Abstract
The AMP-activated kinase (AMPK) is a major energy sensor metabolic enzyme that is activated early during T cell immune responses but its role in the generation of effector T cells is still controversial. Using both in vitro and in vivo models of T cell proliferation, we show herein that AMPK is dispensable for early TCR signaling and short-term proliferation but required for sustained long-term T cell proliferation and effector/memory T cell survival. In particular, AMPK promoted accumulation of effector/memory T cells in competitive homeostatic proliferation settings. Transplantation of AMPK-deficient hematopoïetic cells into allogeneic host recipients led to a reduced graft-versus-host disease, further bolstering a role for AMPK in the expansion and pathogenicity of effector T cells. Mechanistically, AMPK expression enhances the mitochondrial membrane potential of T cells, limits reactive oxygen species (ROS) production, and resolves ROS-mediated toxicity. Moreover, dampening ROS production alleviates the proliferative defect of AMPK-deficient T cells, therefore indicating a role for an AMPK-mediated ROS control of T cell fitness.
Similar content being viewed by others
Introduction
Changes in the proliferative and functional state of T lymphocytes during an immune response are accompanied by profound modifications in the cell metabolism. Whereas naive T cells generate the largest part of their ATP through mitochondrial oxidative phosphorylation (OxPhos), activated lymphocytes rely heavily on glycolysis to fulfill their energetic demands1,2. Long-lived memory T cells that subsist after primary infection convert back to an oxidative metabolism with a marked preference for fatty acid as primary energy source (FAO)1,3.
The AMP-activated protein kinase (AMPK) maintains the energy balance in eukaryotic cells and is a central regulator of cellular responses to metabolic stress4,5,6. Activation of AMPK decreases fatty acid and protein synthesis7,8, and promotes FAO and mitochondrial biogenesis9,10. AMPK also up-regulates the rate of glycolysis through phosphorylation of PFKFB3 and increases glucose uptake11,12. Moreover, AMPK recently emerged as an important sensor and regulator of mitochondrial ROS and a regulator of ROS neutralization by antioxidant cellular systems, thereby maintaining adequate cellular oxidative balance13.
Although T lymphocyte activation results in the rapid activation of AMPK in vitro and in vivo3,14, the role of this enzyme in T cell function is still controversial. AMPK appears dispensable for the generation of effector T cells in two independent studies15,16 while required for the expansion of antigen-specific CD8+ T cells in response to viral or bacterial infections17. Moreover, effector T cells lacking AMPKα1 display reduced mitochondrial bioenergetics and cellular ATP in response to glucose limitation, in support of a role for AMPK in the metabolic adaptations of activated effector T cells17. Despite these observations, mice lacking expression of AMPK in the T cell compartment appear as largely immunocompetent, thus questioning the specific role of this enzyme in regulating immune homeostatis.
We confirm in this report that AMPK is dispensable during the earliest steps of T cell activation. AMPK-deficient cells however display reduced capacity to sustain long term proliferative responses both in vitro and in vivo revealing a potentially important role for this enzyme in the setting of continuous stimulation. We further demonstrate that AMPK expression protects cells from the damaging effects of reactive oxygen species (ROS) that accumulate during rapid cell division, thus revaling the important role of this metabolic sensor in ensuring long term proliferative T cell fitness.
Results
AMPK promotes effector/memory T cells engraftment in the peripheral tissues of bone marrow chimeras
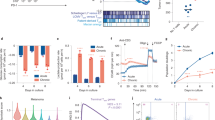
To investigate the role of AMPK in T cell homeostasis, we used conditional KO mice in which loxP-flanked alleles of AMPKα1 (prkaa1f/f) are deleted by the Cre recombinase expressed from the T-cell-specific cd4 promoter (prkaa1f/f–CD4-Cre mice, hereafter referred as AMPKKO-T mice). No major abnormalities in the thymic and splenic lymphoid populations of these mice have been reported15,16. To evaluate whether expression of AMPK modulates the size of T cell compartments in a homeostatic competitive environment, we generated chimeras by reconstituting irradiated CD3ε−/− recipient mice with a 1:1 mixture of congenitally marked bone marrow cells from wild type (CD45.1+) and AMPKKO-T (CD45.2+) donor mice. The average contribution of WT and AMPK-KO cells to the repopulation of the thymus, spleen and lymph nodes CD4 and CD8 T cell compartments was comparable (Fig. 1A,B). Of interest, AMPK-KO splenocytes expressed slightly but significantly lower percentages of CD44hi CD62Llow surface markers characteristic of effector/memory cells (Fig. 1C–F). The AMPK-KO T cell compartment was also markedly deficient in the expression of the tissue homing CXCR3 chemokine receptor (Fig. 1G,H). Accordingly, T cells derived from AMPKKO-T mice contributed only marginally to the repopulation of the gut lymphoid tissues when compared to wild type T cells three months after competitive bone marrow transplantation (Fig. 1A,B and Supplemental Fig. S1A). A reduced reconstitution of the AMPK-KO T cell pool was also observed in the liver of chimeras (Supplemental Fig. S1B). Thus, AMPK is required for T cells to properly exit from naive “progenitor” state and could provide a competitive advantage to effector/memory phenotype T cells in engrafting the niche of non-hematopoietic tissues following adoptive transfer of bone marrow precursors in T cell deficient mice.
AMPK promotes peripheral T cells replenishment upon bone marrow transfer. (A, B) Relative contribution of AMPK-KO CD4+ (A) and CD8+ (B) T cells in the thymus, spleen, MLN, LP and IEL of CD3ε−/− mice reconstituted with a 1:1 mixture of bone marrow cells from WT (CD45.1) and AMPKKO-T (CD45.2) mice. Data are expressed as a ratio of AMPK-KO over WT cells in the CD4+ or CD8+ T cell gate. (C–H) CD62L, CD44 and CXCR3 expression by WT and AMPK-KO T cells recovered from the spleen of mice reconstituted as in (A, B). Representative dot plots (C, E) and histograms showing the mean % ± SD (D, F) in different cell gates as indicated. Data are representative of 3 (A, C, D, G, H) or 2 (B, E, F) independent experiments with n = 6. Statistical analysis: Friedman followed by Dunn multiple comparison (A, B), Mann–Whitney (D, F), Wilcoxon Test (H). *p < 0.05 ; **p < 0.01 ; ***p < 0.001. MLN mesenteric lymph nodes, LP lamina propria, IEL intra-epithelial lymphocytes.
