Abstract
The current study aimed to evaluate the efficacy of linked color imaging (LCI) in improving the visibility of superficial non-ampullary duodenal epithelial tumors (SNADETs). We prospectively evaluated 44 consecutive patients diagnosed with SNADETs. Three trainees and three experts assessed the visibility scores of white light imaging (WLI), LCI, and blue laser imaging-bright (BLI-b) for SNADETs, which ranged from 1 (not detectable without repeated cautious examination) to 4 (excellent visibility). In addition, the L* a* b* color values and color differences (ΔE*) were evaluated using the CIELAB color space system. For SNADETs, the visibility scores of LCI (3.53 ± 0.59) were significantly higher than those of WLI and BLI-b (2.66 ± 0.79 and 3.41 ± 0.64, respectively). The color differences (ΔE*) between SNADETs and the adjacent normal duodenal mucosa in LCI mode (19.09 ± 8.33) were significantly higher than those in WLI and BLI-b modes (8.67 ± 4.81 and 12.92 ± 7.95, respectively). In addition, the visibility score of SNADETs and the color differences in LCI mode were significantly higher than those in WLI and BLI-b modes regardless of the presence of milk white mucosa (MWM). LCI has potential benefits, and it is considered a promising clinical modality that can increase the visibility of SNADETs regardless of the presence of MWM.
This study was registered at the University Hospital Medical Information Network (UMIN000028840).
Similar content being viewed by others
Introduction
Superficial non-ampullary duodenal epithelial tumors (SNADETs) are a rare type of lesion1. However, the number of SNADET cases has been gradually increasing owing to the advancements in endoscopic technology2. Spontaneous duodenal adenomas are precancerous lesions with a high rate of malignant transformation3,4,5,6. Moreover, duodenal tumors are the second leading cause of death in patients with familial adenomatous polyposis7. If SNADETs progress to advanced-stage cancer, pancreaticoduodenectomy (PD) is the standard treatment. PD is a relatively invasive treatment, with a mortality rate ranging from 1 to 4%8,9. Therefore, the early diagnosis of SNADETs is important to facilitate less invasive treatment procedures, such as cold snare polypectomy10, underwater endoscopic mucosal resection11, and endoscopic submucosal dissection12.
In recent years, the Linked color imaging (LCI) system, which is a new endoscopic imaging modality, has been developed13. LCI uses band laser with a wavelength of 410 ± 10 nm in addition to white-light laser. Therefore, this technique can emphasize vascular and surface structures and color differences while maintaining a bright vision13. Previous studies have reported the efficacy of LCI without magnifying endoscopy in the diagnosis of active Helicobacter pylori infection14, gastric cancer15,16,17, colon cancer18, and short-segment barrett’s esophagus19 in clinical practice.
However, the use of LCI to improve the visibility of lesions has not been validated to date. Thus, the current study aimed to investigate whether LCI can enhance the visibility of SNADETs.
Methods
Study design and participants
This prospective study was conducted at Chiba University Hospital (Japan) between September 2017 and June 2019. During this period, consecutive patients diagnosed with SNADETs were prospectively enrolled in this study. The characteristics of the lesions, such as size (mm), location in the duodenum, macroscopic findings, histopathological diagnosis according to the Vienna classification20, and presence of milk white mucosa (MWM)21,22, resected treatment, were assessed. In this study, macroscopic findings were assessed according to the Paris endoscopic classification23. Depressed lesions were defined as IIa + IIc or IIc. This study was reviewed and approved by the institutional review board of Chiba University School of Medicine and was registered at the University Hospital Medical Information Network (UMIN000028840). All methods were performed in accordance with the relevant guidelines and regulations. Informed consent was obtained from all subjects.
