Abstract
Subthreshold depression (StD) affects people who experience clinically relevant depressive symptoms, which does not meet the diagnostic criteria for major depressive disorder (MDD). StD represents an ideal model for understanding the pathophysiological mechanisms of depression. Impaired emotion processing is a core feature of depression; careful investigation is required to better understand the neural correlates of emotion processing in depressed populations. In the current study, we explored whether the resting-state functional connectivity of the amygdala, a hub that taps a wide range of brain areas involved in emotion processing, is altered in individuals with StD when compared with healthy controls. Resting-state imaging data was collected from 59 individuals with StD and 59 age- and gender-matched controls. We found that the resting-state functional connectivity of the left amygdala with the cognitive control network and the left insula was significantly lower in people with StD than that in healthy controls. Such association was not observed in the right amygdala. Furthermore, functional connectivity strength between the left amygdala and the left precuneus was positively associated with depressive symptoms in individuals with StD. Our findings are in line with those reported in subjects with MDD, which may assist in further elucidating the pathophysiological mechanisms of depression, and contribute to the development of tailored treatments for individuals with StD who are at high risk of developing MDD.
Similar content being viewed by others
Introduction
Major depressive disorder (MDD), a debilitating psychiatric condition, is a leading cause of mortality worldwide1. Several studies have suggested that depression is best explained as a spectrum rather than a collection of discrete categories2. Minor and subthreshold depression (StD) affect people who experienced clinically relevant depressive symptoms, which do not meet the diagnosis criteria for MDD. Owing to its high incidence3 and the marked negative affect on quality of life of patients4,5, StD is increasingly becoming a greater health service burden than MDD. A longitudinal study demonstrated that individuals with StD had a fivefold increased risk of experiencing a first lifetime MDD episode compared to healthy controls6. StD is therefore regarded as an ideal model for understanding the pathophysiological mechanisms of depression and aids in the development of tailored treatments for patients with depression at different severity levels.
Deficits in emotion processing are core pathological features of MDD7. Specifically, patients with MDD tend to elicit an abnormally high level of negative emotions and abnormally low level of positive emotions8. Patients with MDD show attentional biases toward cues for sadness or dysphoria9 and have a tendency to interpret neutral or positive information negatively compared with non-depressed individuals10,11. Because these negative biases appear to have a key role in the pathophysiology and maintenance of depressive states, further careful investigation is required to better understand emotion processing in depressed populations12,13.
Depression-related emotion processing deficits have been correlated with aberrant structure and function in the affective network (AN) of the brain14. The amygdala, a critical component of the AN, is a hub that is responsible for a wide range of emotion processing functions, including emotion perception, memory, and regulation15,16. Studies have demonstrated that the connectivity of the amygdala-based networks is involved in critical functions relevant to depression including emotion regulation (through connections to the frontal and insular areas), modulation of sensory information (through connections with visual, auditory, gustatory and olfactory cortices), and processing of visceral information related to emotion stimuli (through connections with the brain stem)17,18. Importantly, depression would increase amygdala reactivity, which biases towards faster processing of negative emotion stimuli across high-order cortical areas that are involved in more complex processing19. Furthermore, depressive symptoms might sensitize affected individuals to both social rejection and social acceptance in everyday experiences20, which is associated with an increased neural reactivity of the amygdala21. Interestingly, there is evidence to support that neurofeedback from neural activity of the amygdala not only substantially reduces depressive symptoms, but also predicts reduction of depressive symptoms22,23.
The aim of the current study was to explore whether resting-state functional connectivity (rs-FC) of the amygdala was altered in individuals with StD compared with individuals without depression symptoms. Because the left and right amygdala might be involved in different emotion regulation processes24 and exhibit different functional connectivities in MDD25, we selected each side of the amygdala as an independent seed region of interest (ROI), and employed whole-brain, voxel-wise functional connectivity analyses to investigate the amygdala functional connectivity pattern in individuals with StD and healthy controls. Considering the close relationship between StD and MDD, we hypothesized that functional connectivity of the amygdala with the frontal and insular areas was impaired in individuals with StD, especially in the emotion regulation system. In addition, we also examined the association between the functional connectivity of the amygdala and self-reported depression symptoms indexed by Beck Depression Inventory within the StD group.
Results
Demographic characteristics
There were no significant differences in demographic characteristics, including age, gender, and head motion profile indexed by frame displacement between StD group and the controls (Ps > 0.05). The StD group showed higher self-reported depressive symptom scores and state-and-trait anxiety scores than the controls (Ps < 0.001). The detailed data is listed in Table 1.