AMPK promotes T cell homeostatic proliferation
We next used a well-established model of homeostatic proliferation to further study the long-term/sustained T cell proliferation of AMPK-KO CD4+ T cells18. CFSE-labeled, congenitally marked, CD44low CD4+ T cells were purified from WT and AMPKKO-T mice and co-transferred into CD3ε−/− lymphopenic recipient mice. Ten days after adoptive transfer, splenic CD4+ T cells expressing high and low CFSE contents, respectively indicative of slow and fast homeostatic proliferation, were recovered in both WT and AMPK-KO subsets. As expected, fast divided cells up-regulated CD44 expression (Supplemental Fig. S2A). Despite starting with similar number of cells, the recovery of donor WT CD4+ T cells was reproducibly greater than that of AMPK-KO cells in each organ tested (Fig. 2A,B). Co-transfer of naive B6.CD45.1 and B6.CD45.2 (both WT genotype) led to similar CD4+ T cell recovery, indicating that CD45 polymorphism does not affect homeostatic proliferation of T lymphocytes (Supplemental Fig. S3A,B). Of interest, subdivision of splenic CD4+ T cell populations based on CFSE content indicated that the relative contribution of AMPK-KO cells to the T cell pool decreased over T cell divisions (Fig. 2C,D; see also Supplemental Fig. S2B for individual mouse representation). The lower percentage of AMPK-KO T cells undergoing fast division rates was further reflected by their lower expression of the Ki67 proliferation marker (Fig. 2E). Wild type fast dividing cells acquired potential for IFNγ secretion as well as cell surface expression of CXCR3 (Fig. 2F,H, upper panels: compare 0–2 and 3 division groups). Both CXCR3 expression and IFNγ secretion were significantly reduced in the AMPK-KO subset whereas comparable IL-2 secretion was observed in WT and KO groups (Fig. 2F–J).
WT CD4+ T cells outcompete their AMPK-KO counterparts during homeostatic proliferation. CFSE labeled CD4+ naive T cells from WT (CD45.1) and AMPKKO-T (CD45.2) mice (1:1 ratio) were i.v. injected into CD3ε−/− mice. Recovered T cells were analyzed on day 10 by flow cytometry. (A, B) Relative contribution of AMPK-KO T cells to the repopulation of the CD4+ T cell subset in the spleen, MLN, LP and IEL (CD4+ gated). Representative dot plots (A) and histograms showing the ratio of AMPK-KO/WT cells (B, gate CD4+ T cells). Data are representative of 3 (LP, IEL), 4 (MLN) or 6 (spleen) independent experiments with n = 6. (C) CFSE dilution versus CD45.1 expression by CD4+ T cells in the spleen. (D) Relative contribution of AMPK-KO T cells in the pool of recovered splenic CD4+ T cells that divided 0–2 or > 3 times, according to CFSE dilution (pool of 4 independent experiments with n = 15). (E) Percentage of WT and AMPK-KO CD4+ T cells that express Ki67 in the spleen (1 experiment representative of 2, with n = 5). (F–H) Percentage of WT and AMPK-KO CD4+ T cells expressing CXCR3 (F) or producing IL-2 and IFNγ (H) according to the number of cell divisions in the spleen. (G–J) Percentage of WT and AMPK-KO CD4+ T cells expressing CXCR3 (G), IL-2 (I) or IFNγ (J) among CD4+ T cells that divided more than 3 times (pool of 2–3 independent experiments, with n = 7–10). Statistical analysis: Friedman followed by Dunn multiple comparison (B), Wilcoxon (D, E, G, I and J). *p < 0.05; **p < 0.01; ***p < 0.001, ****p < 0.0001.
Similar results were obtained when in vitro-derived memory-like Th1 cells were subjected to homeostatic proliferation (see Supplemental Fig. S4A, C–E for details). Moreover undivided WT and AMPK-KO Th1 cells were recovered at similar frequencies upon transfer into lymphoreplete C57BL6 mice, suggesting that AMPK-KO T cells did not experience increased cell death in vivo (Supplemental Fig. S4B).
Graft-versus host disease (GVHD) results from the vigorous proliferation and response of donor T cells to widely distributed alloantigens, leading to T cell mediated pathology19. In this model, we observed that GVHD clinical score and lethalilty were decreased in recipients of AMPK-KO T cells (Supplemental Fig. S5A,B). Decreased percentages of allogeneic CD4+ (but not CD8+ nor Foxp3+Treg cells) were recovered from the spleen of recipient mice transplanted with AMPK-KO T cells (Supplemental Fig. S5C and data not shown). All togheter, these results show that AMPK activation is required to induce T cell homeostatic expansion in vivo as well as T cell pathogenicity and GVHD.
AMPK is dispensable for IL-7 and TCR early signaling but required for optimal T cell expansion in vitro
To further study the role of AMPK in the continuous expansion/survival of CD4+ T cells, we developed an in vitro model of homeostatic T cell proliferation in the presence of autologous dendritic cells and IL-7, based on a recent report of Rosado-Sánchez et al.20. Two weeks in vitro culture of CFSE-labeled naive Th cells with autologous DCs and IL-7, but not DCs or IL-7 alone, led to slow but significant T cell expansion, characterized by increased cell number recovery and the presence of both slow and fast divided cells (Fig. 3A and Supplemental Fig. S6 for details). As previously observed in vivo, only fast dividing cells acquired CD44hi/CD62Llow effector cell marker expression and secrete IFNγ (Supplemental Fig. S6C). Although a 14-day culture in IL-7-containing media already led to a small decrease in AMPK-KO Th cell recovery, the IL-7 + DC-driven proliferation resulted in a further gradual decrease over time (Fig. 3B,C). Accordingly, the KO/WT T cell ratio was further decreased among highly divided (CFSElow) cells (Fig. 3D,E). Similar results have been observed with memory-like Th1 subjected to homeostatic proliferation in vitro (Supplemental Fig. S7). As previously shown in the in vivo model, AMPK-KO Th cells secreted less IFNγ, compared to their wild type counterparts (Fig. 3F).
AMPK promotes homeostatic proliferation in vitro. (A) Naive Th cells from C57BL/6 mice were cultured in the presence of IL-7 and/or syngeneic DCs, as indicated. Culture media were replaced with fresh DCs and IL-7 each 4–5 days. Results indicate the numbers of T cells recovered on day 18, with dashed line corresponding to the number of cells in the input culture (day 0) (data are pooled from 2 independent experiments, n = 3–6). (B) Relative recovery of AMPK-KO T cells after 14 day-culture with IL-7 in the presence or absence of syngenic DC. (C) Relative recovery of AMPK-KO T cells at day 6 and 14 of IL-7 + DC culture. (D) CFSE dilution profiles of WT and AMPK-KO at day 14 of IL-7 + DC culture. (E) Relative contribution of AMPK-KO T cells according to CFSE content. (F) Percentage of WT and AMPK-KO T cells expressing IFNγ at day 14. Data are representative of at least 5 experiments with n = 17 (B) or 8 (C), or are pool of 2 independent experiments with n = 3–6 (A), 13 (E) or 11 (F). Statistical analysis: Mann–Whitney (B), Friedman followed by Dunn multiple comparison (C) Wilcoxon (E–F). *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001.
WT and AMPK-KO naive T cells expressed similar levels of phospho-STAT5 and phospho-STAT1 in response to IL-7 and survived, for at least 14 days, in IL-7-supplemented media (Supplemental Fig. S8 and data not shown), suggesting that defective proliferation does not result from altered IL-7 signaling.
