15,16,17,18,19. TsDCS affects descending pathways20,21,22 and spinal reflexes of different levels23,24,25,26,27 resulting in first therapeutic approaches in patients with restless legs syndrome28, hereditary spastic paraplegia29 or spinal cord injuries24,30,31. Additionally, tsDCS significantly alters amplitudes of motor evoked potentials after paired-pulse transcranial magnetic stimulation32, decreases intracortical inhibition and increases intracortical facilitation33 underlining the impact of tsDCS on motor cortex excitability and descending pathways.
By analyzing TN SEPs, previous studies showed that anodal tsDCS of the lower thoracic spine induces subcortical changes in the neural activity of the brainstem represented by an amplitude depression of the far field potential P30 recorded over the sixth cervical vertebra while cathodal tsDCS tends to increase P3015, both suggesting axonal alterations of transmission. First evidence of tsDCS inducing effects within the somatosensory cortex was recently revealed by illustrating cortical changes of somatosensory functional connectivity in a fMRI study34. However, knowledge of tsDCS’ impact on the somatosensory cortex remains rare.
A more specific approach of deducing intracortical excitability changes of the somatosensory cortex compared to the sole analysis of amplitudes is the evaluation of paired stimulation suppression (PSS) patterns of SEPs. PSS is a common tool to examine neuronal excitability and serves as a model for investigating short-term plasticity35,36,37. By assessing the suppressive effect of a stimulus on a subsequent second stimulus PSS is supposed to measure intracortical excitability35,38,39. Previous studies demonstrated the cortical origin of PSS by using recording techniques at different hierarchical levels of the sensory pathway36,40,41. Based on findings in pharmacological studies, there is strong evidence, that PSS is mainly GABA-mediated39. Furthermore, changes in PSS in the somatosensory system as an indicator of facilitatory and inhibitory effects have been studied in several neurological disorders38,42,43.
Until now, there is no data available concerning the effect of tsDCS on PSS of the somatosensory cortex. To further understand the underlying mechanisms of tsDCS, this prospective volunteer study was designed with the hypothesis that tsDCS will alter the intracortical excitability of the somatosensory cortex expressed by alterations of PSS following TN SEPs.
Results
One out of fifteen participants was excluded from further analysis due to electroencephalogram artefacts. Table 1. Independent from stimulation polarity (Fig. 1), participants did not report any adverse events due to tsDCS. See Supplement 1 for raw data.
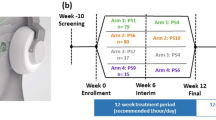
Study design: each study participant underwent three individual sessions of the procedure shown above with a latency of at least 1 week between these sessions. During each session 15 min of either cathodal, anodal or sham transcutaneous spinal direct current stimulation (tsDCS) was applied to the participant. Before tsDCS, a baseline tibial nerve SEP was recorded (Baseline). tsDCS was then applied in randomized individual order. The stimulation was followed by three SEP recordings with the first one being started directly after tsDCS (T0). Another two SEP recordings were performed 30 (T30) and 60 (T60) min after tsDCS.
Single stimulation
Baseline SEPs were comparable throughout all sessions (amplitudes: F2, 39 = 0.484, p = 0.62; latencies: F2, 39 = 0.015, p = 0.985).
No main effects of tsDCS were seen in amplitudes (polarity: F1.88, 15.02 = 0.329, p = 0.712, partial η2 = 0.039; time: F1.55, 12.37 = 1.106, p = 0.345, partial η2 = 0.121). In latencies only a main effect over time was observed (polarity: F1.89, 15.15 = 0.32, p = 0.719, partial η2 = 0.038; time: F2.57, 20.53 = 5.837, p = 0.006, partial η2 = 0.422). Also, no interactions between polarity*time were detected (amplitudes: F3.16, 25.26 = 1.378, p = 0.272, partial η2 = 0.147; latencies: F3.33, 26.62 = 1.416, p = 0.259, partial η2 = 0.15). Figure 2A, Table 2. Polarity sequence did not influence the results.
