Abstract
Various neurovascular bundle-sparing techniques have been introduced to maximize recovery of erectile function after robot-assisted radical prostatectomy (RARP). The clipless intrafascial neurovascular bundle-sparing technique aims to preserve periprostatic structures and neurovascular bundles as much as possible by avoiding clipping of the vascular pedicles. This study reports 1-year functional and oncologic outcomes and postoperative complications in 105 patients with intact preoperative erectile function who underwent a modified clipless intrafascial neurovascular bundle-sparing RARP. Intact erectile function was defined as score ≥ 21 on the Sexual Health Inventory for Men questionnaire or ability to have sexual intercourse. Median follow-up was 26.5 months (IQR 15.25–48). Postoperative erectile function recovery rates were 71.4%, 81.9%, 88.6%, 92.4%, and 94.3% at 1, 3, 6, 9, and 12 months, respectively. The rate of positive surgical margins was 16.2% overall and 11.8% in patients with stage pT2 disease. The biochemical recurrence rate was 6.7% overall. The modified clipless intrafascial neurovascular bundle-sparing technique is safe and feasible and can achieve excellent recovery of erectile function after RARP. Further large-scale prospective comparative studies are warranted.
Similar content being viewed by others
Introduction
Radical prostatectomy is arguably the most common treatment for localized prostate cancer. The goal of radical prostatectomy is to achieve complete cancer control while preserving urinary continence and sexual function. The neural pathways involved in male erectile function were first described by Walsh and Donker1. However, improvement of erectile function outcome after radical prostatectomy requires increased understanding of the anatomy of the responsible nerves. Robot-assisted radical prostatectomy (RARP), with its magnification of the operative field, allows for meticulous dissection of the periprostatic structures and preservation of the neurovascular bundles (NVBs). In efforts to preserve postoperative erectile function, various RARP surgical techniques have been studied2,3,4,5. RARP can achieve consistent functional and oncological outcomes with a low complication rate6,7. Nonetheless, erectile dysfunction after neurovascular bundle-sparing RARP remains a concern, with reported incidence rates ranging from 7.1 to 81.3% at 1-year follow-up8,9. Data from the Prostate Cancer Outcomes Study indicate that post-RARP erectile dysfunction significantly affects health-related quality of life in younger men by affecting everyday interactions with partners and perceptions of their own sexuality10.
After reviewing and employing various erectile function-preserving RARP surgical techniques in our practice, we developed a surgical technique that simultaneously uses athermal dissection and the clipless intrafascial neurovascular bundle-sparing technique. In this study, we describe this modified neurovascular bundle-sparing technique and evaluate its postoperative functional and oncologic outcomes.
Materials and methods
Study population and design
We retrospectively reviewed the electronic medical records of 218 patients with biopsy-proven prostate cancer who underwent treatment for prostate cancer at Hallym University Sacred Heart Hospital between January 2017 and December 2018. Among these patients, 175 who underwent RARP by a single experienced surgeon (> 800 RARPs) were eligible for study inclusion. Patient characteristics and perioperative and oncologic data were recorded. Inclusion criteria were as follows: (1) patient age ≤ 70 years; (2) intact preoperative erectile function, defined as a Sexual Health Inventory for Men (SHIM) questionnaire score ≥ 21; (3) localized low-risk prostate cancer with Gleason score ≤ 7 (cT1–2N0M0); (4) clipless intrafascial neurovascular bundle-sparing RARP; and (5) minimum 1-year follow-up. Exclusion criteria included insufficient data, neoadjuvant hormonal treatment, prior radiation therapy, and prior transurethral resection of the prostate; we also excluded six patients who were transferred to our institution after being diagnosed with prostate cancer in other hospitals. All patient underwent preoperative multiparametric magnetic resonance imaging (mpMRI). Finally, 105 patients were included for analysis.
The study was designed to evaluate functional and oncologic outcomes and the postoperative complications at 12 months. Data were collected in a customized database and analyzed. All methods were carried out in accordance with relevant guidelines and regulations. To evaluate the procedural learning curve, patients were divided into two subgroups based on order of treatment: early (patients 1–52) and late (patients 53–105). Study approval was obtained from the Hallym University Sacred Heart Hospital Ethics Committee (No. 2018-05-012). Written informed consent was obtained from all patients.
