Abstract
The widespread decline of canopy-forming macroalgal assemblages has been documented in many regions during the last decades. This pattern is often followed by the replacement of structurally complex algal canopies by more simplified habitats (e.g., turfs or sea urchin barren grounds). Against all odds, the fucoid Treptacantha elegans, a large Mediterranean brown macroalga, broadened its depth range to deeper and exposed environments and displayed an unexpected range expansion along the northern coast of Catalonia over the last two decades. Here, we reconstruct the spread of T. elegans in time and space and unravel ecological and demographic traits such as population dynamics and genetic patterns to provide a comprehensive and integrated view of the current status and geographical expansion for this species. Fast-growing dynamics, early fertile maturity, and high turnover rate are the main competitive advantages that allow the exposed populations of T. elegans to colonize available substrata and maintain dense and patchy populations. We also provided evidence that the deeper and exposed populations of T. elegans constitute a single group across the Catalan coast, with little genetic differentiation among populations. This seems to support the hypothesis of a unique source of spread in the last decades from the Medes Islands No-Take Zone towards both southern and northern waters.
Similar content being viewed by others
Introduction
How marine populations persist, evolve, and change their geographical distributions as a response to global change is one of the main questions in contemporary ecology1,2. Species may redistribute to keep their preferred environmental conditions3,4,5,6 or adapt their physiology as a response to global and local stressors5,7. Macroalgae are dominant primary producers and ecosystem engineers that provide structure and ecosystem services (e.g., food and fisheries production and carbon storage)8,9,10 to many temperate and sub-temperate shallow coastal habitats where they play a key role in ecosystem functioning11,12. As a response to global and local stressors (i.e., ocean warming or coastal pollution), they may show both geographical expansions, when species colonize new habitats, or contractions, when populations disappear from areas previously inside their distributional range4,13.
In the Mediterranean Sea, Cystoseira sensu lato fucoids (here used to designate Atlantic-Mediterranean fucoid species of Cystoseira sensu stricto, Carpodesmia and Treptacantha14) are late successional species and among the major canopy-forming macroalgae. They provide three-dimensional structure, food, and shelter to many associated species9,15. Decline of these species may drive to a decrease in habitat complexity with important consequences for benthic biodiversity and ecosystem functioning10,15,16,17,18. All Cystoseira sensu latospecies, except C. compressa, appear in the Annex II of the Barcelona convention (2010) among those taxa that are considered threatened or endangered and need protection measures19. Cystoseira sensu lato species show limited dispersal abilities and propagules often disperse over a few meters19,20. This leads to the existence of monospecific stands near parent populations21. This trait combined with local environmental factors may explain the lack of connectivity for many populations of T. elegans in different areas of the Mediterranean Sea22,23, something that seriously jeopardizes their conservation. Nonetheless, the few studies on population genetics suggest that water currents may play a significant role in long-distance dispersal (presumably via drifting of detached reproductive branches) of Cystoseira sensu lato populations24,25,26. Thus, despite the well-documented general decline of local populations16,18,27, evidences for stability and even expansion have been also reported in some areas27,28,29.
Treptacantha elegans (Sauvageau) Orellana and Sansón (until very recently Cystoseira elegans14) is a Mediterranean endemic species. The Mediterranean Sea is oligotrophic, with relatively high salinity, high summer temperatures, and extremely reduced tides, which are mainly regulated by the atmospheric pressure30. T. elegans was first described by Sauvageau for Banyuls-sur-Mer (South-Eastern France)31. This species typically inhabits shallow waters down to 2–3 m depth31, where forms extensive stands at the innermost rocky bottoms of coves and other shallow environments characterized by low to medium water movements32. Recent studies have reported a significant regression of T. elegans in shallow and sheltered environments from the N–W Mediterranean Sea16,18,23. However, in the past two decades, some populations of T. elegans (identification by M. Verlaque, personal communication) have expanded their distribution in the Medes Islands No-Take Zone (NTZ) and the nearby areas (North-Western Mediterranean Sea), where the species can be now found in wave-exposed habitats located between 5 and 15 m depth, with some sparse thalli at 20 m depth. The reasons for this pattern are yet to be determined.
Here we investigate this recent spread throughout the North Catalan coast. Concretely, our goals are: (1) provide insights about the main traits of deeper and exposed populations of T. elegans and the species population ecology; (2) assess its historical and current distribution in the Catalan coast; and (3) provide an estimate of the patterns of genetic diversity and differentiation among populations including ancient and more recently established zones.
Results
Phenological and ecological traits
The seawater temperature in the north Catalan coast is characterized by strong seasonal variability. In 2018, the mean annual temperature at 5 m depth was 17.27 °C ± 1.26 (Mean ± SE). In accordance with the well-known thermal regime of this area33, minimum winter temperatures were recorded in February and March (12.35 °C ± 0.3, Mean ± SE) and the maximum in August (23.82 °C ± 0.9, Mean ± SE) just before the gradual drop throughout autumn.