(A) Example of a single stimulation tibial nerve somatosensory evoked potential recorded over Cz’. “A” shows the amplitude (µV) and “Lat” the latency (ms) of the P40-N50 peak. (B/C) Examples of a paired stimulation tibial nerve somatosensory evoked potentials recorded over Cz’ with an interstimulus interval of 60 (PS60; B) and 90 (PS90; C) ms. “A1” shows the amplitude (P40-N50; µV) of the first peak as “A2” shows the amplitude of the second peak. “A2s” displays the amplitude of the second peak after linear subtraction of the response to a single stimulation. y-axis: amplitude [µV]. x-axis: time [ms].
Paired stimulation (PS60 and PS90)
An interaction of polarity*time was revealed for PS60 (F3.54, 28.34 = 3.857, p = 0.015, partial η2 = 0.325), but no main effects on polarity (F1.84, 14.75 = 0.268, p = 0.751, partial η2 = 0.032) or time (F2.25, 18.02 = 3.208, p = 0.059, partial η2 = 0.286). Figures 2B and 3A, Table 3. Focusing on PS90, no interaction between polarity*time could be explored (F3.07, 24.6 = 2.86, p = 0.056, partial η2 = 0.263). Also, there were no effects of tsDCS polarity (F1.81, 14.48 = 0.886, p = 0.424, partial η2 = 0.1) or over time (F1.4, 11.21 = 4.021, p = 0.06, partial η2 = 0.334). Figures 2C and 3B, Table 3. Polarity sequence did not influence the results.
(A/B) The plots show the progress of paired stimulation suppression ratios (A2s/A1) recorded over Cz’ following stimulation of the tibial nerve with an interstimulus interval of 60 (PS60; A) and 90 (PS90; B) ms. The black line shows progress after cathodal, the dotted line after anodal and the grey line after sham transcutaneous spinal direct current stimulation. Recordings took place before (baseline), immediately (T0) as well as 30 (T30) and 60 (T60) min after stimulation. y-axis: amplitude ratios (A2s/A1). x-axis: points of time.
Discussion
Previously unaddressed, the present study aimed to evaluate cortical modulatory effects of tsDCS in healthy subjects. Against expectations, tsDCS did not alter the intracortical excitability of the primary somatosensory cortex assessed by paired stimulation of TN SEPs.
Even though there is evidence of tsDCS influencing cortical circuits and functional connectivity offside its site of application15,16,32,33,34,44, there is no data available yet on its effect on the intracortical excitability of the primary somatosensory cortex. By evaluating the suppressive effect of a precursory stimulus on a subsequent stimulus of an evoked cortical potential, PSS is a commonly used tool to estimate intracortical excitability35,36,45,46,47 and seems to reflect inhibitory cortical processes36,48. Especially presynaptic mechanisms are believed to be essential for PSS39,49. In animal trials presynaptic GABAA50 and GABAB51,52 receptors have been identified to be involved in PSS, which is dynamic and declines with age in humans46.
The missing effect is surprising, as clinically established invasive procedures on the spinal cord such as pulsed SCS have already been proven to affect cortical TN SEP components in terms of amplitude depressions11 deducing that pulsed stimulation—may it be invasive or transcutaneous53—seems to be one major factor of efficacy. Specifying on transcutaneous SCS over the cervical spinal cord, the carrier frequency is essential for modulating intracortical inhibition of the motor cortex53.
Yet, non-invasive anodal tsDCS has been demonstrated to attenuate TN SEP amplitudes on a subcortical level whereas cortical SEP components were unaffected15, which is in line with our findings. The authors argued, that the far field potential P30, originated in the cervicomedullary junction54, is more sensitive than the near field potential P40-N50, generated within the primary sensory cortex54,55,56,57, for spatial and temporal synaptic summation may have equalized possible cortical effects15,58. However, in our setup, which was designed to be a more specific approach of deducing intracortical excitability by analyzing PSS rather than sole amplitudes, after both cathodal and anodal tsDCS there were no relevant changes in neither amplitudes nor latencies of SS nor ratios of paired stimulation amplitudes of the P40-N50 response over Cz’ up to one hour after tsDCS. Hence, we must conclude that tsDCS does not affect inhibitory cortical processes of the somatosensory cortex.