Data collection and outcomes
The following patient data was obtained: age, body mass index (BMI), American Society of Anesthesiologist (ASA) score, prostate volume, prostate-specific antigen (PSA) level, Gleason score of prostate biopsy specimen, and D'Amico risk classification. Baseline erectile function was assessed using an abridged Korean version of the SHIM questionnaire11. Intraoperative variables included overall operative time, estimated blood loss, blood transfusion, nerve-sparing laterality, complications (intraoperative and postoperative), and pelvic lymph node dissection (PLND). Postoperative complications were recorded and evaluated using the Clavien–Dindo classification12. Postoperative erectile function was evaluated using the SHIM questionnaire at 1, 3, 6, 9, and 12 months after RARP. Recovery of postoperative erectile function was defined as score ≥ 21 on the SHIM questionnaire or the ability to have successful sexual intercourse. Pathologic variables included pathologic cancer stage, Gleason score, and positive surgical margins (PSMs). Biochemical recurrence (BCR) was defined according to the European Association of Urology guidelines as two consecutive PSA values ≥ 0.2 ng/mL13.
Surgical techniques
All patients underwent transperitoneal RARP. Standard patient positioning and port placement have been described previously14. The da Vinci Xi Surgical System (Intuitive Surgical, Sunnyvale, CA, USA) was used in all cases.
-
1.
Athermal dissection of the vas deferens and seminal vesicles
After bladder neck dissection, we performed athermal dissection of the vas deferens and seminal vesicles using Hem-o-Lok clips or 4 mm hemoclips to avoid thermal damage to the tissue; the use of monopolar electrocautery lateral to the seminal vesicles was avoided. The artery to the vas deferens, located between the seminal vesicles and the vas deferens, was resected en bloc (Fig. 1A).
-
2.
Clipless intrafascial neurovascular bundle-sparing technique
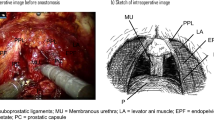
The endopelvic fascia was preserved in patients with clinical stage ≤ T2c disease. In patients with indications for clipless intrafascial neurovascular bundle-sparing, the robotic grasper arm was used to carefully expose the prostate capsule to detach the overlying fat and periprostatic fascia (Fig. 1B). Arterial pulsations from the cavernous vessels within the NVB were easily noted with further lateral dissection. These vessels were preserved by gently pushing them posterolaterally toward the rectum. The prostatic pedicles were further mobilized off the prostate capsule anteriorly to the most distal ends of the vascular pedicles (Fig. 1C). The identified vascular pedicles were then swept off the prostate, further mobilizing the NVBs, which were then gently eased off of the posterolateral surface of the prostate capsule with a combination of blunt and sharp dissection. We resumed antegrade dissection by peeling off the periprostatic fascia, NVBs, and prostatic pedicles en bloc until reaching the urethra (Fig. 1D). The use of electrocautery and clips during the nerve-sparing procedure was avoided. When bleeding from the periprostatic vessels occurred, brief pressure was applied using SURGICEL hemostatic gauze; mild venous bleeding was left uncontrolled. When pulsatile arterial bleeding was encountered, ligation was performed with 4–0 V-Loc suture. After the prostate dissection was complete, the preserved NVBs and prostatic pedicles were clearly visible and appeared thick (Fig. 2).
-
3.
PLND
PLND was performed in all patients. In patients with a > 5% risk of lymph node involvement in the Briganti nomogram, extended PLND to the common iliac artery area was performed15. Limited PLND to the obturator fossa was performed in those with estimated risk < 5%. Hem-o-Lok clips were used instead of cauterization during lymph node dissection to prevent lymphocele formation.
Operative steps. (A) The vas deferens and seminal vesicles were dissected athermally (artery of the vas deferens, white arrow). (B) The prostate capsule was exposed by detaching the overlying periprostatic fascia and prostatic pedicles. (C) The prostatic pedicles were further mobilized, including the neurovascular bundles, using antegrade dissection (distal end of the prostatic pedicles, white arrow). (D) Combined blunt and sharp dissection of the neurovascular bundles was performed as far distal to the apex as possible until the urethra was reached (prostate capsule, white star; urethra, white arrow).