Branches of Treptacantha elegans from deeper and exposed infralittoral habitats (5–15 m depth) began to grow from the perennial basal disc (or holdfast) in early spring when sea water temperature starts to rise (Fig. 1). The receptacles located at the base of each spiny branch began to mature progressively as branches approached their maximum development. The reproductive period spanned from the end of May to August, individuals being fertile mainly when water temperature rose above 18 °C. In June and July, 90% of the sampled individuals showed receptacles (Fig. 1). All fertile individuals measured more than 4.5 cm. Like the thalli from more sheltered environments, specimens of T. elegans from deeper, exposed habitats shed their branches after the reproductive period and remained attached to the substrate through the perennial holdfast until the following spring34,35.
Monthly presence of fertile Treptacantha elegans individuals. Light blue bars represent the percentage of immature individuals, without the presence of reproductive structures. Medium-blue bars represent the percentage of the individuals beginning the reproductive stage, with reproductive receptacles in the maturing process. Dark blue bars represent the percentage of fertile and reproductive individuals. The red line shows the mean monthly temperature at 5 m depth. Pictures at the top illustrate the monthly canopy-forming branches development stage.
The deeper, exposed populations monitored in the Medes Islands showed similar densities (51.3 ± 4.26 ind/m2, Mean ± SE) over the three years. No differences were found in density (χ22 = 4.8, p = 0.09) and mean size of individuals (χ22 = 3.7, p = 0.16) across the three sites. When grouped into 1 cm size classes, the size distribution of T. elegans individuals mostly ranged from 1 to 23 cm (Fig. 2) but unusual larger sizes (up to 42 cm), like those found in specimens from shallow, sheltered environments36, were also found. The mean size of T. elegans individuals sampled over the 3 years in the 6 permanent plots was 10.92 ± 0.21 cm (Mean ± SE). The mean growth rate within first-year individuals was 8.6 cm ± 0.6 (Mean ± SE). Some specimens could reach relatively large sizes, being 28 cm the maximum growth observed for an individual recruited the previous spring. New, annually appeared recruits were about one-third of the total counts in the permanent plots (33.7% ± 7.9, Mean ± SE), 82% of which exceeded the fertile minimum size (4.5 cm). Mean annual mortality rate within the 3 populations was about 36% (35.9% ± 9.8, Mean ± SE). Small specimens showed higher annual mortality rates than the larger ones (Fig. 3, Table 1).
Spatial and temporal distribution
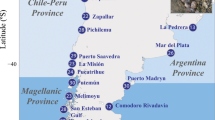
Since their first observation in the Medes Islands NTZ37, T. elegans populations from deeper and exposed shores have progressively expanded to other areas of the coast of Catalonia (Fig. 4). The farthest populations were detected 30 km away from the Medes Islands in 2011 and 2012 (Table 2). All the 17 populations detected over the last 2 decades were still present in 2018 on rocky and exposed bottoms between 5 and 15 m depth.
Geographical distribution of deep Treptacantha elegans over the studied years in the Catalan coast. Colored dots indicate the temporal sequence of their first report and circled numbers identify the populations where molecular analyses were conducted. Map was created using ArcMap 10.7 (https://desktop.arcgis.com/en/arcmap).
Population genetic structure and diversity
The 8 microsatellite loci showed moderate polymorphism, ranging from 3 to 14 total alleles per locus (64 in total). Allelic richness was higher in the Medes Islands NTZ population and lowest in the northern population (Messina; Table 3). Although it showed the lowest number of private alleles, marginally higher genetic diversity was also observed in Medes Islands NTZ (Table 3). All populations showed positive inbreeding coefficients (FIS) resulting from heterozygote deficiency (Table 3). Mean FST across all populations and loci was 0.07. The analyses of Molecular Variance (AMOVA) revealed that most genetic variation occurred within populations (93%, Supplementary Table S1). Pairwise FST estimates revealed no differentiation between the Medes Islands and Begur (separated only by 9 km, Fig. 4 locations 1 and 3). Pairwise estimates between the Medes Islands with Messina and Messina with Begur were significant (Table 4). According to Structure Harvester the best K was 3. Structure results showed negligible population differentiation within T. elegans populations across the Catalan coast, i.e., revealed a genetically homogeneous region (Fig. 5, Supplementary Table S2).
Discussion
Most species of Cystoseira sensu lato are often considered late successional species, are especially sensitive to environmental and man-induced stressors38, and show limited dispersal abilities19,21. For these reasons, they can be considered less competitive species which makes the results of this study surprising. Despite its decline in shallow and sheltered environments from neighboring areas16,18, the brown alga Treptacantha elegans has remarkably increased both its depth range and spatial distribution over the last two decades along the Catalan coast.