Esophagogastroduodenoscopy
Esophagogastroduodenoscopy was conducted using the LASEREO system (FUJIFILM, Tokyo, Japan) with an EG-L600WR7 or EG-L600ZW7 endoscope. To investigate the efficacy of LCI in increasing the visibility of SNADETs, we prospectively collected one image acquired using white light imaging (WLI), LCI, and blue laser imaging-bright (BLI-bright) at the same site and angle. SNADETs were resected via endoscopy and were pathologically confirmed thereafter. Both endoscopic resection and pathological examination were performed in our institution.
Visibility score of the three modalities for SNADETs
In total, 44 images were prepared for each modality and were presented to six endoscopists for interpretation. The six endoscopists included three experts and three trainees. The six endoscopists were independent of those who performed esophagogastroduodenoscopy. In this study, an expert was defined as an endoscopist with > 5 years of experience in using image-enhanced endoscopy and a trainee as an endoscopist with < 1 year of experience. The images were presented randomly to each endoscopist on a black background with a similar size. The visibility score of the three modalities for SNADETs were evaluated using previously reported visibility scoring systems with the following scores: 4, excellent visibility (easily detectable); 3, good visibility (detectable with cautious observation); 2, fair visibility (hardly detectable without cautious examination); and 1, poor visibility (not detectable without repeated cautious examination)18,24.
L* a* b* color values and color differences (ΔE*) between SNADETs and the adjacent normal duodenal mucosa
To evaluate the color differences between SNADETs and the adjacent normal duodenal mucosa, the images were assessed and scored for an objective evaluation based on L* a* b* (L* = light/dark, a* = red/green, and b* = yellow/blue) color values in the CIELAB color space system25 using ADOBE Photoshop CC 2017, as previously described19,26. The color difference (ΔE* = [(ΔL*)2 + (Δa*)2 + (Δb*)2]1/2) of the pixel values based on L* a* b* color spaces within the region of interest was analyzed to evaluate the visibility of each color image19,26.
Intra- and inter-observer agreement for the visibility of SNADETs
To evaluate the degree of intra-observer agreement for the visibility of the SNADETs, each endoscopist performed inspection twice. The kappa coefficient of reliability was calculated within each endoscopist in WLI, BLI-b, and LCI. To evaluate the degree of inter-observer agreement for the visibility of SNADETs, the kappa coefficient of reliability was calculated for each subject group in WLI, BLI-b, and LCI.
The kappa coefficient of reliability was judged according to following definition.
0.0–0.2 (slight agreement), 0.21–0.40 (fair agreement), 0.41–0.60, (moderate agreement), 0.61–0.80 (substantial agreement), 0.81–1.0 (almost perfect or perfect agreement).
Sample size calculation
No previous report has examined the color differences between SNADETs and the normal duodenal mucosa. Therefore, the sample size was determined using preliminary data (5 patients each) to fit the paired Wilcoxon rank-sum test, and the color difference (ΔE*) between SNADETs and the duodenal mucosa was considered the outcome of interest. Regarding the sample size of the patients with SNADETs, the mean color differences (ΔE*) in WLI and LCI modes were 10.2 and 14.5, respectively, and the response within each subject group had a normal distribution with a standard deviation (SD) of 7.1. If the actual difference in the experimental and control means is equal to 4.3, the study would require a sample of 40 patients with SNADETs to reject the null hypothesis with a probability of 95% and a significance level of 0.05. The drop-out rate was approximately 10%, and 44 patients with SNADETs were finally included in the analysis.
Statistical analysis
The baseline data were presented as mean ± SD. The differences in visibility scores and L* a* b* color values among the groups were analyzed using the Wilcoxon rank-sum test. The tumor size was compared using the Mann–Whitney U test. The intra- and inter-observer agreement for the visibility of SNADETs was assessed using the Fleiss kappa. All statistical analyses were performed using the Statistical Package for the Social Sciences software version 26 (SPSS Inc., Chicago, IL, the USA). P values < 0.05 were considered statistically significant.
Results
Characteristics of the patients and lesions
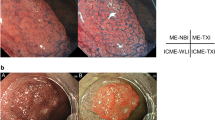
In total, 44 patients and 44 lesions were evaluated. The characteristics of the patients and lesions are shown in Table 1. The representative SNADET cases are shown in Fig. 1A,B. The tumor size was compared according to macroscopic findings, as shown in Table 2.