Statistical analyses
Statistical analyses were conducted using SPSS Statistics for Windows version 26.0 (IBM Corp., Armonk, NY, USA). Continuous variables are reported as medians with interquartile range (IQR); categorical variables are reported as percentages. Erectile function was analyzed using the Kaplan–Meier method; its association with preoperative and intraoperative variables was tested using logistic regression analysis. Subgroup analyses were performed in patients with suspected posterolateral tumor on preoperative mpMRI. P < 0.05 was considered significant.
Results
Patient characteristics
Baseline demographic, clinical, and pathologic data for the patients are summarized in Table 1.
Operative outcomes and complications
Median operative time was 210 min (IQR, 140–230). Median intraoperative blood loss was 200 mL (IQR, 150–450); only one patient with cardiovascular disease received an intraoperative blood transfusion. Pelvic lymphadenopathy was not found in any patient. Median length of hospital stay was 7 days (IQR, 7–7). Mean number of days with urinary catheter was seven (IQR, 7–7). The overall complication rate was < 10% (Table 2). No intraoperative complications occurred. Hematoma or lymphocele that required further procedures did not occur. At 12 months follow-up, no patients reported urinary retention or leakage.
Erectile function outcomes
All patients received penile rehabilitation with regular use of oral phosphodiesterase type 5 (PDE5) inhibitors from 1 week postoperatively until recovery of erectile function. Rates of recovery of erectile function were 71.4%, 81.9%, 88.6%, 92.4%, and 94.3% at 1, 3, 6, 9, and 12 months after RARP, respectively. Figure 3 shows the Kaplan–Meier curve of erectile function recovery. Median time to recovery was 21 days (IQR, 21–90). Regarding the learning curve analysis, progressive change in the number of patients with recovered function and operative time at each time point was not recorded. Of the 105 study patients, 86 (82%) experienced recovery of erectile function by 3 months; the remaining 19 could achieve a partial erection insufficient for penetration with use of PDE5 inhibitors.
Multivariate logistic regression analysis showed a significantly higher rate of erectile function recovery at 1 and 3 months after RARP with the bilateral clipless intrafascial neurovascular bundle-sparing approach (p < 0.001). Patients with low-risk D'Amico classification had a significantly higher rate of erectile function recovery at 1, 3, and 6 months (p < 0.05). However, at 6 months, the significant independent predictors were BMI, prostate volume, low or intermediate D'Amico risk classification, and bilateral neurovascular bundle-sparing approach (p < 0.05; Table 3).
Pathologic findings and oncological results
Table 2 shows the histopathologic data. Overall, 17 of 105 patients (16.2%) exhibited PSMs on postoperative pathology; PSMs were found in the apical and posterolateral margins in nine and five patients, respectively. In the cohort of patients with stage pT2 disease, the PSMs rate was lower (11.8%; 11 of the 93 patients). Subanalysis of 30 patients with suspected posterolateral tumor on preoperative mpMRI showed posterolateral PSMs in 5 (16.7%).
Median follow-up was 26.5 months (IQR, 15.25–48). BCR occurred in seven patients (6.7%). Median PSA level at the time of BCR was 0.3 ng/mL (IQR, 0.2–0.95). Patients with BCR underwent pelvic MRI, bone scintigraphy, and chest and abdominal computed tomography. Four patients were treated with luteinizing hormone-releasing hormone. The other three patients exhibited persistently elevated or increasing PSA levels without evidence of metastasis and were treated with salvage pelvic radiotherapy and androgen deprivation therapy.
Discussion
RARP has been increasingly accepted as a surgical treatment option for younger and healthier patients with localized prostate cancer over the last decade16,17. RARP achieves consistent oncological outcomes and is associated with a low risk of complications18,19. Nevertheless, post-prostatectomy erectile dysfunction is a common adverse effect. Recent studies suggest that the course of NVBs is more complex than Walsh described previously1. Tewari et al. described that the prostate rests on a hammock-like nerve distribution, demonstrating that the NVBs are more a network of multiple finely dispersed nerves than a distinct structure20,21. Furthermore, Eichelberg et al. showed that only 46%–66% of all nerves are found in the classical posterolateral location in relation to the prostate, while 21%–29% are identified on the anterolateral surface22. Therefore, preservation of the surrounding periprostatic structures and NVBs should be attempted as much as possible. The mechanism of postoperative erectile function recovery is complex and not completely understood. However, it is universally accepted that preserving the original anatomical structures related to the prostate is the key to ensuring functional recovery. The intrafascial neurovascular bundle-sparing technique is important in determining functional outcomes.