AMPK has been shown to restrain mTOR activity, yet other AMPK-independent pathways of regulation of mTOR have also been described21,22. Similar increase in S6K phosphorylation, a direct mTOR substrate, were observed in WT and KO activated Th cells and IL-7-driven memory Th1 cells (Supplemental Fig. S9). In agreement with normal mTOR activity and with our previous report15, AMPK-KO T cells expressed early T cell activation markers and displayed normal short-term TCR-dependent proliferative response to anti-CD3 or allogeneic APCs (Supplemental Fig. S10A–C). However, co-culture of WT and AMPK-KO T cell in the presence of anti-CD3/CD28 mAbs revealed a small decrease in the AMPK-KO versus WT Th cell recovery. Futhermore WT Th cells largely outcompete their AMPK-KO counterparts when further expanded with IL-2 (Supplemental Fig. S10D). Collectively, these data indicate that AMPK sustains prolonged cytokine-driven T cell proliferation and/or viability.
AMPK regulates mitochondrial fitness in T cells
RNA-seq analysis revealed no difference in the pattern of gene expression between WT and AMPK-KO Th cells recovered at the end of a 14-day culture with DCs and IL-7 (Supplemental Fig. S11), indicating a negligible role of AMPK in the regulation of gene transcription in this experimental setting.
WT and AMPK-KO CD4+ T cells were assessed for mitochondrial bioenergetics. Freshly purified (or rested in IL-7-supplemented media) AMPK-KO naive Th cell expressed a slight reduced mitochondrial membrane potential (Fig. 4A and data not shown).This defect was exacerbated in IL-7 + DC-expanded T cells and in in vitro-derived memory like Th1 (Fig. 4B,C and Supplemental Fig. S12A,B). Although AMPK-KO T cells recovered after 14 days of homeostatic proliferation exhibited decreased mitochondrial membrane potential, this was not associated with changes in oxygen consumption rate (OCR) or extra-cellular acidification rate (ECAR) (Fig. 4D–F). Transmission electron microscopy (TEM) revealed that AMPK-KO T cells did not show difference in mitochondria size and number (Fig. 4G–I). Yet, we noticed an increased percentage of mitochondria harboring localized increased membrane density and membrane budding in AMPK-KO cells (Fig. 4J,K).
AMPK promotes mitochondrial fitness of T cells. (A–C) Mitochondrial membrane potential measured as a ratio of JC1 Red/Green fluorescence ratio in naive T cells (A), homeostatic proliferation-recovered T cells (B) and memory-like Th1 cells (C), with WT ratio set to 1. (D–F) Basal OCR (D), maximal OCR (E) and ECAR (F) of WT and AMPK-KO T cells recovered after 14 days of homeostatic proliferation. (G–I) TEM analysis of WT and AMPK-KO naive T cells. Representative images (G), numbers (H) and areas (I) of mitochondria (a total of 179 (WT) and 170 (KO) mitochondria from 27 (WT) and 29 (AMPK-KO) T cells analyzed are represented). (J, K) Image representative (J) and percentage (K) of mitochondria presenting a budding protrusion (WT : 3 out of 179, AMPK-KO : 16 out 170 mitochondria). Data are representative of 7 (A, B) or 2 (H, I, K) experiments or are pooled from 9 (C) or 2 (D–F, n = 6) independent experiments. Statistical analysis: paired t-test (A–C), Mann–Whitney (D–F, H–I), Chi2 (K). **p < 0.01; ***p < 0.001; ****p < 0.0001.
Turnover of damaged mitochondria is part of the mitochondrial quality control process and it has recently been shown that enhanced mitophagy can slow-down the cell cycle progression23. To measure mitochondria turnover in IL-7-treated WT and AMPK-KO Th cell cultures, we pulsed-chased mitochondria with the Mito-Green Tracker probe and followed the Mito-Green loss over time. We chose to perform these experiments on non-dividing IL-7-treated naive Th cells to avoid interferences between mitochondria turnover (on a per cell basis) and mitochondria dilution over cell divisions. As a control, cells were re-stained with the mitochondrial probe at each timing to check for total mitochondrial content. As shown in Fig. 5A,B, Mito-Green staining gradually decreased over time and AMPK-KO Th cells exhibited increased mitochondrial turnover at each time point.
Accelerated mitochondrial clearance and defective mitochondrial stress-induced glycolytic switch in AMPK-KO T cells. (A) Representative FACS profiles of total mitochondrial mass (left panel) and pulse-chase remaining mitochondrial staining (right panels) in WT and AMPK-KO Th cells after 4, 6 and 9 day in IL-7-supplemented media. (B) Kinetic of mitochondrial mass loss, calculated from gate indicated in (A, right panels). (C, D) Kinetic of ECAR upon oligomycin treatment of WT and AMPK-KO Th1 memory-like cells (C) or IL-7 + DC-expanded T cells (D). Data are expressed as fold increase relative to time point 0. Raw data are also provided in supplemental Figure S13. Data are pooled from 3 independent experiments (n = 9–16, B) or 2 independent experiments (n = 6, D), or are representative of 7 individual experiments (n = 16, C). Statistical analysis: Wilcoxon (B), Mann-Withney (C, D). **p < 0.01; ****p < 0.0001.
Mitochondrial fitness is essential for the rapid induction of glycolysis during re-activation of memory CD8+ T cells24. Interestingly, WT but not AMPK-KO Th cells recovered from long term in vitro cultures showed increased ECAR upon both mitochondria stress and anti-CD3/CD28 re-stimulation (Fig. 5C,D, Supplemental Figs. S12C and S13).
Dampening ROS production partially restores the competitive proliferative capacity of AMPK-KO T cells
Mitochondria are major cellular sources of reactive oxygen species (ROS)25,26. In agreement with a role for AMPK in maintaining redox balance in cancer cells27,28, AMPK-KO homeostatic proliferating T cells and memory-like Th1 cells generated increased amounts of ROS (Fig. 6A,B). Although freshly purified or IL-7 cultured AMPK-KO naive Th cells expressed similar levels of intra-cellular ROS at steady state (Fig. 6C and data not shown), they were highly sensitive to ROS-mediated cell death (Fig. 6D), further indicating that AMPK regulates ROS balance and protects Th cells from ROS-mediated toxicity. WT and AMPK-KO Th cells exhibited similar sensitivity to a pro-apoptotic drug staurosporine (Fig. 6E), confirming the selectivity of AMPK for ROS-driven cell death. Similar results were obtained with memory Th1-like cells (Supplemental Fig. S14).
AMPK dampens ROS production and toxicity in T cells. (A–C) Cellular ROS staining with DHR123 probe in IL-7 + DC-expanded T cells (A), in memory-like Th1 cells (B), and in naive T cells (C). FACS staining of a representative experiments (left panels) and histogram showing MFI fold increase in AMPK-KO cells in comparison to WT cells set to 1 (right panels). (D, E) Percentages of cell viability after treatement of WT and AMPK-KO naive Th cells with H2O2 (0.5 mM for 2 h, D) or with staurosporine (STS, 5 µM for 4 h, E). (F, H) Cellular ROS staining of naive Th cells after 4 days in IL-7-supplemented culture medium with or without 2-ME (F) and supplemented with MitoTEMPO (H). (G, I) Relative recovery of AMPK-KO T cells after 14 days of homeostatic proliferation in vitro in a culture medium with or without 2-ME (G) and with or without MitoTEMPO (I). Data are pooled from 7 (n = 11, A), 9 (n = 8, B), (n = 7, C), 3 (n = 5, D), 4 (n = 4, E), 4 (n = 20, G) or 6 (n = 13, I) independent experiments or are representative of 3 individal experiments (F, H). Statistical analysis: Wilcoxon (A-C), Mann–Whitney (D, E, G, I). *p < 0.05; **p < 0.01; ****p < 0.0001.