Supporting our results, recent research showed a selective effect of tsDCS on nociception models which were predominantly forwarded by thinly myelinated Aδ fibers16,17,18,25. In other words, it is likely that thickly myelinated Aβ nerve fibers remain largely unaffected by tsDCS, as seen by SEP transmission. Doing so, tsDCS may lead to quantifiable subcortical changes causing an altered pain perception but leaving the intracortical excitability of the somatosensory cortex unchanged.
Regardless of our findings and the exact mechanisms, tsDCS has been shown to be a promising technique in pathological conditions like pain16,17,19, post-stroke-conditions44, restless legs syndrome28 or spinal cord injuries24,31. Those effects of tsDCS rely mainly on pathological or sensitized preconditions also seen in experimental settings for presupposed pain18 or in combination e.g. with locomotor learning59. Approaches to assess effects of SCS on SEPs also premised pain11,12,13. Additionally, invasive spinal direct current stimulation in rats caused changes in cortical evoked potentials which were dependent on cortical spontaneous activity14. The dependency of cortical plasticity on cofactors is renowned60 as is the pharmaceutical modulation of PSS on for example the somatosensory39 or the visual cortex61 depicting hypothetic requirements that were not available in our setup. Concluding that tsDCS predominantly affects specific or pathological preconditions, further research should be conducted especially in patients.
PSS of the TN is not as common as of the median nerve. Literature is not providing standard procedures and parameter settings. Yet this setup was chosen to be comparable with the one of Cogiamanian et al. who earlier demonstrated an effect of tsDCS on SEPs at cervicomedullary level15. In addition, there are no universal standards defining the amplitudes of TN SEPs. In order to focus on intracortical excitability, the presented study—contrasting15—not only takes the approach of analyzing PSS instead of mere SS amplitudes but also defines the SEP amplitude as peak to peak of P40 to N50 as these potentials are assumed to be generated intracortically11,55,62,63. Furthermore, even in accordance with the guidelines of the International Confederation of Clinical Physiology, there is a vast number of possible electrode configurations and recording techniques involving equipment, montages, filters, interfering signals, stimulus rates or the type of stimulation electrodes64. Limiting the time of data acquisition for each SEP to 10 min for the repetitive setup of a single stimulation followed by two paired stimulations at 2 Hz would allow the acquisition of 400 cycles which is comparably less considering the possible alterations of the second phase.
Additionally, the sample size of fourteen participants is low, but was based on comparable tsDCS studies, which are typically around 12–15 subjects15,16,23,25,26. However, effect sizes underline our results. Single changes found over time without any effect due to tsDCS seem to be inconclusively and should be neglected.
Furthermore, the exact spread of the electric field of tsDCS in the human body is unknown yet as are the mechanisms and the targets of the technique leading to a lack of standardization of electrode characteristics, electrode placement and energy charge during application. Considering the spread of the electric field, body positioning during the application seems to be a crucial factor as a significant increase of motor evoked potentials after cathodal tsDCS has been shown when tsDCS was applied to the proband in supine position whereas in seated position there were no significant effects65. Again, stimulation patterns (e.g., pulsed11,13 versus continuous DCS, stimulation repetition, duration34,65 and intensity) may vary the effects explaining heterogenous results. As SEPs were not recorded during tsDCS, an early effect may have been missed. The latter aspects should be addressed in future research.
In conclusion, against expectations, the current results did not reveal any relevant impact of tsDCS on the intracortical excitability of the somatosensory cortex by altering PSS patterns after stimulation of the TN. However, as evidence for supraspinal effects of tsDCS has been provided, further research is required to reveal the specific mechanisms of tsDCS focusing on patients’ conditions and thereby strengthening the implementation for clinical use of this promising non-invasive and low-cost technique.
Methods
The protocol of this single-blinded, randomized and placebo-controlled crossover study was approved by the Ethics Committee of the Medical Faculty of the Ruhr-University Bochum, Germany (No. 4358-12; approved on 25 May 2012). All actions were in accordance with the Declaration of Helsinki and CONSORT guidelines were followed (Supplement 2). The trial was registered at German Clinical Trials Register (DRKS-ID: DRKS00012651; registered 04/08/2017). Written and informed consent of all participants was obtained before data acquisition.