Functional connectivity results
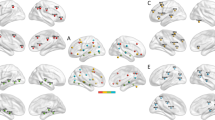
Compared with the controls, the StD group exhibited decreased functional connectivity between the left amygdala and regions of the cognitive control network, including the bilateral middle frontal gyri and the salience network, i.e. the left insula (Figs. 1, 2 and Table 2). However, there were no significant differences in the functional connectivity of the right amygdala between the StD group and controls.
Functional connectivity of the left amygdala in individuals with StD compared with healthy controls (Ctrl). (a), (c), and (e) Compared with the control, the StD group showed decreased functional connectivity between the left amygdala seed region and the left insula (a), the left middle frontal gyrus (c), and the right middle frontal gyrus (e). (b), (d), and (f) Average functional connectivity of the left insula (b), the left middle frontal gyrus (d), and the right middle frontal gyrus (f) in both groups.
We examined the association between the depressive symptoms indexed by BDI and the functional connectivity of the amygdala within the StD group. We found that depressive symptom severity was positively associated with functional connectivity between the left amygdala and left precuneus (maximum r = 0.51, Fig. 3). However, there were no significant associations between the functional connectivity map of the right amygdala and BDI scores.
Functional connectivity of the left amygdala as a function of depression severity as indexed by BDI scores. (a) For individuals with StD, higher BDI scores were associated with increased functional connectivity between the left amygdala and left precuneus. (b) Correlation between BDI and strength of functional connectivity between the left amygdala and left precuneus.
Supplementary analysis
We repeated the analysis after regressing out the global signal during data preprocessing, and found that clusters in the left insula still showed significant decreased functional connectivity with the left amygdala in the StD group compared with the controls (Supplementary Fig. S1) and the depressive symptom severity was associated with stronger connectivity between the left amygdala and left precuneus (Supplementary Fig.S2).
Discussion
In this study, we characterized the functional connectivity profile of the amygdala, a key region for emotion processing, in 59 individuals with StD and 59 age- and gender-matched controls. We found that the rs-FC of the left amygdala with the bilateral middle frontal gyri and the left insula showed a significant decrease in individuals with StD compared with that in healthy controls, which was not observed in the right amygdala. Moreover, the rs-FC between the left amygdala and left precuneus was positively associated with the BDI scores in individuals with StD. Our study demonstrated that, compared with healthy controls, the rs-FC between the amygdala and prefrontal cortex that is responsible for cognitive control, was disrupted in subjects with StD, while hyperconnectivity between the left amygdala and left precuneus was correlated with depressive symptom severity.
The bilateral middle frontal gyri are the core areas of the cognitive control network26,27, which is involved in cognitive and executive functions. Decreased amygdala functional connectivity within the cognitive control network, as well as imbalanced amygdala rsFC (both hyper and hypo-connected) within the default mode network have been observed in patients with MDD25. According to the cognitive model of depression28,29,30, patients with MDD incline to become trapped in cognitive dysregulation (e.g., memory impairment, difficulty making decisions, and loss of cognitive flexibility), negative attention focus, and negative rumination. Several studies have demonstrated that the middle frontal gyrus, part of the cognitive control network, is involved in top-down cognitive control31,32. Decreased middle frontal gyrus activation has been observed in patients with MDD when performing cognitive inhibitory processing during emotion processing33, suggesting that these patients have cognitive vulnerability to depression34. Importantly, Pannekoek et al.35 found that impaired functional connectivity between the amygdala and the cognitive control network could dysregulate top-down cognitive control from the prefrontal cortex to the amygdala and explain affective cognition processing deficits among MDD patients. Notably, at behavioral level, individuals with StD showed emotion processing deficits characterized by spending more time on negative faces and producing less accurate responses compared to controls36. At the neural level, individuals with StD showed a significant decreased functional connectivity in the cognitive control network, especially the functional connectivity of the dorsolateral prefrontal cortex with the insula and the regions associated with the temporo-parietal junction compared with controls37. Additionally, Li et al.38 found that the failure of successful response inhibition could lead to a reduced activation of the prefrontal cortex in response to negative stimuli in individuals with StD. Therefore, impaired connectivity between the left amygdala and bilateral middle frontal gyri could help explain why individuals with StD show cognitive inhibition deficits in emotion processing.