Whereas low concentrations of ROS are essential for T cell activation and proliferation, high concentration of ROS affect cell survival, thereby highlighting a major role for anti-oxidative processes in the tight control of intra-cellular ROS equilibrium and cell proliferation29. To test whether increased ROS levels expressed by AMPK-KO Th cells would impede their competitive proliferation, we set up homeostatic proliferation cultures in the absence or presence of antioxidants. In agreement with our working hypothesis, lack of 2-mercaptoethanol (2-ME), a potent reducing agent that prevents toxic levels of oxygen radicals through stabilization of the intracellular cysteine and GSH levels30,31, led to a reduced proliferative capacity and/or survival of AMPK-KO when compared to WT cells (Fig. 6F,G). Accordingly, supplementation of the culture media with the ROS scavenger MitoTEMPO reduced intra-cellular ROS contents and partially restored the proliferative competence/survival of AMPK-KO Th cells (Fig. 6H,I).
Discussion
AMPK plays important roles in cellular energy metabolism and orchestrates cellular antioxidant defenses, thereby acting as a safeguard against prolonged and potently detrimental oxidative damages27. We show herein that AMPK dampens intra-cellular ROS levels in homeostatic proliferating T cells and protects Th cells from ROS-mediated cell mortality, thereby sustaining prolonged proliferation both in vitro and in vivo. Moreover, during the course of GVHD, AMPK expression in alloreactive T cells drives T cell expansion and accelerates the onset of the disease. Because hematopoietic stem cell transplantation is likely associated with both beneficial (Graft versus Tumor) and pathological (Graft versus Host Disease) side effects19,32, understanding the mechanisms regulating the chronic T cell proliferation in response to continuously present alloantigens is of great importance.
Reactive oxygen species are generated as by-products of mitochondrial metabolism and eliminated via antioxidant systems. It is well established that, upon antigenic stimulation, T cells produce mitochondrial ROS that regulate secondary signaling molecules to bring about optimal TCR activation33,34. However, excessive mitochondrial ROS production or defective ROS neutralization by antioxidant cellular systems can also lead to T cell death, and antioxidant reagents can improve the survival of activated T cells35,36,37,38. Cellular ROS levels increase with enhanced mitochondrial metabolic activity but are also produced under conditions of metabolic stress, such as hypoxia39,40.
In agreement with the well-known role of AMPK in regulating mitochondrial fitness41, we observed that AMPK-deficient T cells displayed mitochondrial depolarization and accumulated mitochondrial membrane damages. Although AMPK has been reported to promote mitochondrial biogenesis through activation of the transcription factor PGC1 in distinct cell types41, AMPK-KO T cells displayed similar mitochondrial mass and pgc1 expression, indicating that the mitochondrial defects observed in this experimental model occurred independently of total mitochondrial cell content. In vitro-expanded AMPK-KO T cells exhibit a similar mitochondrial respiration rate, compared to their wild type counterparts. This contrasts with data showing that cytotoxic T effector cells isolated from AMPK-deficient mice infected with Listeria monocytogenes displayed reduced mitochondrial respiratory rates relative to control cells17. Intriguingly, maximal OCR only slightly increased above basal OCR (low spare respiratory capacity, SRC), indicating that Th cell operate at their maximal oxidative rate in our proliferation settings. Use of SRC by naive T cells to energetically support in vivo spontaneous homeostatic proliferation has been recently reported42. Utilization of SRC might thus increase oxygen consumption to maximal levels independently of mitochondrial potential. A sustained maximal respiration rate combined with defects in mitochondria membranes could therefore lead to increased ROS production in AMPK-KO proliferating T cells.
Freshly purified AMPK-KO naive T cells exhibited reduced mitochondrial potential without sign of excessive cellular ROS concentration, indicating that a mitochondrial defect preceded ROS production during proliferation of AMPK-deficient T lymphocytes. Alternatively, a defect in ROS scavenging mechanisms could also contribute to the gradual accumulation of ROS in proliferating AMPK-KO T cells overtime. In this view, the lower proliferation rate/survival capacity of AMPK-KO T cells could result from reduced activity of the pentose phosphate pathway and/or other cytosolic pathways that, together with mitochondrial enzymes, supply the cells with the NADPH reducing power43. Although the mRNA encoding these enzymes (glucose 6-phosphate dehydrogenase, isocitrate dehydrogenase 1,…) were expressed at similar levels between WT and KO cells, further experiments would be required to test their activities and to measure the NADPH/NADP+ and GSH/GSSG contents in homeostatic-expanded T cells.
Although in vitro homeostatic proliferating and IL-7-driven memory-like AMPK-KO Th cells accumulated ROS, we did not detect elevated ROS levels in the remaining KO T cells recovered during homeostatic proliferation in vivo (data not shown). As AMPK-KO T cells are highly sensitive to ROS-mediated cell death, it is conceivable that cells expressing higher ROS levels would be prone to cell dead and, consequently, be masked by the dynamics of apoptotic cell clearance in vivo44. Moreover, such dying cells could also be lost during organ dissociation and the centrifugation steps necessary for cell recovery and cytometry staining.
One of the most striking observations of this study is the inability of homeostatic-expanded AMPK-deficient Th cells to shift towards a glycolytic metabolism in response to a mitochondrial poison or TCR signaling. To date, the role of AMPK in the control of the Warburg effect has been controversial. Inactivation of AMPKα in both transformed and non-transformed cells promoted a HIF-1α-dependent reprogramming of glucose metabolism characteristic of the Warburg effect45. In contrast, loss of the tumor suppressor folliculin (FLCN) gene, a negative regulator of AMPK, promoted constitutive AMPK activation in cancer cells, leading to increased mitochondrial biogenesis and a ROS-induced HIF transcriptional activity, thereby coupling AMPK-dependent mitochondrial biogenesis to HIF-dependent Warburg metabolic reprogramming46. Moreover, other reports identified AMPK as a major driver of glycolysis either through direct phosphorylation of PFKFB347,48 or indirectly, through mitochondrial ATP-driven hexokinase activation24.
Our study thus posits AMPK as a driver of the Warburg effect in T lymphocytes. Whether the inability of AMPK-deficient T cells to switch back to glycolysis account for their defective proliferation awaits further experimentations.