Participants
Fifteen healthy and right-handed young men were recruited via local advertisement and consecutively included. To eliminate sex differences only men were included. Exclusion criteria were any previous medical condition (such as obesity, diabetes, chronic pain, seizures or other neurological or psychiatric comorbidities) and any regular use of medication.
TN SEP and paired stimulation
SEPs of the tibial nerve were acquired using a block electrode (distance between anode and cathode: 20 mm) placed over the tibial nerve stem at the right medial malleolus. To ensure a reliable supramaximal nerve stimulation, current intensity of the square wave (0.2 ms) was set approximately 2 mA above motor threshold intensity inducing a muscular twitch of the plantar muscles38. Table 1. A Digitimer DS7A Current Stimulator (Digitimer Ltd, Hertfordshire, UK) timed by a custom-built timing device (microcontroller board, Arduino) generated stimuli according to the following fixed scheme: a single stimulus (SS) was followed by paired stimuli with an interstimulus interval (ISI) of 60 ms (PS60), which were again followed by paired stimuli with an ISI of 90 ms (PS90). Previous studies demonstrated that an ISI of 60–90 ms leads to a reliable PSS (see below)66. As the recovery time of the tibial nerve after paired stimulation is around 250 ms, stimulation frequency was set to 2 Hz giving enough time to bridge the refractory phase of cortical potentials at an ISI of 90 ms66. SEPs were recorded by an electrode placed at Cz’ referenced to frontopolar cortex (Fp) and with the ground electrode placed above the forehead (Fpz). Electrode locations were accurately cleaned and degreased before electrode placement. Resistances were kept below 5 kOhm. For averaging, 400 repetition cycles were recorded using Brain Vision Recorder software (Version 1.02, Brain Products GmbH, Gilching, Germany). We used Brain Vision Analyzer software (Version 1.05, Brain Products GmbH, Gilching, Germany) for further offline analysis of the SEPs. Averaged peak-to-peak amplitudes and latencies (P40-N50 component55) of each SS SEP were manually detected and analyzed. Figure 2A. For the acquisition of PSS ratios, we analyzed peak-to-peak amplitudes evoked by the second stimulus (A2) after digital subtraction of the single stimulus (A2s) and divided it by the response of the first stimulus before linear subtraction (A1). Figure 2B,C. PSS for PS60 and PS90 was expressed as a ratio of these amplitudes (A2s/A1)36,38,47.
TsDCS
TsDCS was generated by a neuroConn DC-Stimulator Plus (neuroConn GmbH, Ilmenau, Germany) and applied by a pair of rubber electrodes (5 × 7 cm; 35 cm2; 2 mm thick) each positioned longitudinally). Analogously to Schweizer et al.34,67, the spinal electrode was centered with its lower end above the spinous process of the twelfth thoracic vertebra, the reference electrode was attached above the left scapula spine with its medial edge aligned with the medial margin of the scapula. Ten20 conductive paste (Weaver and Company, Aurora, Colorado, USA) was used to minimize resistances and to contribute to an even skin contact of the electrodes. Using a maximum current of 2.5 mA at a current density of 0.071 mA/cm2, a total charge of 63.9 mC/cm2 was supplied complying to general safety criteria68. Stimulation duration was set to 870 s with an additional ramp up of ten seconds and a ramp down of 20 s, hence a total stimulation time of 15 min. The spinal electrode determined the polarity (cathodal/anodal). Cathodal setup was used for sham procedure with the stimulation being limited to 15 s of 1.5 mA preceded by a ramp up of 10 s and followed by a ramp down of 20 s as well as a shammed stimulation of 855 s, resulting in a cumulative pretended stimulation time of 15 min. In detail, subjects were told that the stimulation would last for 15 min.
Experimental design
Trial execution took place in the laboratories of the BG-University Hospital Bergmannsheil in Bochum, Germany. Participants were seated comfortably and semi-prostrated with their heads slightly flexed. Lights were dimmed, acoustics were quiet and room temperature was acclimated to 22 °C. Blinded subjects underwent cathodal, anodal and sham stimulation each during an individual out of three sessions. Sessions were scheduled at least 7 days apart at an analog time of day to avoid residual and daytime effects. The sequence of tsDCS polarity was randomized computer-generated with variable block length for each subject. SEPs were recorded before (baseline), immediately (T0) as well as 30 (T30) and 60 min (T60) after tsDCS (Fig. 1).