A better knowledge of the population dynamics of a species is crucial for understanding, explaining, and predicting species distributions. In the Medes Islands NTZ, deeper and exposed T. elegans forests showed fast-growing dynamics. During their first year of life, more than 80% of their stands were capable to reach fertile maturity and the relatively largest sizes that may ensure survival rates higher than 50%. These uncommon traits among species of the same genus clearly represent an advantage for colonizing new available substrates. The observed similar mean annual mortality and recruitment rates suggest a high population turnover rate, which might also represent a major advantage to T. elegans.
Our molecular analyses of the three exposed and deeper T. elegans populations across the Catalan coast showed great genetic homogeneity despite relatively high intra-population variation and inbreeding. The first T. elegans population from exposed shores was detected in the Medes Islands NTZ in 1996 and its origin has been thought to be related to the protection effect39, specifically to the lower density of herbivores in the reserve40. Since then, only a slight expansion (< 1 km) has been observed during the following 15 years. The southern (Begur) and northernmost (Messina) populations analyzed here have not been described until 2010 and 2011 respectively, even though regular monitoring was carried out in these locations. Hence, we assume that the Medes Islands may represent the source population from which the species expansion across the exposed shores in the Catalan coast begun. The Medes Islands population also exhibited the highest allelic richness, which supports our assumption of it being a mature population thus a reasonable source population. As in other Cystoseira sensu lato species, our results may suggest a common ancestral gene pool but loosely connected populations22,26,41. This is supported by the patchy distribution of T. elegans, a pattern likely due to the scattered presence of rocky and suitable habitats in the area and the limited dispersal range of gametes in fucoids19,20,21,42. Unfortunately, our data are not sufficient to detect a clear correlation between distance and genetic differentiation within populations.
While the dispersal distance of early life stages is less than 10 m from source populations in T. elegans43, here we show the existence of two populations 30 km away from the Medes Islands NTZ. This may be explained by the fact that this species, as other fucoids, is capable to disperse through detached or drifting fertile parts of mature individuals21,27,44 or by animals18. This may explain the southern spreading through the Northern Current (NC), which flows with a permanent southwestward circulation in the Northwestern Mediterranean basin45. Although a NC mediated spread in the opposite direction is unlikely, other processes such as wind-induced currents or extreme meteorological conditions play important roles in the water circulation of this complex hydrodynamical region. This may explain the passive transportation of T. elegans from the exposed shores of the Medes Islands to the northern studied exposed areas46.
Increased tolerance and adaptation and/or migration to different locations within their specific niche are the main responses to environmental changes in macroalgae7. Morphological differences have been reported in T. elegans individuals between populations from sheltered and exposed infralittoral habitats23 such as the presence of thicker and spinier branches in the individuals from exposed shores, a trait that may be the result of a phenotypic plasticity response (acclimation rather than adaptation) to such environments, as no evidence for genetic differences has been detected in preliminary assays of these morphotypes (Authors, unpublished data). The potential source of the first deeper T. elegans population observed in the exposed shores of the Medes Islands is unknown. However, it may have arrived from the original shallow population in Banyuls-sur-Mer (France), where this species was described for the first time31, or from other shallow littoral populations from the north of Catalonia where the species was more abundant in the past23.
Several environmental drivers and the interaction of some favorable conditions may have promoted the spread observed. Seawater eutrophication is one of the major causes for the disappearance of Cystoseira sensu lato populations in the Mediterranean Sea29,47. Evidence of a general improvement in coastal water quality, particularly during the 1990s48, is a possible explanation for the expansion of Cystoseira sensu lato species in the studied area49. Besides, the Medes Islands NTZ might have worked as a refuge for T. elegans populations during the period of improvement of environmental conditions, mainly because of the lower density of herbivores due to the predatory fish control in the reserve40. In addition to these local stressors, global warming and extreme climatic events (such as storms and heatwaves) are also threatening marine coastal life, especially in shallower waters50. The increase in sea-surface temperature over the last decades51,52 may have led to species responses in macroalgal assemblages such as the observed here53. While we know that T. elegans may withstand high summer temperatures as an adaptation to the shallow environment23, our results suggest that a shift in the depth-range distribution and a subsequent acclimation to the new environments may be a result of warming.
Our results shed new light on the ability of a Cystoseira sensu lato species to persist or even widen their geographical distributions under global change scenarios. For this to happen, however, it is important to improve the water quality and to protect population sources that ensure dispersal like those found in the Medes Islands NTZ, especially in organisms with short-dispersing propagules. Finally, given its fast and stable population dynamics (early fertile maturity and high turnover rate), we highlight that T. elegans represent an ideal organism to conduct habitat restoration actions in previously degraded systems such as sea urchin barrens or turf-dominated habitats43.