In the absence of AMPK, homeostatic proliferating Th cells secreted reduced amount of IFNγ, compared to their wild type counterparts. This contrast with data showing that AMPK dampened IFNγ production in CD8+ T cells activated in vitro, in particular when cells are exposed to low glucose concentration17. This discrepancy could be easily explained by the differences in the experimental models between the studies. Reduced IFNγ production did not reflect a general defect in cytokine secretion as Th cells of both genotypes produced similar amounts of IL-2. During homeostatic proliferation, IFNγ was secreted by fast dividing cells. It is therefore possible that higher IFNγ secretion in the wild type cell compartment simply reflected the presence of cells that underwent more division in this group. Although we excluded slow-divided cells (0–3 cell divisions, based on CFSE contents) from the analysis, we could not test this hypothesis further as CFSE dilutions did not allow us to discriminate cells undergoing more divisions. Decreased IFNγ secretion by AMPK-KO T cells could also result from their defective glycolytic switch as IFNγ production was intimately connected with a glycolytic metabolism24,49,50.
To date, the role of AMPK in T cell activation has been controversial. T cells purified from AMPKα1-deficient mice displayed normal responses to mitogenic stimulations in vitro15,51, while impaired central memory T cell generation was reported in T cell-specific AMPKα1-deficient mice16. Of interest, in the latter study, the disability to generate central memory AMPK-deficient T cells was revealed upon co-transfer of a mixture of WT and KO T cells in immunized recipient mice. All together, these data are best explained by assuming that AMPK might be dispensable for immune responses as long as cells do not experience metabolic stress but could confer a proliferative/survival advantage to effector (and central memory) cells in situations of metabolic crisis and/or nutriment competition.
The observation that AMPK deficiency did not preclude Th cell proliferation in response to a strong mitogenic anti-CD3/CD28 signaling confirms and extends previous data showing that AMPK is dispensable for a short term effector cell differentiation15,16. A strong Akt/mTOR-driven glycolytic program might thus compensate for AMPK deficiency and sustain a rapid proliferative response52. However, anti-CD3/CD28 activated AMPK-KO T cells exhibited defective proliferation when expanded with IL-2 for longer periods. Taken together, our data thus indicate that the homeostatic proliferative defect of AMPK-KO T cells does not reflect a specific defective response to homeostatic signals but rather results from the progressive accumulation of uncontrolled ROS production, leading to cell damages and premature senescence. In support of this assumption, we observed that antioxidant treatments partially alleviated the competitive proliferative/survival defect of AMPK-KO T cells.
The higher mitochondria turnover in AMPK-KO T cells seems at odds with the conventional role of AMPK in promoting mitochondrial fission and mitochondria renewal41. However, fusion with healthy surrounding mitochondria helps mitigate stress by mixing the contents of partially damaged mitochondria as a form of complementation, without requiring a complete recycling53. Although the role of AMPK in the resistance to this “minor mitochondrial crisis” is still ill described, AMPK has been shown to prevent mitochondrial fission in adipose tissue, through enhanced phosphorylation of Drp1on the inhibitory serine 63754. In this context, an accelerated mitochondrial component turnover could thus reflect an increased need for recycling damaged mitochondria in AMPK-KO T cells, although this hypothesis still warrants further investigations.
AMPK was recently shown to down-regulate PD-1 expression, thereby promoting memory-like activities of CAR-T cells in vivo55. Of interest, PD-1 mediated regulation of T cell function through repressed mitochondrial metabolism and production of ROS, potentially leading to cell death of activated T cells56,57. A fine tuning of the AMPK mediated control over cell metabolism and ROS equilibrium would thus open new avenues for medical interventions in order to prevent GVHD or to sustain CAR-T cell homeostatic expansion in vivo.
Materials and methods
Mouse model
AMPKα1fl/fl mice were kindly provided by Dr. B. Viollet (Institut Cochin, Paris—France) and bred to CD4-Cre transgenic mice, obtained from Dr. G. Van Loo (University of Gand—Belgium). C57BL/6 and Balb/c mice were purchased from Envigo (Horst—Nederland). CD45.1 were purchased from Jackson Laboratory (Bar Harbor—USA) and CD3ε−/− were obtained from EMMA (Orléans—France). Control mice were littermate AMPKα1fl/fl Cre- or AMPKα1+/fl Cre- or sex- and age-matched C57BL/6 or CD45.1 mice. Mice were housed under specific pathogen-free (SPF) conditions. The experiments were performed in compliance with the relevant laws and institutional guidelines and were approved by the Local Ethic Committee. We received specific approval for this study from the Université Libre de Bruxelles Institutional Animal Care and Use Committee (protocol numbers CEBEA-31 and 91).
Cell purifications
Bone marrow cells from tibia and femur were purified from naive mice by flushing. When required (GVHD), T cells were magnetically depleted. Briefly, bone marrow cells were incubated 20 min at 4 °C with anti-CD4 (L3T4)-, anti-CD8α (Ly-2)- and anti-CD90.2-MicroBeads (Miltenyi Biotec, Bergisch-Gladbach, Germany) and then separated over an auto-MACS column using the DepleteS program.
Total T cells (CD4+ and CD8+) were purified from the spleen of naive mice by Stemcell negative selection, according to manufacturer’s recommendations. Briefly, spleen cells were incubated 30 min at 4 °C with 0.5 µg/ml of anti-Ter119-biot (Ly-76), anti-CD19-biot (MB19-1), anti-Gr1-biot (RB6-8C5), anti-CD49b-biot (DX5), anti-CD11c-biot (N418) and anti-MHCII (I-A/I-E)-biot (M5/114) antibodies. Antibodies are from BD Biosciences or BioXCell. Cells were then incubated with Biotin selection cocktail 2 (Stemcell) for 17 min at 4 °C. D2 Magnetic Particles (Stemcell) were added and cells were incubated for an additional 7 min at 4 °C before placing them in the Stemcell magnet.
Naive T cells (CD4+ CD44-) were purified from the spleen of naive mice by MACS column enrichment followed by cells sorting. Spleen cells were incubated at 4 °C with anti-CD4 (L3T4)-MicroBeads (Miltenyi Biotec) after 10 min CD4 (RM4-5)-PB, CD44 (IM7)-PE-Cy7, CD62L (MEL-14)-A700 and CD25 (PC61)-BB515 were added for an additional 15 min. These antibodies are all from BD. The cells were first separated over an LS-column (Miltenyi Biotec) and the CD4+ fraction was then sorted as CD4+ CD25- CD62L+ CD44- by FACS ARIA III (BD Biosciences).
For dendritic cell purification, spleens were treated with collagenase (Gestimed). Recovered splenocytes were resuspended in Nicodenz (DO360 = 0.666, Lucron Bioproducts). RPMI-medium with 3% FCS was added on the top of Nicodenz. After 15 min centrifugation at 4 °C and 1700G without brake, DC fraction were recovered from the gradient interface and incubated with CD11c-MicroBeads (Miltenyi Biotec) for 15 min at 4 °C. The excess of beads was washed out and cells were then separated over an LS-column (Miltenyi Biotec).