Similar to Chien et al.23, we have been performing the athermal clipless intrafascial neurovascular bundle-sparing technique in an antegrade fashion. This approach is athermal, completely eliminating the use of monopolar electrocautery. In addition, we dissect the NVBs off the prostate in a medial to lateral direction without clipping the vascular pedicles. Although distinguishing the plane between the prostate and the periprostatic fascia where the pedicle enters can be challenging, eliminating bulk clipping of the pedicles is possible provided meticulous dissection of the pedicle vessels as they enter the prostate. We believe that eliminating the use of electrocautery and bulk clipping of the prostatic pedicles may provide better tissue preservation with the NVBs during intrafascial neurovascular bundle-sparing RARP (Supplementary Video S1).
The risk for PSMs is certainly possible when approaching the posterolateral plane using the clipless intrafascial neurovascular bundle-sparing technique. However, this technique did not appear to affect oncologic outcomes in this study. Although our overall rate of PSMs (16.2%) was higher than that noted in a previously reported study24, our rate in the cohort of patients with pT2 stage disease (11.8%) was similar to or lower than that noted in another RARP study25. In our subanalysis of 30 patients with suspected posterolateral tumor on preoperative mpMRI, posterolateral PSMs were noted in 16.7%; thus, the clipless intrafascial technique does not pose a high risk of PSMs in patients with a posterolateral tumor.
The postoperative functional outcomes in this study are promising. The clipless intrafascial neurovascular bundle-sparing technique aims to accomplish an early return to erectile function via maximal preservation of the periprostatic anatomical structures in the pelvic cavity, which is associated with functional normalcy. Using a validated sexual function SHIM questionnaire at 1 month after RARP, 71.4% of our patients returned to their baseline preoperative sexual function scores, which increased to 81.9%, 88.6%, 92.4%, and 94.3% at 3, 6, 9, and 12 months, respectively. These recovery rates are favorable compared with a previous RARP study that used the same questionnaire (53.1%, 69.9%, 82.3%, and 86.7% at 1, 3, 6, and 12 months, respectively)26. Although the surgical techniques used in the clipless intrafascial neurovascular bundle-sparing approach are not particularly novel, we found that their combined use can result in excellent postoperative functional outcomes.
Several limitations of this study include its retrospective nature, non-comparative design, and small sample size. In addition, all surgeries were performed by a single surgeon at a single institution; the surgeon’s extensive experience may have influenced functional outcomes. Nonetheless, our initial results suggest excellent erectile function recovery after antegrade clipless intrafascial neurovascular bundle-sparing RARP. A long-term large-scale multicenter study comparing erectile function after standard and clipless intrafascial neurovascular bundle-sparing RARP is necessary to validate our findings.
Conclusion
In summary, the clipless intrafascial neurovascular bundle-sparing technique is safe and feasible and can achieve excellent recovery of postoperative erectile function without compromising oncologic outcomes after RARP. Further comparative prospective studies are warranted.
References
Walsh, P. C. & Donker, P. J. Impotence following radical prostatectomy: Insight into etiology and prevention. J. Urol. 128, 492–497 (1982).
Basiri, A. et al. Comparison of retropubic, laparoscopic and robotic radical prostatectomy: WHO is the winner?. World J. Urol. 36, 609–621 (2018).
Menon, M. et al. Vattikuti Institute prostatectomy: Technical modifications in 2009. Eur. Urol. 56, 89–96 (2009).
Finley, D. S., Osann, K., Skarecky, D. & Ahlering, T. E. Hypothermic nerve-sparing radical prostatectomy: Rationale, feasibility, and effect on early continence. Urology. 73, 691–696 (2009).
Patel, V. R. et al. The role of the prostatic vasculature as a landmark for nerve sparing during robot-assisted radical prostatectomy. Eur. Urol. 61, 571–576 (2012).
Wallerstedt, A. et al. Short-term results after robot-assisted laparoscopic radical prostatectomy compared to open radical prostatectomy. Eur. Urol. 67, 660–670 (2015).
Chang, P. et al. Expanded prostate cancer index composite for clinical practice: Development and validation of a practical health related quality of life instrument for use in the routine clinical care of patients with prostate cancer. J. Urol. 186, 865–872 (2011).