Methods
Phenology and demographic data
Measurement of the main axis has been reported as a good indicator of algae size in morphometric studies of other Cystoseira sensu lato species54. However, as Treptacantha elegans is characterized by a tiny main axis (1–3 cm length55), this trait hinders accurate measurements in situ unless invasive techniques are used. To discern the best less invasive size indicator for in situ measurements, we collected 27 specimens of Medes Islands NTZ and measured several morphometric parameters. Correlation analyses between the parameters showed high correlation between the maximum length of the longest axis and the rest of the morphometric variables measured (Supplementary Table S3). The maximum length of the longest axis was then selected as the best parameter for easy and robust measurements from different observers.
To accurately describe the T. elegans annual life cycle (i.e. growth of branches, reproductive period, and branch shedding) throughout the annual temperature cycle, 20 T. elegans individuals covering a wide size range (2–23 cm) were sampled monthly in the Medes Islands NTZ in 2018. Their fertile maturity status was discerned by pooling the individuals sampled within three stages: (1) immature individuals without receptacles; (2) individuals beginning their reproductive period, with receptacles but still immature; (3) fertile and reproductive individuals. Annual seawater temperatures were assessed by high-resolution hourly temperature recordings at 5 m depth in the Medes Islands obtained from the T-MEDNet platform (https://www.t-mednet.org).
To untangle the population dynamics of T. elegans from exposed shores (5–15 m depth), we performed a survey at the Medes Islands NTZ in 2017, 2018, and 2019 on three populations. These populations thrive a few hundred meters apart (see the 3 green dots in the Medes Islands, Fig. 4B). All three sites were south facing and showed healthy populations of T. elegans of similar extension thriving on top of the rocky boulders. At each site, two permanent plots of 1–1.5 m2 (depending on boulder size) were placed a few meters apart at around 10 m depth. Thanks to the high density of T. elegans populations in the patches, these tiny 6 permanent plots allowed to monitor 684 specimens. Generalized linear mixed models (GLMMs) with Poisson error distribution were applied to test the similarity of the three selected sites. Density and mean size of T. elegans individuals in 2017 were treated as independent response variables, the site as the fixed response variable and the plots as the random response variable. For the two fitted models we applied a Type II Wald χ2 test to show a non-significant effect of sites. This led us to pool together all specimens from the three sites of the Medes Islands location (Fig. 4B), which also correspond with the oldest and most mature T. elegans populations found in this study. All specimens in the 6 permanent plots were mapped and individually monitored yearly from 2017 to 2019 in early summer during the macroalgal maximum development period. All specimens inside the plots were measured using a caliper by SCUBA divers. Population density and size structure were calculated by pooling the individuals into 0.9 cm binned size intervals54,56,57. Differences in density measures between two consecutive annual surveys within the studied period were used to estimate the mortality and recruitment rates. For each survey transition, mortality was quantified as the disappearance of individuals between census and, recruits were identified as new individuals appearing in the permanent plots at each census regardless of their size. Mortality rates for each size class were compared using a generalized linear model (GLM) fitted with a negative binomial distribution and a logarithm link function after visually checking the most likely distribution of the data and residuals58. GLM analyses were conducted using the package “MASS” for R software59 and GLMMs using the package “lme4”60 with R version 3.3.361.
Spatial and temporal distribution
Past and present distribution of shallow (≤ 1 m depth) and sheltered Treptacantha elegans in the Catalan coast has been recently reported23. Although descriptive studies have been carried out in the shorelines here investigated since the seventies62,63, T. elegans was not found in deeper and more exposed environments until the nineties, when Sala reported its presence37 (as Cystoseira spinosa23) for the Medes Islands. All available information about the spatial and temporal distribution of this species along the Catalan coast is reported in Table 2.
Molecular analysis
Samples for molecular analysis were collected in three study locations along the Catalan coast, thus covering the whole distribution range for the study area (Fig. 4, locations: 1 Medes Islands NTZ, 2 Messina, and 3 Begur). We collected twenty Treptacantha elegans samples corresponding to branches from 20 individuals haphazardly selected at each location, at a maximum distance among samples of 10 m. The samples were individually preserved by drying them in silica gel until DNA extraction.
Genomic DNA for each population was extracted from the dried samples using the NucleoSpin Plant II kit (Macherey–Nagel Duren, Germany) according to the manufacturer’s protocol. Eight microsatellite loci formerly developed for Treptacantha baccata (unpublished data from Biogeographical Ecology and Evolution research group, CCMAR) were used for genotyping. Polymerase chain reactions (PCRs) were performed in 15 µL total volume containing 1· GoTaq Flexi buffer (Promega, Madison, WI, USA), 1.5 mm MgCl2, 0.07 mm each dNTP, 0.4 µM of labelled (FAM, ATT0-550, ATT0-565 or HEX) forward primers, 0.4 µM of reverse primers, 1U GoTaq Flexi DNA Polymerase (Promega), and 5 µL of 1:100 diluted DNA template. In all PCRs, an initial denaturation step (95 °C, 2 min) was followed by 30 cycles of 95 °C for 30 s, a primer specific annealing temperature (Ta) for 30 s and 72 °C for 30 s, ending with a final elongation step at 72 °C for 5 min. Amplified fragments were separated using an ABI PRISM 3130 xl (Applied Biosystems, CCMAR, Portugal) automated capillary sequencer. Alleles were manually scored in STRand64 using the GeneScan 500 LIZ dye size standard (Applied Biosystems).