Representative cases of SNADETs: WLI, LCI, and BLI-b modes at the same site in a fully extended condition without zooming. SNADETs, superficial non-ampullary duodenal epithelial tumors; WLI, white light imaging; LCI, linked color imaging; BLI-b, blue laser imaging-bright. (A) 8-mm, 0-IIa HGA with MWM. MWM, milk white mucosa; HGA, high-grade adenoma. (B) 13-mm, 0-IIa IMC without MWM. MWM, milk white mucosa; IMC, intramucosal carcinoma.
Visibility score
The mean visibility scores (± standard deviation (SD)) of WLI, LCI, and BLI-b for SNADETs are shown in Table 3 (first time evaluation is shown). The scores obtained using LCI were significantly higher than those obtained using WLI (P < 0.01) and BLI-b (P < 0.01). This difference was also recognized by the experts (P < 0.01). The scores obtained using LCI were not significantly higher than those obtained using BLI-b among trainees.
In lesions with MWM, the scores obtained using LCI were significantly higher than those obtained using WLI (P < 0.01) and BLI-b (P < 0.01). This difference was also recognized by experts (P < 0.01). The scores obtained using LCI were not significantly higher than those obtained using BLI-b among trainees. In lesions without MWM, the scores obtained using LCI were significantly higher than those obtained using WLI (P < 0.01) and BLI-b (P = 0.034). This difference was also recognized by experts (P < 0.01 and 0.046, respectively). The scores obtained in LCI mode was not significantly higher than those obtained in BLI-b mode among trainees.
In depressed lesions, the scores obtained using LCI were significantly higher than those obtained using WLI (P < 0.01) and BLI-b (P < 0.01). This difference was also recognized by experts (P < 0.01) and trainees (P < 0.01 and 0.034, respectively). In non-depressed lesions, the scores obtained in LCI mode were significantly higher than those obtained in WLI (P < 0.01) and BLI-b (P < 0.01) modes. This difference was also recognized by experts (P < 0.01). The scores obtained in LCI mode was not significantly higher than those obtained in BLI-b mode among trainees.
Color difference between SNADETs and the adjacent duodenal mucosa
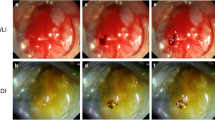
The representative cases used in analyzing the color difference are shown in Fig. 2. The color differences (ΔE*) between the SNADETs and the adjacent normal duodenal mucosa in WLI, LCI, and BLI-b modes are shown in Table 4. Color differences (ΔE*) among each modality are shown in Table 4A and color differences (ΔE*) between lesions in oral and anal side of the major papilla are shown in Table 4B.
Representative cases for evaluating color difference: The color difference (ΔE*) of the pixel values based on L* a* b* color spaces within the region of interest was analyzed to evaluate the visibility of each color image. The green line squares indicate the region of interest in the normal duodenal mucosa. The yellow line squares represent the region of interest in SNADETs. SNADETs, superficial non-ampullary duodenal epithelial tumors; WLI, white light imaging; LCI, linked color imaging; BLI-b, blue laser imaging-bright.
The color differences in LCI mode were significantly higher than those in WLI and BLI-b modes for each subtype. In lesions without MWM, the color difference was not significantly different between WLI and BLI-b modes. The color differences for each modality were not significantly different between lesions in oral and anal side of the major papilla.