Statistical analysis
Statistical analysis was carried out using IBM SPSS Statistics software (Version 25; IBM Corp., Armonk, New York, USA) by a blinded statistician. To check for inconsistencies and carryover effects, baseline data for amplitudes and latencies of each single stimulation SEP were compared by calculating a one-way analysis of variance (ANOVA) with the factor polarity (three levels: cathodal, anodal, sham). Based on normal distribution (histograms; see example in Supplement 3), data of amplitudes and latencies for SS and amplitude ratios for both PS60 and PS90 were analyzed using ANOVA for repeated measurements (rmANOVA) with Greenhouse–Geisser correction to test for main effects [’polarity’ (three levels: cathodal, anodal or sham) and ‘time’ (four levels: baseline, T0, T30, T60)]. The main factors and interactions (polarity*time) were specifically of interest to analyze for temporal effects (time) due to different tsDCS (polarity) on SS and PSS. A test for polarity sequence interaction as part of the crossover design was performed. The level of significance was set to p < 0.05.
Data availability
The datasets generated and analyzed during this study are available from the corresponding author on reasonable request.
References
Lefaucheur, J. P. et al. Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS). Clin. Neurophysiol. 128, 56–92 (2017).
Priori, A., Ciocca, M., Parazzini, M., Vergari, M. & Ferrucci, R. Transcranial cerebellar direct current stimulation and transcutaneous spinal cord direct current stimulation as innovative tools for neuroscientists. J. Physiol. 592, 3345–3369 (2014).
Sparing, R. & Mottaghy, F. M. Noninvasive brain stimulation with transcranial magnetic or direct current stimulation (TMS/tDCS)—From insights into human memory to therapy of its dysfunction. Methods 44, 329–337 (2008).
Priori, A., Berardelli, A., Rona, S., Accornero, N. & Manfredi, M. Polarization of the human motor cortex through the scalp. NeuroReport 9, 2257–2260 (1998).
Gobbelé, R. et al. P21.3 Transcranial direct current stimulation applied over the somatosensory cortex—Differential effect on low and high frequency SEPs. Clin. Neurophysiol. 117, 217 (2006).
Nitsche, M. A. & Paulus, W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J. Physiol. 527, 633–639 (2000).
Nitsche, M. A. & Paulus, W. Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology 57, 1899–1901 (2001).
Woods, A. J. et al. A technical guide to tDCS, and related non-invasive brain stimulation tools. Clin. Neurophysiol. 127, 1031–1048 (2016).
Nitsche, M. A. et al. Transcranial direct current stimulation: State of the art 2008. Brain Stimul. 1, 206–223 (2008).
Brunoni, A. R. et al. Clinical research with transcranial direct current stimulation (tDCS): Challenges and future directions. Brain Stimul. 5, 175–195 (2012).
Wolter, T., Kieselbach, K., Sircar, R. & Gierthmuehlen, M. Spinal cord stimulation inhibits cortical somatosensory evoked potentials significantly stronger than transcutaneous electrical nerve stimulation. Pain Physician 16, 405–414 (2013).
Grabow, T. S., Tella, P. K. & Raja, S. N. Spinal cord stimulation for complex regional pain syndrome: An evidence-based medicine review of the literature. Clin. J. Pain 19, 371–383 (2003).
Poláček, H., Kozák, J., Vrba, I., Vrána, J. & Stančák, A. Effects of spinal cord stimulation on the cortical somatosensory evoked potentials in failed back surgery syndrome patients. Clin. Neurophysiol. 118, 1291–1302 (2007).
Aguilar, J. et al. Spinal direct current stimulation modulates the activity of gracile nucleus and primary somatosensory cortex in anaesthetized rats. J. Physiol. 589, 4981–4996 (2011).
Cogiamanian, F., Vergari, M., Pulecchi, F., Marceglia, S. & Priori, A. Effect of spinal transcutaneous direct current stimulation on somatosensory evoked potentials in humans. Clin. Neurophysiol. 119, 2636–2640 (2008).