Bone marrow transfer
Bone marrow cells purified from WT (CD45.1) and AMPKKO-T (CD45.2) mice were mixed at ratio 1:1 and i.v. injected (4 to 5 × 106 cells in total) into 1200 cGy (split dose) irradiated CD3ε-/- mice. 8 to 12 weeks later, WT and AMPK-KO T cells from different organs were analyzed by flow cytometry.
In vivo homeostatic proliferation
Naive (CD4+ CD44−) cells obtained from WT (CD45.1) and WT or AMPKKO-T (CD45.2) mice were mixed at ratio 1:1 and i.v. injected (1–2 × 106 cells in total) into CD3ε−/− mice. 10 to 12 days later, WT and AMPK-KO T cells from different organs are analyzed by flow cytometry. In some experiments cells were CFSE-labelled (Invitrogen) before i.v. injection.
GVHD
Irradiated Balb/c mice (750 cGy, day − 1) were i.v. injected with 5 × 106 C57BL/6T cell depleted bone marrow with or without 106 total T cells from either WT or AMPKKO-T mice (day 0). Clinical score was followed three times a week and calculated as previously described by Cooke et al.58. 7 to 10 days later, WT and AMPK-KO T cells from different organs are analyzed by flow cytometry.
Isolation of intestinal lymphocytes
Intestines were collected and cleared from residual connective tissue, Peyer’s patches and feces. Intestines were then cut into small pieces and incubated for 30 min at 37 °C with agitation in a medium containing EDTA (2.5 mM, VWR) and DTT (72.5 µg/ml, Sigma-Aldrich). IEL were recovered by washing and shaking the intestines 3 times in a medium with EDTA and one time without EDTA. To obtain LP lymphocytes, intestines were further chopped and incubated for 30 min at 37 °C in medium containing liberase (20 µg/ml, Roche) and DNAse (400 µg/ml, Roche). Enzymes were neutralized with EDTA (2.5 mM) and the intestines were crushed and filtered. IEL and LP fractions were finally washed and submitted to 15 min centrifugation at 500G, 20 °C without break in a 30% Percoll (GE Healthcare) solution. Lymphocytes are in the pellet.
In vitro differentiation of memory-like Th cells
Naive T cells (CD4+ CD44−) were polarized into Th1 cells for 3 days using plate-bound anti-CD3 (5 µg/ml, clone 145-2C11, BioXCell), anti-CD28 (1 µg/ml, clone 37.51, BioXCell), recombinant IL-12 (10 ng/ml, Peprotech) and anti-IL-4 (10 µg/ml, BioXCell). Th1 cells were then cultured, for 4 to 7 additional days, into IL-7-supplemented (20 ng/ml, Peprotech) media to obtain memory-like cells.
In vitro homeostatic proliferation
Naive T cells (CD4+ CD44−) purified from WT (CD45.1) and AMPKKO-T (CD45.2) mice were mixed at a 1:1 ratio, CFSE-labelled and seeded in a 96-well plate (105 cell/well). T cells were cultured at 37 °C in RPMI supplemented with 5% heat-inactivated FBS (Sigma-Aldrich), 1% non-essential amino acids (Invitrogen), 1 mM sodium pyruvate (Invitrogen), 2 mM l-glutamin (Invitrogen), 500 U/ml penicillin/500 μg/ml streptomycin (Invitrogen), and 50 μM β-mercaptoethanol (Sigma-Aldrich). The homeostatic stimuli were provided by 2 × 104 DC purified from autologous C57BL/6 (H-2b) mice and IL-7 (20 ng/ml, Peprotech). Each 4–5 days of culture, half of the medium volume was refreshed with new IL-7-supplemented medium and freshly purified DC. Cells were analyzed on day 13–15. For antioxidant supplementation, MitoTEMPO (20 µM, Sigma) was added to culture medium and refreshed at the same time than IL-7.
Memory-like Th1 from WT (CD45.1) and AMPKKO-T (CD45.2) mice were mixed at ratio 1.1 and were cultured with 2 × 104 syngeneic DC and IL-7 (20 ng/ml, Peprotech) for 9 additional days. Half of the medium volume was refreshed with new IL-7-supplemented medium and freshly purified DC at day 5.
Flow cytometry
Specific cell-surface staining was performed using standard procedure with the following monoclonal antibodies conjugated to fluorochrome or biotin: CD4 (RM4-5), CD44 (IM7), CD45.1 (A20) from BD Bioscience, and CD8α (53-6.7), CD183 (CXCR3-173), CD62L (MEL-14), TCRβ (H57-597) from eBioscience. Strepatividin was purchased from BD Bioscience. Dead cells were excluded by LIVE/DEAD (Invitrogen) staining and non-specific, Fc-mediated interactions were blocked by anti-Mouse CD16/CD32 (2.4G2 BioXCell). For intracellular staining, cells were incubated with a fixing solution (Fixation/Permeabilization, eBioscience) and stained with directly conjugated monoclonal antibodies anti-IFNγ (XMG1.2), IL-2 (JES6-5H4), Ki67 (B56) from BD-Bioscience, and FoxP3 (FJK-16 s) from eBioscience. For cytokine production assessment, cells were pre-incubated for 4 h with PMA 50 ng/ml (Sigma), ionomycin 1 µg/ml (Sigma) and brefeldin A (eBiosciences).
For mitochondrial parameters analysis, cells were incubated 25 min at 37 °C with the Mito-Green dye (100 nM, Invitrogen), TMRE (5 nM, Invitrogen) or JC-1 probe (2 µM, Invitrogen) in complete medium.
For the mitochondria renewal assay, naive T cells were stained with Mito-Green dye (2 µM, Invitrogen) for 20 min at 37 °C (106 cells per 100 µL). Then the loss of this staining was recorded by flow cytometry at day 4, 6 and 9. At each time point, fresh staining of Mito-Green dye was performed to measure total mitochondria content on unstained cells (100 nM).
For ROS detection, cells were incubated 30 min at 37 °C with Dihydrorhodamine (DHR) 123 (2 µM, Sigma) in complete medium.
Cells were analyzed by FACS Canto II or ARIA III (BD Bioscience) and data were analyzed with the FlowJo Software (Tree Star).
Seahorse
OCR and ECAR were determined using a Seahorse XFp or XF96 Extracellular Flux Analyser following user guide. Plate were pre-coated with poly-d-lysine (200 µg/ml, Millipore) for cell adhesion. 1 mM pyruvate, 2 mM de glutamine and 10 mM glucose were added to XF Base Medium and pH was adjusted to 7.4. Drugs were added to reach a final concentration of: oligomycin 1 µM, FCCP 0.5 µM, rotenone/antimycin A 0.5 µM and 2-DG 50 mM. For CD3/CD28 beads (Dynabeads Mouse T-Activator CD3/CD28, LifeTechnologies) injection, beads:cells ratio was 4:1. Seahorse protocol was adjusted with the following settings: mix 2 min, wait 2 min and measure 4-5 min. At the end of the Seahorse analysis, cells were lysed and Bradford (Biorad) protein dosage was performed.