All individuals with missing data at three or more loci were excluded from analyses. These corresponded to 4 individuals, 2 from the southern population (Begur) and 2 from the norther one (Messina). Identification of genotyping errors due to null alleles, large allele dropout and the scoring errors were screening with the MICRO-CHECKER software v. 2.2.365. When evidence of null alleles was significant, a correction for null alleles was applied. Estimates of genetic diversity were calculated for each locus and population using the GENETIX software v. 4.0566. This included the mean number of alleles per locus (allelic richness), the non-biased expected heterozygosity (HE), the observed heterozygosity (HO), the number of private alleles, and the inbreeding coefficient (FIS). Pair-wise differentiation, as assessed with FST67, was calculated. Analyses of molecular variance components (AMOVA) were conducted using ARLEQUIN v3.168.
Population structure was inferred using STRUCTURE v2.44469 considering admixture and no a priori population assignments. The correlated allele frequency model was run with a burning time of 250,000 repetitions, 1,000,000 iterations and considering a range of genetic clusters (K) from 1 to 5. The model was run 14 times for each K to check the consistency. The best K was estimated using the program Structure Harvester70 which ranks K’s according to Evanno method71.
Data availability
The datasets generated for this study are available on request to the corresponding author.
References
Koehn, J. D., Hobday, A. J., Pratchett, M. S. & Gillanders, B. M. Climate change and Australian marine and freshwater environments, fishes and fisheries: synthesis and options for adaptation. Mar. Freshw. Res. 62(9), 1148–1164 (2011).
Munday, P. L., Warner, R. R., Monro, K., Pandolfi, J. M. & Marshall, D. J. Predicting evolutionary responses to climate change in the sea. Ecol. Lett. 16, 1488–1500 (2013).
Perry, A. L., Low, P. J., Ellis, J. R. & Reynolds, J. D. Climate change and distribution shifts in marine fishes. Science 308(5730), 1912–1915 (2005).
Wernberg, T. et al. Impacts of climate change in a global hotspot for temperate marine biodiversity and ocean warming. J. Exp. Mar. Biol. Ecol. 400(1–2), 7–16 (2011).
Poloczanska, E. S. et al. Global imprint of climate change on marine life. Nat. Clim. Change 3(10), 919 (2013).
Pecl, G. T. et al. Biodiversity redistribution under climate change: impacts on ecosystems and human well-being. Science 355(6332), eaai9214 (2017).
Harley, C. D. et al. Effects of climate change on global seaweed communities. J. Phycol. 48(5), 1064–1078 (2012).
Smale, D. A. et al. Threats and knowledge gaps for ecosystem services provided by kelp forests: a northeast Atlantic perspective. Ecol. Evol. 3(11), 4016–4038 (2013).
Cheminée, A. et al. Nursery value of Cystoseira forests for Mediterranean rocky reef fishes. J. Exp. Mar. Biol. Ecol. 442, 70–79 (2013).
Mineur, F. et al. European seaweeds under pressure: consequences for communities and ecosystem functioning. J. Sea. Res. 98, 91–108 (2015).
Wernberg, T. et al. Seaweed communities in retreat from ocean warming. Curr. Biol. 21(21), 1828–1832 (2011).
Wahl, M. et al. The responses of brown macroalgae to environmental change from local to global scales: direct versus ecologically mediated effects. PiP 2(1), 11–29 (2015).
Bates, A. E. et al. Defining and observing stages of climate-mediated range shifts in marine systems. Glob. Environ. Change 26, 27–38 (2014).
Orellana, S., Hernández, M. & Sansón, M. Diversity of Cystoseira sensu lato (Fucales, Phaeophyceae) in the eastern Atlantic and Mediterranean based on morphological and DNA evidence, including Carpodesmia gen. emend. and Treptacantha gen. emend. Eur. J. Phycol. 54(3), 447–465 (2019).
Blanfuné, A., Boudouresque, C. F., Verlaque, M. & Thibaut, T. The fate of Cystoseira crinita, a forest-forming Fucale (Phaeophyceae, Stramenopiles), in France (North Western Mediterranean Sea). Estuar. Coast. Shelf. Sci. 181, 196–208 (2016).
Thibaut, T., Pinedo, S., Torras, X. & Ballesteros, E. Long-term decline of the populations of Fucales (Cystoseira spp. and Sargassum spp.) in the Alberes coast (France, North-western Mediterranean). Mar. Pollut. Bull. 50(12), 1472–1489 (2005).