Truini, A. et al. Transcutaneous spinal direct current stimulation inhibits nociceptive spinal pathway conduction and increases pain tolerance in humans. Eur. J. Pain 15, 1023–1027 (2011).
Meyer-Frießem, C. H. et al. Transcutaneous spinal DC stimulation reduces pain sensitivity in humans. Neurosci. Lett. 589, 153–158 (2015).
Lenoir, C., Jankovski, A. & Mouraux, A. Anodal transcutaneous spinal direct current stimulation (tsDCS) selectively inhibits the synaptic efficacy of nociceptive transmission at spinal cord level. Neuroscience 393, 150–163 (2018).
Perrotta, A. et al. Modulation of temporal summation threshold of the nociceptive withdrawal reflex by transcutaneous spinal direct current stimulation in humans. Clin. Neurophysiol. 127, 755–761 (2016).
Bocci, T. et al. Transcutaneous spinal direct current stimulation modulates human corticospinal system excitability. J. Neurophysiol. 114, 440–446 (2015).
Lim, C. Y. & Shin, H. I. Noninvasive DC stimulation on neck changes MEP. NeuroReport https://doi.org/10.1097/WNR.0b013e32834b939d (2011).
Yamaguchi, T., Fujimoto, S., Otaka, Y. & Tanaka, S. Effects of transcutaneous spinal DC stimulation on plasticity of the spinal circuits and corticospinal tracts in humans. 2013 6th Int. IEEE/EMBS Conf. Neural Eng. (NER) https://doi.org/10.1109/NER.2013.6695925 (2013).
Winkler, T., Hering, P. & Straube, A. Spinal DC stimulation in humans modulates post-activation depression of the H-reflex depending on current polarity. Clin. Neurophysiol. 121, 957–961 (2010).
Hubli, M., Dietz, V., Schrafl-Altermatt, M. & Bolliger, M. Modulation of spinal neuronal excitability by spinal direct currents and locomotion after spinal cord injury. Clin. Neurophysiol. 124, 1187–1195 (2013).
Cogiamanian, F. et al. Transcutaneous spinal cord direct current stimulation inhibits the lower limb nociceptive flexion reflex in human beings. Pain 152, 370–375 (2011).
Lamy, J.-C., Ho, C., Badel, A., Arrigo, R. T. & Boakye, M. Modulation of Soleus H-reflex by spinal DC stimulation in humans. J. Neurophysiol. 108, 906–914 (2012).
Lamy, J.-C. & Boakye, M. BDNF Val66Met polymorphism alters spinal DC stimulation-induced plasticity in humans. J. Neurophysiol. 110, 109–116 (2013).
Heide, A. C. et al. Effects of transcutaneous spinal direct current stimulation in idiopathic restless legs patients. Brain Stimul. 7, 636–642 (2014).
Ardolino, G. et al. Spinal direct current stimulation (tsDCS) in hereditary spastic paraplegias (HSP): A sham-controlled crossover study. J. Spinal Cord Med. 1–8. https://doi.org/10.1080/10790268.2018.1543926 (2018).
Powell, E. S., Carrico, C., Salyers, E., Westgate, P. M. & Sawaki, L. The effect of transcutaneous spinal direct current stimulation on corticospinal excitability in chronic incomplete spinal cord injury. NeuroRehabilitation 43, 125–134 (2018).
Sayenko, D. G. et al. Self-assisted standing enabled by non-invasive spinal stimulation after spinal cord injury. J. Neurotrauma 36, 1435–1450 (2019).
Bocci, T. et al. Spinal direct current stimulation modulates short intracortical inhibition. Neuromodulat. Technol. Neural Interface 18, 686–693 (2015).
Murray, L. M. & Knikou, M. Repeated cathodal transspinal pulse and direct current stimulation modulate cortical and corticospinal excitability differently in healthy humans. Exp. Brain Res. 237, 1841–1852 (2019).
Schweizer, L., Meyer-Frießem, C. H., Zahn, P. K., Tegenthoff, M. & Schmidt-Wilcke, T. Transcutaneous spinal direct current stimulation alters resting-state functional connectivity. Brain Connect. 7, 357–365 (2017).