ROS/staurosporine-sensitivity
2–5 × 105 cells were incubated for 2 h with different concentrations of H2O2 (Merck) or for 2, 4 or 6 h with staurosporine (5 µg/ml, Sigma) at 37 °C. The percentage of survival was measured after Propidium iodide staining (Sigma). To avoid loss of dying cells during the cell recovery procedure, propidium iodide was added directly into the culture wells at the end of treatment. Cells were then harvested and analysed without centrifugation and washing.
Transmission electron microscopy (TEM)
A total of 27 (WT) and 29 (AMPK-KO) T cells were analyzed by transmission electron microscopy. Naive T cells were maintained 4 days in IL-7-supplemented medium and then fixed overnight in 2.5% glutaraldehyde (Electron Microscopy Sciences, UK) at 4 °C and post-fixed with 1% osmium tetroxide (Electron Microscopy Sciences, UK) and 1.5% ferrocyanide (Electron Microscopy Sciences, UK) in 0.15 M cacodylate buffer. The cells were further stained with 1% uranyl acetate (Electron Microscopy Sciences, UK), serially dehydrated and embedded in epoxy resin (Agar 100 resin; Agar Scientific, UK). Ultrathin 70-nm sections were produced with a Leica EM UC6 ultramicrotome and mounted on copper–Formvar–carbon grids (Electron Microscopy Sciences). Observations were made on a Tecnai 10 electron microscope (FEI, Eindhoven, Netherlands), and images were captured with a Veleta camera and processed with SIS iTEM sofware (Olympus, Germany).
Statistical analysis
Statistical analysis were performed using Prism6 (GraphPad Software, San Diego, CA). Datasets were first tested for Gaussian distribution with the D’Agostino & Pearson omnibus normality test. Statistical analysis performed are detailed for each graph in the figure legend. n.s.: non significant; *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001.
References
Chang, C.-H. & Pearce, E. L. Emerging concepts of T cell metabolism as a target of immunotherapy. Nat. Immunol. 17, 364 (2016).
Vander Heiden, M. G., Cantley, L. C. & Thompson, C. B. Understanding the warburg effect: the metabolic requirements of cell proliferation. Science 324, 1029–1033 (2009).
Pearce, E. L. et al. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature 460, 103–107 (2009).
Hardie, D. G., Ross, F. A. & Hawley, S. A. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat. Rev. Mol. Cell Biol. 13, 251–262 (2012).
Andris, F. & Leo, O. AMPK in lymphocyte metabolism and function. Int. Rev. Immunol. 1, 67–81 (2014).
Ke, R., Xu, Q., Li, C., Luo, L. & Huang, D. Mechanisms of AMPK in the maintenance of ATP balance during energy metabolism. Cell Biol. Int. 42, 384–392 (2017).
Inoki, K., Zhu, T. & Guan, K.-L. TSC2 mediates cellular energy response to control cell growth and survival. Cell 115, 577–590 (2003).
Hardie, D. G. & Pan, D. A. Regulation of fatty acid synthesis and oxidation by the AMP-activated protein kinase. Biochem. Soc. Trans. 30, 1064–1070 (2002).
Jäger, S., Handschin, C., St. Pierre, J. & Spiegelman, B. M. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1α. Proc. Natl. Acad. Sci. 104, 12017–12022 (2007).
Fullerton, M. D. et al. Single phosphorylation sites in Acc1 and Acc2 regulate lipid homeostasis and the insulin-sensitizing effects of metformin. Nat. Med. 19, 1649 (2013).
Marsin, A.-S., Bouzin, C., Bertrand, L. & Hue, L. The stimulation of glycolysis by hypoxia in activated monocytes is mediated by AMP-activated protein kinase and inducible 6-phosphofructo-2-kinase. J. Biol. Chem. 277, 30778–30783 (2002).
Fryer, L. G. D. et al. Characterization of the role of the AMP-activated protein kinase in the stimulation of glucose transport in skeletal muscle cells. Biochem. J. 363, 167–174 (2002).
Rabinovitch, R. C. et al. AMPK maintains cellular metabolic homeostasis through regulation of mitochondrial reactive oxygen species. Cell Rep. 21, 1–9 (2017).
Tamás, P. et al. Regulation of the energy sensor AMP-activated protein kinase by antigen receptor and Ca2+ in T lymphocytes. J. Exp. Med. 203, 1665–1670 (2006).
Mayer, A., Denanglaire, S., Viollet, B., Leo, O. & Andris, F. AMP-activated protein kinase regulates lymphocyte responses to metabolic stress but is largely dispensable for immune cell development and function. Eur. J. Immunol. 38, 948–956 (2008).
Rolf, J. et al. AMPKα1: a glucose sensor that controls CD8 T-cell memory. Eur. J. Immunol. 43, 889–896 (2013).
Blagih, J. et al. The energy sensor AMPK regulates T cell metabolic adaptation and effector responses invivo. Immunity 42, 41–54 (2015).
Boyman, O., Krieg, C., Homann, D. & Sprent, J. Homeostatic maintenance of T cells and natural killer cells. Cell. Mol. Life Sci. 69, 1597–1608 (2012).
Pidala, J. Graft-vs-host disease following allogeneic hematopoietic cell transplantation. Cancer Control 18, 268–276 (2011).
Rosado-Sánchez, I. et al. An in vitro system of autologous lymphocytes culture that allows the study of homeostatic proliferation mechanisms in human naive CD4 T-cells. Lab. Investig. 98, 500–511 (2018).
Yang, K., Neale, G., Green, D. R., He, W. & Chi, H. The tumor suppressor Tsc1 enforces quiescence of naive T cells to promote immune homeostasis and function. Nat. Immunol. 12, 888–897 (2011).
Efeyan, A. et al. Regulation of mTORC1 by the Rag GTPases is necessary for neonatal autophagy and survival. Nature 493, 679–683 (2013).
Sarraf, S. A. et al. PINK1/parkin influences cell cycle by sequestering TBK1 at damaged mitochondria, inhibiting mitosis. Cell Rep. 29, 225-235.e5 (2019).
van der Windt, G. J. W. et al. CD8 memory T cells have a bioenergetic advantage that underlies their rapid recall ability. Proc. Natl. Acad. Sci. USA. 110, 14336–14341 (2013).
Turrens, J. F. Mitochondrial formation of reactive oxygen species. J. Physiol. 552, 335–344 (2003).
Sena, L. A. et al. Mitochondria are required for antigen-specific T cell activation through reactive oxygen species signaling. Immunity 38, 225–236 (2013).
Zhao, Y. et al. ROS signaling under metabolic stress: cross-talk between AMPK and AKT pathway. Mol. Cancer 16, 79 (2017).
Jeon, S.-M., Chandel, N. S. & Hay, N. AMPK regulates NADPH homeostasis to promote tumour cell survival during energy stress. Nature 485, 661–665 (2012).
Mak, T. W. et al. Glutathione primes T cell metabolism for inflammation. Immunity 46, 675–689 (2017).
Ishii, T., Bannai, S. & Sugita, Y. Mechanism of growth stimulation of L1210 cells by 2-mercaptoethanol in vitro. Role of the mixed disulfide of 2-mercaptoethanol and cysteine. J. Biol. Chem. 256, 12387–12392 (1981).