Mangialajo, L., Chiantore, M. & Cattaneo-Vietti, R. Loss of fucoid algae along a gradient of urbanization, and structure of benthic assemblages. Mar. Ecol. Progr. Ser. 358, 63–74 (2008).
Thibaut, T., Blanfuné, A., Boudouresque, C. F. & Verlaque, M. Decline and local extinction of Fucales in French Riviera: the harbinger of future extinctions?. Mediterr. Mar. Sci. 16(1), 206–224 (2015).
Gianni, F. et al. Conservation and restoration of marine forests in the Mediterranean Sea and the potential role of Marine Protected Areas. AIOL J. 4(2), 83–101 (2013).
Capdevila, P., Linares, C., Aspillaga, E., Riera, J. L. & Hereu, B. Effective dispersal and density-dependence in mesophotic macroalgal forests: insights from the Mediterranean species Cystoseira zosteroides. PLoS ONE 13(1), e0191346 (2018).
Mangialajo, L. et al. Zonation patterns and interspecific relationships of fucoids in microtidal environments. J. Exp. Mar. Biol. Ecol. 412, 72–80 (2012).
Buonomo, R. et al. Predicted extinction of unique genetic diversity in marine forests of Cystoseira spp. Mar. Environ. Res. 138, 119–128 (2018).
Mariani, S. et al. Past and present of Fucales from shallow and sheltered shores in Catalonia. Reg. Stud. Mar. Sci. 32, 100824 (2019).
Susini, M. L. Statut et biologie de Cystoseira amentacea var. stricta (Doctoral dissertation, 2006).
Thibaut, T. et al. Unexpected temporal stability of Cystoseira and Sargassum forests in Port-Cros, one of the oldest Mediterranean marine National Parks. Cryptogamie Algol. 37(1), 61–91 (2016).
Buonomo, R. et al. Habitat continuity and stepping-stone oceanographic distances explain population genetic connectivity of the brown alga Cystoseira amentacea. Mol. Ecol. 26(3), 766–780 (2017).
Thibaut, T. et al. Unexpected abundance and long-term relative stability of the brown alga Cystoseira amentacea, hitherto regarded as a threatened species, in the north-western Mediterranean Sea. Mar. Pollut. Bull. 89(1–2), 305–323 (2014).
Thibaut, T. et al. Connectivity of populations of the seaweed Cystoseira amentacea within the Bay of Marseille (Mediterranean Sea): genetic structure and hydrodynamic connections. Cryptogamie Algol. 37(4), 233–256 (2016).
Iveša, L., Djakovac, T. & Devescovi, M. Long-term fluctuations in Cystoseira populations along the west Istrian Coast (Croatia) related to eutrophication patterns in the northern Adriatic Sea. Mar. Pollut. Bull. 106(1–2), 162–173 (2016).
Ros, J. D., Romero, J., Ballesteros, E. & Gili, J. M. Diving in blue water. The benthos. In Western Mediterranean (ed. Margalef, R.) 233–295 (Pergamon Press, Oxford, 1985).
Sauvageau, C. A propos des Cystoseira de Banyuls et de Guéthary. Bulletin de la Station Biologique d’Arcachon (1912).
Feldmann, J. Les algues marines de la cote des Albères I–III: cyanophycées, chlorophycées phéophycées (1937).
Pascual, J., Bensoussan, N., Salat, J., & Garrabou, J. Clima i règim tèrmic de les aigües de les illes Medes i el Montgrí. El fons marí de les illes Medes i el Montgrí: quatre dècades de recerca per a la conservació. (ed. Càtedra d’Ecosistemes Litorals Mediterranis) 63 (2012).
Gómez-Garreta, A., et al. Flora phycologica iberica. I. Fucales. (ed. Servicio de Publicaciones, Universidad de Murcia) (2001).
Rodríguez-Prieto, C., Ballesteros, E., Boisset, F., & Afonso-Carrillo, J. Guía de las macroalgas y fanerógamas marinas del Mediterráneo occidental (ed. Omega) (2013).
Barceló, M.C., et al. Flora Phycologica Iberica Vol. 1 Fucales (ed. Servicio de Publicaciones, Universidad de Murcia) (2001).
Sala, E. The role of fishes in the organization of a Mediterranean sublittoral community (Doctoral dissertation, 1996).
Ballesteros, E. et al. A new methodology based on littoral community cartography dominated by macroalgae for the implementation of the European Water Framework Directive. Mar. Pollut. Bull. 55(1–6), 172–180 (2007).
Sala, E. et al. The structure of Mediterranean rocky reef ecosystems across environmental and human gradients, and conservation implications. PLoS ONE 7(2), e32742 (2012).
Medrano, A. et al. No-take marine reserves control the recovery of sea urchin populations after mass mortality events. Mar. Environ. Res. 145, 147–154 (2019).