Höffken, O. et al. Excitability in somatosensory cortex correlates with motoric impairment in amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. Front. Degener. 20, 192–198 (2019).
Höffken, O., Lenz, M., Tegenthoff, M. & Schwenkreis, P. Multichannel SEP-recording after paired median nerve stimulation suggests origin of paired-pulse inhibition rostral of the brainstem. Neurosci. Lett. 468, 308–311 (2010).
Gatica Tossi, M. A., Lillemeier, A.-S. & Dinse, H. R. Influence of stimulation intensity on paired-pulse suppression of human median nerve somatosensory evoked potentials. NeuroReport 24, 451–456 (2013).
Höffken, O. et al. Influence of parameter settings on paired-pulse-suppression in somatosensory evoked potentials: A systematic analysis. Clin. Neurophysiol. 124, 574–580 (2013).
Stude, P., Lenz, M., Höffken, O., Tegenthoff, M. & Dinse, H. A single dose of lorazepam reduces paired-pulse suppression of median nerve evoked somatosensory evoked potentials. Eur. J. Neurosci. 43, 1156–1160 (2016).
Creutzfeldt, O., Hellweg, F.-C. & Schreiner, C. Thalamocortical transformation of responses to complex auditory stimuli. Exp. Brain Res. 39, 87–104 (1980).
Miller, L. M., Escabí, M. A., Read, H. L. & Schreiner, C. E. Spectrotemporal receptive fields in the lemniscal auditory thalamus and cortex. J. Neurophysiol. 87, 516–527 (2002).
Hallett, M. Transcranial magnetic stimulation: A primer. Neuron 55, 187–199 (2007).
Kobayashi, M. & Pascual-Leone, A. Transcranial magnetic stimulation. In The Clinical Neurophysiology Primer (eds Blum, A. S. & Rutkove, S. B.) 499–515 (Humana Press, Totowa, 2007).
Bocci, T. et al. An unexpected target of spinal direct current stimulation: Interhemispheric connectivity in humans. J. Neurosci. Methods 254, 18–26 (2015).
Goto, S. et al. Disinhibitory shift of recovery curve of somatosensory-evoked response in elderly: A magnetoencephalographic study. Clin. Neurophysiol. 126, 1228–1233 (2015).
Lenz, M. et al. Increased excitability of somatosensory cortex in aged humans is associated with impaired tactile acuity. J. Neurosci. 32, 1811–1816 (2012).
Schwartz, M. & Shagass, C. Recovery functions of human somatosensory and visual evoked potentials*. Ann. N. Y. Acad. Sci. 112, 510–525 (2006).
Nakagawa, K., Inui, K., Yuge, L. & Kakigi, R. Inhibition of somatosensory-evoked cortical responses by a weak leading stimulus. Neuroimage 101, 416–424 (2014).
Hashimoto, K. & Kano, M. Presynaptic origin of paired-pulse depression at climbing fibre-Purkinje cell synapses in the rat cerebellum. J. Physiol. 506, 391–405 (1998).
Schmidt, S., Redecker, C., Bruehl, C. & Witte, O. W. Age-related decline of functional inhibition in rat cortex. Neurobiol. Aging 31, 504–511 (2010).
Hickmott, P. & Dinse, H. Effects of aging on properties of the local circuit in rat primary somatosensory cortex (S1) in vitro. Cereb. Cortex 23, 2500–2513 (2013).
Porter, J. T. & Nieves, D. Presynaptic GABA B receptors modulate thalamic excitation of inhibitory and excitatory neurons in the mouse barrel cortex. J. Neurophysiol. 92, 2762–2770 (2004).
Benavides, F. D. et al. Cortical and subcortical effects of transcutaneous spinal cord stimulation in humans with tetraplegia. J. Neurosci. 40, 2633–2643 (2020).
Tinazzi, M. et al. Subcortical P30 potential following tibial nerve stimulation: Detection and normative data. Ital. J. Neurol. Sci. 16, 623–628 (1995).
Miura, T., Sonoo, M. & Shimizu, T. Establishment of standard values for the latency, interval and amplitude parameters of tibial nerve somatosensory evoked potentials (SEPs). Clin. Neurophysiol. 114, 1367–1378 (2003).