Intra- and inter-observer agreement for the visibility of SNADETs
Intra-and inter-observer agreement for the visibility of SNADETs were performed. The kappa coefficients of reliability for the visibility of SNADETs within each endoscopist (WLI/LCI/BLI-b) (intra-observer agreement) were trainee A (0.57/0.40/0.40), trainee B (0.51/0.31/0.44), trainee C (0.50/0.25/0.22), expert A (0.49/0.40/0.45), expert B (0.63/0.40/0.21), and expert C (0.26/0.35/0.25). Intra-observer agreement of LCI was judged as fair for both trainees and experts. The kappa coefficients of reliability for the visibility of SNADETs in WLI/LCI/BLI-b modes among trainees and experts (inter-observer agreement) were 0.33/0.27/0.29 and 0.20/0.33/0.21, respectively. In WLI, LCI, and BLI-b modes, the inter-observer agreements for SNADETs except for WLI among experts were fair for both trainees and experts. The kappa coefficient of WLI among experts was interpreted as slight.
Discussion
To the best of our knowledge, this is the first study that assessed the efficacy of LCI in increasing the visibility of SNADETs using visibility score and color difference.
Typically, malignant SNADETs and some adenomas have an orange color on LCI13. In our study, almost all SNADETs had an orange or orange-red color on LCI. This modality can emphasize vascular and surface structures. In addition, LCI has the greatest brightness, followed by WLI and BLI-bright13. These factors could contribute to the highest color difference in LCI mode compared with WLI and BLI-b modes. The color difference in LCI could have a higher visibility score among all endoscopists and experts. By contrast, the visibility score of LCI and BLI-b did not significantly differ among trainees. Both LCI and BLI have a high emission intensity at short wavelengths (410 nm)27,28. However, BLI-bright has blue and green color information, and LCI has additional red color information. In addition, LCI has more color patterns in the mucosa due to the emission intensity at wavelengths different from those of WLI13. For trainees, both BLI and LCI might help to visualize SNADETs.
Tanaka et al. reported that a whitish villus is considered as lipids in epithelial cells at the villi tips29. Whitish villus and MWM have a similar appearance30. Toya et al. reported that MWM was less frequently observed for SNADETs in duodenal bulb than in second portion31. In our study, MWM presented as a white mucosa in WLI, LCI, and BLI-b modes. The color difference in LCI mode was significantly higher than that in WLI and BLI-b modes in lesions with and without MWM. These results were also identified between the lesions in oral and the lesions in anal side of the major papilla. In lesions without MWM, the color difference between WLI and BLI-b was not significantly different. These results indicate that LCI emphasized the color patterns of SNADETs regardless of the presence of MWM. Of note, among all endoscopists and experts, the visibility of SNADETs, even those without MWM, was significantly higher in LCI mode than in WLI and BLI-b modes. The clinical characteristic of SNADETs without MWM has not been completely validated. In this study, five of six SNADETs without MWM were HGA and IMC. Hence, it is important to identify SNADETs without MWM, and LCI may help detect these lesions by increasing their visibility irrespective of the presence of MWM.
The color difference in depressed lesions was significantly higher than that in non-depressed lesions. The depressed lesions were significantly smaller than the non-depressed lesions. However, the visibility score of LCI for depressed lesions was significantly higher than that of WLI and BLI-b among all endoscopists, experts, and trainees. LCI might emphasize the color patterns of depressed-type SNADETs, leading to a higher visibility score. Depressed-type duodenal tumors have a higher cancerous component32,33. Furthermore, Goda et al. reported that of 139 SNADETs, 46 (33%) were mucosal carcinomas and 1 submucosal carcinoma with a diameter of 6–10 mm34. Taken together, the detection of depressed or relatively small lesions are considered important. LCI is more effective than WLI and BLI-b as it increases the visibility of these lesions.
With respect to kappa coefficient, the intra- and inter observer agreements for SNADETs on LCI were fair in both trainees and experts. The kappa coefficient of reliability was more likely to show a lower value in the analysis with a larger number of observers and a high number of evaluation scores. If we analyzed the kappa coefficient with a smaller number of evaluation scores for two observers, the kappa coefficient could be higher.
This study had several limitations. First, although we calculated the sample size, the proportion of some subtype lesions, including those without MWM, was small. Second, this study conducted an evaluation using still images. The actual visibility of SNADETs was assessed through video. Thus, the use of a video may facilitate a more accurate assessment. Third, this study only analyzed color difference and visibility score. Although LCI may help in the detection of SNADETs, a prospective study that assesses the actual detection rate of SNADETs on LCI must be conducted.