Susini, M. L., Thibaut, T., Meinesz, A. & Forcioli, D. A preliminary study of genetic diversity in Cystoseira amentacea (C. Agardh) Bory var. stricta Montagne (Fucales, Phaeophyceae) using random amplified polymorphic DNA. Phycologia 46(6), 605–611 (2007).
Sales, M. & Ballesteros, E. Shallow Cystoseira (Fucales: Ochrophyta) assemblages thriving in sheltered areas from Menorca (NW Mediterranean): relationships with environmental factors and anthropogenic pressures. Estuar. Coast. Shelf. Sci. 84(4), 476–482 (2009).
Medrano, A. et al. From marine deserts to algal beds: Treptacantha elegans revegetation to reverse stable degraded ecosystems inside and outside a no-take marine reserve. Restor. Ecol. 28(3), 632–644 (2020).
Deysher, L. & Norton, T. A. Dispersal and colonization in Sargassum muticum (Yendo) Fensholt. J. Exp. Mar. Biol. Ecol. 56, 179–195 (1982).
Calvo, E. et al. Effects of climate change on Mediterranean marine ecosystems: the case of the Catalan Sea. Clim. Res. 50(1), 1–29 (2011).
André, G., Garreau, P., Garnier, V. & Fraunié, P. Modelled variability of the sea surface circulation in the North-western Mediterranean Sea and in the Gulf of Lions. Ocean Dyn. 55(3–4), 294–308 (2005).
Sales, M., Cebrian, E., Tomas, F. & Ballesteros, E. Pollution impacts and recovery potential in three species of the genus Cystoseira (Fucales, Heterokontophyta). Estuar. Coast. Shelf. Sci. 92(3), 347–357 (2011).
Pinedo, S., Zabala, M. & Ballesteros, E. Long-term changes in sublittoral macroalgal assemblages related to water quality improvement. Bot. Mar. 56(5–6), 461–469 (2013).
Ricart, A. M. et al. Long-term shifts in the north western Mediterranean coastal seascape: the habitat-forming seaweed Codium vermilara. Mar. Pollut. Bull. 127, 334–341 (2018).
Blanfuné, A., Boudouresque, C. F., Verlaque, M. & Thibaut, T. The ups and downs of a canopy-forming seaweed over a span of more than one century. Sci. Rep. 9(1), 1–10 (2019).
Nykjaer, L. Mediterranean Sea surface warming 1985–2006. Clim. Res. 39(1), 11–17 (2009).
Sicre, M. A. et al. Sea surface temperature variability in the North Western Mediterranean Sea (Gulf of Lion) during the Common Era. Earth Planet. Sci. Lett. 456, 124–133 (2016).
Gallon, R. K. et al. Twenty years of observed and predicted changes in subtidal red seaweed assemblages along a biogeographical transition zone: inferring potential causes from environmental data. J. Biogeogr. 41(12), 2293–2306 (2014).
Ballesteros, E. et al. Deep-water stands of Cystoseira zosteroides C. Agardh (Fucales, Ochrophyta) in the Northwestern Mediterranean: insights into assemblage structure and population dynamics. Estuar. Coast. Shelf. Sci. 82(3), 477–484 (2009).
Cormaci, M., Furnari, G., Catra, M., Alongi, G. & Giaccone, G. Flora marina bentonica del Mediterraneo: Phaeophyceae. Bollettino dell’accademia Gioenia di scienze naturali di Catania 45(375), 1–508 (2012).
Capdevila, P. et al. Recruitment patterns in the Mediterranean deep-water alga Cystoseira zosteroides. Mar. Biol. 162(6), 1165–1174 (2015).
Verdura, J., Sales, M., Ballesteros, E., Cefalì, M. E. & Cebrian, E. Restoration of a canopy-forming alga based on recruitment enhancement: methods and long-term success assessment. Front. Plant Sci. 9, 1832 (2018).
McCullagh, P. & Nelder, J. A. Generalized Linear Models 2nd edn, 532 (Chapman and Hall, London, 1989).
Venables, W. N. & Ripley, B. D. Modern Applied Statistics with S 4th edn. (Springer, Berlin, 2002).
Bates, D. et al. Package ‘lme4’. Convergence 12(1), 2 (2015).
R Core Team. R: a language and environment for statistical computing. R Foundation for Statistical Computing. https://www.R-project.org/ (2017).
Ballesteros, E., Pérez, M. & Zabala, M. Aproximación al conocimiento de las comunidades algales de la zona infralitoral superior de la costa catalana. Collect. Bot. 15, 69–100 (1984).
Ballesteros, E., Romero, J., Gili, J. M. & Ros, J. D. L’estatge infralitoral de les Illes Medes: les algues fotófiles. In Els Sistemes Naturals de les Illes Medes (eds Ros, J. et al.) (Institut d’Estudis Catalans, Barcelona, 1984).