Kakigi, R. et al. Topography of somatosensory evoked magnetic fields following posterior tibial nerve stimulation. Electroencephalogr. Clin. Neurophysiol. 95, 127–134 (1995).
Ziegler, D., Muhlen, H., Dannehl, K. & Gries, F. A. Tibial nerve somatosensory evoked potentials at various stages of peripheral neuropathy in insulin dependent diabetic patients. J. Neurol. Neurosurg. Psychiatry 56, 58–64 (1993).
Tinazzi, M. & Mauguiére, F. Assessment of intraspinal and intracranial conduction by P30 and P39 tibial nerve somatosensory evoked potentials in cervical cord, brainstem, and hemispheric lesions. J. Clin. Neurophysiol. https://doi.org/10.1097/00004691-199505010-00003 (1995).
Awosika, O. O. et al. Transcutaneous spinal direct current stimulation improves locomotor learning in healthy humans. Brain Stimul. 12, 628–634 (2019).
Kirkwood, A., Rozas, C., Kirkwood, J., Perez, F. & Bear, M. F. Modulation of long-term synaptic depression in visual cortex by acetylcholine and norepinephrine. J. Neurosci. 19, 1599–1609 (1999).
Höffken, O., Lenz, M., Höckelmann, N., Dinse, H. R. & Tegenthoff, M. Noradrenergic modulation of human visual cortex excitability assessed by paired-pulse visual-evoked potentials. NeuroReport 23, 707–711 (2012).
Seyal, M. & Gabor, A. J. The human posterior tibial somatosensory evoked potential: Synapse dependent and synapse independent spinal components. Electroencephalogr. Clin. Neurophysiol. Potentials Sect. 62, 323–331 (1985).
Beric, A. & Prevec, T. S. The early negative potential evoked by stimulation of the tibial nerve in man. J. Neurol. Sci. 50, 299–306 (1981).
Seyal, M., Emerson, R. G. & Pedley, T. A. Spinal and early scalp-recorded components of the somatosensory evoked potential following stimulation of the posterior tibial nerve. Electroencephalogr. Clin. Neurophysiol. 55, 320–330 (1983).
Murray, L. M., Tahayori, B. & Knikou, M. Transspinal direct current stimulation produces persistent plasticity in human motor pathways. Sci. Rep. 8, 717 (2018).
Saito, T. et al. Recovery functions of common peroneal, posterior tibial and sural nerve somatosensory evoked potentials. Electroencephalogr. Clin. Neurophysiol. 85, 337–344 (1992).
Schweizer, L. M. et al. Influence of transcutaneous spinal stimulation on human LTP-like pain amplification. A randomized, double-blind study in volunteers. Clin. Neurophysiol. 128, 1413–1420 (2017).
Nitsche, M. A. et al. Safety criteria for transcranial direct current stimulation (tDCS) in humans. Clin. Neurophysiol. 114, 2220–2223 (2003).
Acknowledgements
The manuscript is part of JHB’s doctoral thesis.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
All authors meet all criteria for authorship recommended by ICMJE and agree with the final contents of the manuscript. J.H.B.: Investigator, did statistical analyses, interpreted data, drafted manuscript. C.M.F.: Investigator, drafted ethical proposal and manuscript, interpreted data. L.M.S.: Investigator, did statistical analyses, interpreted data, revised manuscript. L.S.: Interpreted data, revised manuscript. P.K.Z.: Responsible for concept and made critical revision, interpreted data, agreed with the final manuscript. M.T.: Responsible for concept, participated in drafting the manuscript, agreed with the final manuscript. O.H.: Gave advice, made critical revisions, interpreted data, drafted manuscript.
Corresponding author
Ethics declarations
Competing interests
This work was supported by the Deutsche Forschungsgemeinschaft/German Research Foundation (DFG) (SFB 874, Project No.: 122679504) to MT (A1), OH and LS (A5). The other authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bettmann, J.H., Meyer-Frießem, C.H., Schweizer, L.M. et al. Transcutaneous spinal direct current stimulation shows no effect on paired stimulation suppression of the somatosensory cortex. Sci Rep 10, 22010 (2020). https://doi.org/10.1038/s41598-020-79131-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-79131-2
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.