In conclusion, LCI has potential benefits, and it is considered a promising modality that can increase the visibility of SNADETs regardless of the presence of MWM. Moreover, it may improve the detection rate of these lesions.
Abbreviations
- SNADETs:
-
Superficial non-ampullary duodenal epithelial tumors
- PD:
-
Pancreaticoduodenectomy
- CSP:
-
Cold snare polypectomy
- UEMR:
-
Underwater endoscopic mucosal resection
- LCI:
-
Linked color imaging
- MWM:
-
Milk white mucosa
- WLI:
-
White light imaging
- BLI-bright:
-
Blue laser imaging-bright
- SD:
-
Standard deviation
- LGA:
-
Low-grade adenoma
- HGA:
-
High-grade adenoma
- IMC:
-
Intramucosal carcinoma
References
Nakayama, A. et al. How I do it, endoscopic diagnosis for superficial non-ampullary duodenal epithelial tumors. Dig. Endosc. 32, 417–424 (2020).
Yamasaki, Y. et al. Differentiation between duodenal neoplasms and non-neoplasms using magnifying narrow-band imaging. Do we still need biopsies for duodenal lesions?. Dig. Endosc. 32, 84–95 (2020).
Galandiuk, S. et al. Villous tumors of the duodenum. Ann. Surg. 207, 234–239 (1988).
Miller, J. H. et al. Upper gastrointestinal tract, villous tumors. Am. J. Roentgenol. 134, 933–936 (1980).
Perzin, K. H. & Bridge, M. F. Adenomas of the small intestine, a clinicopathologic review of 51 cases and a study of their relationship to carcinoma. Cancer 48, 799–819 (1981).
Sellner, F. Investigations on the significance of the adenoma-carcinoma sequence in the small bowel. Cancer 66, 702–715 (1990).
Johnson, J. C., DiSario, J. A. & Grady, W. M. Surveillance and treatment of periampullary and duodenal adenomas in familial adenomatous polyposis. Curr. Treat. Options Gastroenterol. 7, 79–89 (2004).
Yeo, C. J. et al. Six hundred fifty consecutive pancreaticoduodenectomies in the 1990s, Pathology, complications, and outcomes. Ann. Surg. 226, 248–57 (1997) ((discussion 257–260)).
Cameron, J. L., Riall, T. S., Coleman, J. & Belcher, K. A. One thousand consecutive pancreaticoduodenectomies. Ann. Surg. 244, 10–15 (2006).
Maruoka, D. et al. Cold polypectomy for duodenal adenomas, a prospective clinical trial. Endoscopy 49, 776–783 (2017).
Kenneth, F. et al. “Underwater” EMR of sporadic laterally spreading nonampullary duodenal adenomas (with video). Gastrointest. Endosc. 78, 496–502 (2013).
Miura, Y. et al. Duodenal endoscopic submucosal dissection is feasible using the pocket-creation method. Endoscopy 49, 8–14 (2017).
Osawa, H. et al. Linked color imaging and blue laser imaging for upper gastrointestinal screening. Clin. Endosc. 51, 513–526 (2018).
Dohi, O. et al. Linked color imaging improves endoscopic diagnosis of active Helicobacter pylori infection. Endosc. Int. Open 4, E800–E805 (2016).
Ono, S., Abiko, S. & Kato, M. Linked color imaging enhances gastric cancer in gastric intestinal metaplasia. Dig. Endosc. 29, 230–231 (2017).
Kanzaki, H. et al. Linked color imaging (LCI), a novel image-enhanced endoscopy technology, emphasizes the color of early gastric cancer. Endosc. Int Open 5, E1005–E1013 (2017).
Fukuda, H. et al. Linked color imaging technology facilitates early detection of flat gastric cancers. Clin. J. Gastroenterol. 8, 385–389 (2015).