Toonen, R. J. & Hughes, S. Increased throughput for fragment analysis on an ABI Prism 377 automated sequencer using a membrane comb and STRand software. Biotechniques 31(6), 1320–1325 (2001).
Van Oosterhout, C., Hutchinson, W. F., Wills, D. P. & Shipley, P. MICRO-CHECKER: software for identifying and correcting genotyping errors in microsatellite data. Mol. Ecol. Notes 4(3), 535–538 (2004).
Belkhir, K., Borsa, P., Chikhi, L., Raufaste, N., & Bonhomme, F. GENETIX 4.05, logiciel sous Windows TM pour la génétique des populations. https://www.genetix.univ-montp2.fr/genetix/genetix.htm (1996–2004).
Weir, B. S. & Cockerham, C. C. Estimating F-statistics for the analysis of population structure. Evolution 38, 1358–1370 (1984).
Excoffier, L., Laval, G. & Schneider, S. Arlequin an integrated software package for population genetics data analysis. Evol. Bioinform. https://doi.org/10.1177/117693430500100003 (2005).
Pritchard, J. K., Stephens, M. & Donnelly, P. Inference of population structure using multilocus genotype data. Genetics 155(2), 945–959 (2000).
Earl, D. A. STRUCTURE HARVESTER: a website and program for visualizing STRUCTURE output and implementing the Evanno method. Conserv. Genet. Resour. 4(2), 359–361 (2012).
Evanno, G., Regnaut, S. & Goudet, J. Detecting the number of clusters of individuals using the software STRUCTURE: a simulation study. Mol. Ecol 14(8), 2611–2620 (2005).
Hereu, B., Zabala, M. & Sala, E. Multiple controls of community structure and dynamics in a sublittoral marine environment. Ecology 89(12), 3423–3435 (2008).
Medrano, A. et al. Long-term monitoring of temperate macroalgal assemblages inside and outside a No take marine reserve. Mar. Environ. Res. 153, 104826 (2019).
Nei, M. Estimation of average heterozygosity and genetic distance from a small number of individuals. Genetics 89(3), 583–590 (1978).
Acknowledgements
We thank Marc Verlaque for help on T. elegans taxonomic identification and Kike Ballesteros for his helpful and constructive discussions on this study. We also thank Mikel Zabala, Ignasi Montero-Serra, and Jordi Solà for their help on the fieldwork, and to ‘Macroalgae and invasive species monitoring group’, especially María García, for untangling the presence of T. elegans populations among their data across the Catalan coast. This work was partially funded by the long-term monitoring programme of the Natural Park of Cap de Creus and the Natural Park of Montgrí, Medes Islands, and Baix Ter protected areas (Public Agreement PTOP- 2017-130, Departament de Territori i Sostenibilitat of the Generalitat de Catalunya) and the European Union's Horizon 2020 research and innovation programme under the Grant Agreement No 689518 (MERCES) and the Grant Agreement No 730984, ASSEMBLE Plus to the Portuguese node of EMBRC-ERIC (EMBRC.PT ALG-01-0145-FEDER-022121). Cristina Linares gratefully acknowledges the financial support by ICREA under the ICREA Academia programme. Simone Mariani was supported by the Spanish INTRAMURAL CSIC PIE201730E033. Work at CCMAR was funded by FCT—Foundation for Science and Technology (Portugal) UID/Multi/04326/2019, PTDC/MAR-EST/6053/2014, Biodiversa/0004/2015, SFRH/BPD/88935/2012 (DL 57/2016/CP1361/CT0010), SFRH/BSAB/150485/2019, and a Pew Marine Fellowship. This output reflects only the authors' view, and the European Union cannot be held responsible for any use of the information contained herein that may be made.
Author information
Authors and Affiliations
Contributions
A.M., B.H., and C.L. conceived and designed the research; A.M., B.H., C.L., S.M., M.P., and G.R. conducted the field monitoring; A.M., J.N., C.P., and E.S. conducted the molecular analyses; A.M. analyzed the data and wrote the first version of the manuscript with contributions from all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Medrano, A., Hereu, B., Mariani, S. et al. Ecological traits, genetic diversity and regional distribution of the macroalga Treptacantha elegans along the Catalan coast (NW Mediterranean Sea). Sci Rep 10, 19219 (2020). https://doi.org/10.1038/s41598-020-76066-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-76066-6
This article is cited by
-
Comparative population genetics of habitat-forming octocorals in two marine protected areas: eco-evolutionary and management implications
Conservation Genetics (2024)
-
The succession of epiphytic microalgae conditions fungal community composition: how chytrids respond to blooms of dinoflagellates
ISME Communications (2023)
-
All shallow coastal habitats matter as nurseries for Mediterranean juvenile fish
Scientific Reports (2021)
-
Highly restricted dispersal in habitat-forming seaweed may impede natural recovery of disturbed populations
Scientific Reports (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.