Ishii, T. & Mann, G. E. Redox status in mammalian cells and stem cells during culture in vitro: critical roles of Nrf2 and cystine transporter activity in the maintenance of redox balance. Redox Biol. 2, 786–794 (2014).
Dummer, W. et al. T cell homeostatic proliferation elicits effective antitumor autoimmunity. J. Clin. Invest. 110, 185–192 (2002).
Devadas, S., Zaritskaya, L., Rhee, S. G., Oberley, L. & Williams, M. S. Discrete generation of superoxide and hydrogen peroxide by T cell receptor stimulation: selective regulation of mitogen-activated protein kinase activation and fas ligand expression. J. Exp. Med. 195, 59–70 (2002).
Kamiński, M. M. et al. Mitochondrial reactive oxygen species control T cell activation by regulating IL-2 and IL-4 expression: mechanism of ciprofloxacin-mediated immunosuppression. J. Immunol. 184, 4827–4841 (2010).
Dumont, A. et al. Hydrogen peroxide-induced apoptosis is CD95-independent, requires the release of mitochondria-derived reactive oxygen species and the activation of NF-κB. Oncogene 18, 747–757 (1999).
Cossarizza, A. et al. Mitochondria alterations and dramatic tendency to undergo apoptosis in peripheral blood lymphocytes during acute HIV syndrome. AIDS 11, 19–26 (1997).
Gelderman, K. A., Hultqvist, M., Holmberg, J., Olofsson, P. & Holmdahl, R. T cell surface redox levels determine T cell reactivity and arthritis susceptibility. Proc. Natl. Acad. Sci. USA 103, 12831–12836 (2006).
Belikov, A. V., Schraven, B. & Simeoni, L. T cells and reactive oxygen species. J. Biomed. Sci. 22, 85 (2015).
Valko, M. et al. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 39, 44–84 (2007).
Wellen, K. E. & Thompson, C. B. Cellular metabolic stress: considering how cells respond to nutrient excess. Mol. Cell 40, 323–332 (2010).
Herzig, S. & Shaw, R. J. AMPK: guardian of metabolism and mitochondrial homeostasis. Nat. Rev. Mol. Cell Biol. 19, 121–135 (2018).
Previte, D. M. et al. Lymphocyte activation gene-3 maintains mitochondrial and metabolic quiescence in naive CD4+ T cells. Cell Rep. 27, 129-141.e4 (2019).
Nóbrega-Pereira, S. et al. G6PD protects from oxidative damage and improves healthspan in mice. Nat. Commun. 7, 10894 (2016).
Elliott, M. R. & Ravichandran, K. S. The dynamics of apoptotic cell clearance. Dev. Cell 38, 147–160 (2016).
Faubert, B. et al. AMPK is a negative regulator of the Warburg effect and suppresses tumor growth in vivo. Cell Metab. 17, 113–124 (2013).
Yan, M. et al. The tumor suppressor folliculin regulates AMPK-dependent metabolic transformation. J. Clin. Invest. 124, 2640–2650 (2014).
Marsin, A. S. et al. Phosphorylation and activation of heart PFK-2 by AMPK has a role in the stimulation of glycolysis during ischaemia. Curr. Biol. 10, 1247–1255 (2000).
Hart, P. C. et al. MnSOD upregulation sustains the Warburg effect via mitochondrial ROS and AMPK-dependent signalling in cancer. Nat. Commun. 6, 6053 (2015).
Gubser, P. M. et al. Rapid effector function of memory CD8+T cells requires an immediate-early glycolytic switch. Nat. Immunol. 14, 1064–1072 (2013).
Bantug, G. R., Galluzzi, L., Kroemer, G. & Hess, C. The spectrum of T cell metabolism in health and disease. Nat. Rev. Immunol. 18, 19–34 (2018).
MacIver, N. J. et al. The liver kinase B1 is a central regulator of T cell development, activation, and metabolism. J. Immunol. 187, 4187–4198 (2011).
Adams, W. C. et al. Anabolism-associated mitochondrial stasis driving lymphocyte differentiation over self-renewal. Cell Rep. 17, 3142–3152 (2016).
Youle, R. J. & van der Bliek, A. M. Mitochondrial fission, fusion, and stress. Science 337, 1062–1065 (2012).
Li, A. et al. Metformin and resveratrol inhibit Drp1-mediated mitochondrial fission and prevent ER stress-associated NLRP3 inflammasome activation in the adipose tissue of diabetic mice. Mol. Cell. Endocrinol. 434, 36–47 (2016).
Zhang, Z. et al. Metformin enhances the antitumor activity of CD8 and gd T lymphocytes via the AMPK–miR-107–eomes–PD-1 pathway. J. Immunol. https://doi.org/10.4049/jimmunol.1901213 (2020).
Bengsch, B. et al. Bioenergetic insufficiencies due to metabolic alterations regulated by the inhibitory receptor PD-1 are an early driver of CD8(+) T cell exhaustion. Immunity 45, 358–373 (2016).
Tkachev, V. et al. Programmed death-1 controls T cell survival by regulating oxidative metabolism. J. Immunol. 194, 5789–5800 (2015).
Cooke, K. R. et al. An experimental model of idiopathic pneumonia syndrome after bone marrow transplantation: I. The roles of minor H antigens and endotoxin. Blood 88, 3230–3239 (1996).
Acknowledgements
We especially thank Muriel Moser for her great support all along this work, for stimulating discussions and for careful revision of the manuscript. We also thank Caroline Abdelaziz and Véronique Dissy for animal care and for technical support. This work was supported by the European Regional Development Fund (ERDF) and the Walloon Region (Wallonia-Biomed portfolio, 411132-957270), grant from the Fonds Jean Brachet and research credit from the National Fund for Scientific Research, FNRS, Belgium. The CMMI is supported by the European Regional Development Fund and the Walloon Region. F.A. is a Research Associate at the FNRS. T.P. is recipient of a research fellowship from the FNRS/Télévie. A.L. is supported by a Belgian FRIA fellowship.
Author information
Authors and Affiliations
Contributions
F.A. conceived and supervised the research program and experiments, and wrote the manuscript. A.L. and T.P. performed the experiments, acquired, and analyzed data. S.D., D.P.M. and M.V. performed experiments and contributed to the analysis of the data. O.L. conceived the research program, provided financial support and revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lepez, A., Pirnay, T., Denanglaire, S. et al. Long-term T cell fitness and proliferation is driven by AMPK-dependent regulation of reactive oxygen species. Sci Rep 10, 21673 (2020). https://doi.org/10.1038/s41598-020-78715-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-78715-2
This article is cited by
-
Inhibition of choline metabolism in an angioimmunoblastic T-cell lymphoma preclinical model reveals a new metabolic vulnerability as possible target for treatment
Journal of Experimental & Clinical Cancer Research (2024)
-
Metabolic Reprogramming of Anti-cancer T Cells: Targeting AMPK and PPAR to Optimize Cancer Immunotherapy
Indian Journal of Clinical Biochemistry (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.