Sooriakumaran, P. et al. Erectile dysfunction and oncologic outcomes following open retropubic and robot-assisted radical prostatectomy: Results from the LAParoscopic Prostatectomy Robot Open trial. Eur. Urol. 73, 618–627 (2018).
Ficarra, V. et al. Retropubic, laparoscopic, and robot-assisted radical prostatectomy: A systematic review and cumulative analysis of comparative studies. Eur. Urol. 55, 1037–1063 (2009).
Penson, D. F. et al. General quality of life 2 years following treatment for prostate cancer: What influences outcomes? Results from the prostate cancer outcomes study. J. Clin. Oncol. 21, 1147–1154 (2003).
Rosen, R. C. et al. Development and evaluation of an abridged, 5-item version of the International Index of Erectile Function (IIEF-5) as a diagnostic tool for erectile dysfunction. Int. J. Impot. Res. 11, 319–326 (1999).
Dindo, D., Demartines, N. & Clavien, P. A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 240, 205–213 (2004).
Heidenreich, A. et al. EAU guidelines on prostate cancer. Part II: treatment of advanced, relapsing, and castration-resistant prostate cancer. Eur Urol. 65, 467–479 (2014).
Porpiglia, F. et al. Total anatomical reconstruction during robot-assisted radical prostatectomy: Implications on early recovery of urinary continence. Eur. Urol. 69, 485–495 (2016).
Briganti, A. et al. Updated nomogram predicting lymph node invasion in patients with prostate cancer undergoing extended pelvic lymph node dissection: The essential importance of percentage of positive cores. Eur. Urol. 61, 480–487 (2012).
Heidenreich, A. et al. EAU guidelines on prostate cancer. Part 1: screening, diagnosis, and local treatment curative intent-update 2013. Eur. Urol. 65, 124–137 (2014).
Saika, T. et al. Role of robot-assisted radical prostatectomy in locally advanced prostate cancer. Int. J. Urol. 25, 30–35 (2018).
Bill-Axelson, A. et al. Radical prostatectomy versus watchful waiting in early prostate cancer. N. Engl. J. Med. 364, 1708–1717 (2011).
Froehner, M. et al. Perioperative complications after radical prostatectomy: Open versus robot-assisted laparoscopic approach. Urol. Int. 90, 312–315 (2013).
Tewari, A. K. et al. Improving time to continence after robot-assisted laparoscopic prostatectomy: Augmentation of the total anatomic reconstruction technique by adding dynamic detrusor cuff trigonoplasty and suprapubic tube placement. J. Endourol. 26, 1546–1552 (2012).
Tewari, A. K. et al. Anatomical grades of nerve sparing: A risk-stratified approach to neural-hammock sparing during robot-assisted radical prostatectomy (RARP). BJU Int. 108, 984–992 (2011).
Eichelberg, C. et al. Nerve distribution along the prostatic capsule. Eur. Urol. 51, 105–110 (2007).
Chien, G. W. et al. Modified clipless antegrade nerve preservation in robotic-assisted laparoscopic radical prostatectomy with validated sexual function evaluation. Urology. 66, 419–423 (2005).
Patel, V. R. et al. Pentafecta: A new concept for reporting outcomes of robot-assisted laparoscopic radical prostatectomy. Eur. Urol. 59, 702–707 (2011).
Srougi, V. et al. Surgical method influences specimen margins and biochemical recurrence during radical prostatectomy for high-risk prostate cancer: A systematic review and meta-analysis. World J. Urol. 35, 1481–1488 (2017).
de Carvalho, P. A. et al. Retrograde release of the neurovascular bundle with preservation of dorsal venous complex during robot-assisted radical prostatectomy: Optimizing functional outcomes. Eur. Urol. S0302–2838, 30481 (2018).
Acknowledgements
This research was supported by Hallym University Research Fund 2017 (HURF-2017-36).
Author information
Authors and Affiliations
Contributions
T.Y.S. interpreted the patient data and was a major contribution in writing the manuscript and performed all statistical analyses of the reported data. Y.S.L. performed protocol/project development, data collection, manuscript writing, and manuscript editing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Video S1.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shin, T.Y., Lee, Y.S. Robot-assisted radical prostatectomy with clipless intrafascial neurovascular bundle-sparing approach: surgical technique and one-year functional and oncologic outcomes. Sci Rep 10, 17595 (2020). https://doi.org/10.1038/s41598-020-74513-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-74513-y
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.