Suzuki, T. et al. Linked-color imaging improves endoscopic visibility of colorectal nongranular flat lesions. Gastrointest. Endosc. 86, 692–697 (2017).
Takeda, T. et al. Improved visibility of Barrett’s esophagus with linked color imaging, Inter- and intra-rater reliability and quantitative analysis. Digestion 97, 183–194 (2018).
Dixon, M. F. Gastrointestinal epithelial neoplasia, Vienna revisited. Gut 51, 130–131 (2002).
Fujinami, H. & Inatsuchi, S. Endoscopic diagnosis of duodenal adenoma and early cancers. Stomach Intestine (Tokyo) 50, 629–639 (2015) ((in Japanese with English abstract)).
Yoshimura, N. et al. Endoscopic features of nonampullary duodenal tumors with narrow-band imaging. Hepatogastroenterology 57, 462–467 (2010).
Participants in the Paris Workshop. The Paris endoscopic classification of superficial neoplastic lesions, esophagus, stomach, and colon. Gastrointest. Endosc. 58 (suppl) (2003).
Yoshida, N. et al. Improvement in the visibility of colorectal polyps by using blue laser imaging (with video). Gastrointest. Endosc. 82, 542–549 (2015).
Kuehni, R. G. Color-tolerance data and the tentative CIE 1976 L a b formula. J. Opt. Soc. Am. 66, 497–500 (1976).
Dohi, O. et al. Recognition of endoscopic diagnosis in differentiated-type early gastric cancer by flexible spectral imaging color enhancement with indigo carmine. Digestion 86, 161–170 (2012).
Osawa, H. & Yamamoto, H. Present and future status of flexible spectral imaging color enhancement and blue laser imaging technology. Dig. Endosc. 26, 105–115 (2014).
Osawa, H. et al. Blue laser imaging provides excellent endoscopic images of upper gastrointestinal lesions. Video J. Encycl. GI Endosc. 1, 607–610 (2014).
Tanaka, M. et al. Significance of magnifying endoscopy in diagnosis of duodenal elevated lesions. Stomach Intestine 38, 1709–1720 (2003) ((in Japanese with an English abstract)).
Tsuji, S. et al. Preoperative endoscopic diagnosis of superficial non-ampullary duodenal epithelial tumors, including magnifying endoscopy. World J. Gastroenterol. 21, 11832–11841 (2015).
Toya, Y. et al. Clinicopathological features and magnifying chromoendoscopic findings of non-ampullary duodenal epithelial tumors. Digestion 97, 219–227 (2018).
Yamanaka, T., Yamamichi, N. & Konishi, F. Clinicopathological study of duodenal adenoma. Gastroenterol Endosc. 29, 3070–3079 (1987) ((in Japanese with an English abstract)).
Tanaka, K. et al. Depressed-type early duodenal carcinoma (carcinoma in situ) observed by enhanced magnification endoscopy. Endoscopy 39, E125-126 (2007).
Goda, K. et al. Endoscopic diagnosis of superficial non-ampullary duodenal epithelial tumors in Japan, Multicenter case series. Dig. Endosc. 26, 23–29 (2014).
Acknowledgements
The authors would like to thank the staff of the Endoscopy Center of Chiba University Hospital for providing technical assistance and helping in the documentation in this study.
Author information
Authors and Affiliations
Contributions
K.O., D.M.: designed the study, conducted the experiment, collected, analyzed, and interpreted data, and wrote the manuscript. T.M., designed the study, analyzed and interpreted data, and assisted in writing the manuscript. M.T., T.K., H.O., A.N., Y.O., K.S., and M.A. assisted in conducting the experiment and collecting data. J.K., N.K. assisted in interpreting data and writing the manuscript. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Okimoto, K., Maruoka, D., Matsumura, T. et al. Linked color imaging can improve the visibility of superficial non-ampullary duodenal epithelial tumors. Sci Rep 10, 20667 (2020). https://doi.org/10.1038/s41598-020-77726-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-77726-3
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.