In addition to the aberrant connectivity between the amygdala and the cognitive control network, we also found that compared with controls, individuals with StD exhibited decreased functional connectivity between the left amygdala and the left insula. The insula is a region that underpins the processing of interoceptive states39, and has functional interconnections to regions associated with the experience of emotion40, hubs of the default mode, cognitive control, and frontostriatal networks41. Hence, it is crucial to generate a current emotion awareness state for the integration of stimulus-driven, bottom-up interoceptive signals with top-down predictions. Notably, patients with MDD exhibit heightened interoceptive awareness, which affects their ability to filter both exogenous and endogenous stimuli for adaptive regulation, such as an increase in negative self-focused thought, or rumination that impairs the shifting from internally focused to externally focused attention in individuals with MDD. Studies have suggested that aberrant functional connectivity between the amygdala and insula can lead to stronger anticipation of negative events and a tighter functional link between visceral perception and emotional response42. He et al.43 also demonstrated that individuals with StD presented negative anticipation and negative attention bias. Moreover, longitudinal studies have also demonstrated that individuals at ultra-high risk of developing an affective disorder, and who subsequently transition to an affective disorder, have reduced volumes in the insula and amygdala, thereby providing further evidence that these regions may be trait markers for affective disorders44. Our finding suggests the decreased amygdala–insula functional connectivity at rest might play a role for maladaptive coupling of emotion processing and autonomic regulation in StD.
We also found that the strength of the functional connectivity between the amygdala and left precuneus positively correlated with the BDI scores. The precuneus is linked to reflective self-awareness45,46, and is reliably activated in MDD when instructed with self-related information14. Importantly, increased functional connectivity between the amygdala and the precuneus has been frequently reported in MDD (see meta-analysis25), and this pattern has been associated with recursive self-referential thinking pattern characterized by increased responses to negative stimuli47,48. Our finding that there was a positive association between the amygdala and precuneus indicates that StD may be a suitable model for exploring the pathophysiological mechanisms of depression; moreover, this model may aid in the development of tailored treatments for patients at different stages of the disorder.
Interestingly, we found that the functional lateralization of amygdala connectivity in individuals with StD was limited to the left amygdala. A previous report had indicated that the left amygdala was more frequently activated than the right amygdala during emotion processing49. Moreover, Wager et al.50 demonstrated that under negative-value emotional stimuli, the neural activity pattern of the amygdala showed lateralization. Although the right amygdala was reported to be activated when encoding an emotional stimulus51, different study showed that top-down regulation may involve only the left amygdala, whereas bottom-up responses involve both left and right52. Considering the cognitive model of depression28,29,30, our findings further suggest that depressed individuals have cognitive inhibition deficits in emotion processing. Nevertheless, the results of our study suggest that using only the left or the right amygdala as the seed, or combining the results from both sides, may lead to potential differences being overlooked.
In the current study, we only recruited individuals with StD and age- and gender-matched controls to investigate the rs-FC of the amygdala. Although our findings are similar to those observed in MDD, future studies should directly explore potential differences in the amygdala between individuals with StD and MDD. Moreover, studies have suggested that StD may be a risk factor for the development of MDD, it is important to investigate the neural signatures associated with transitioning between mild/moderate depression and severe depression, through a longitudinal study following subjects with StD to recovery or full-blown MDD.
In conclusion, we mapped the functional connectivity profile of the amygdala in individuals with StD, and found that compared with healthy controls, the functional connectivity between the left amygdala and both the cognitive control network and left insula was significantly decreased in individuals with StD, which was not observed in the right amygdala. Moreover, the rs-FC between the left amygdala and the left precuneus was positively associated with the BDI scores in individuals with StD. These findings are similar to those reported in patients with MDD, indicating both emotion processing and emotional regulation deficits in depression.
Methods
Participants
All data used in this study were obtained from the Southwest University Longitudinal Imaging Multimodal (SLIM) Brain Data Repository (https://fcon-1000.projects.nitrc.org/indi/retro/southwestuni-qiu-index.html); this is a project focuses on the neuroscience of creativity and affective disorders, and was approved by the Research Ethics Committee of the Brain Imaging Center of Southwest University53. Informed written consent was obtained from each participant. The data acquisition protocol was carried out in accordance with the Declaration of Helsinki revised in 1989. All the participants in this project were Chinese undergraduate students with no history of psychiatric or neurological disorders. None of the participants fulfilled the DSM-IV criteria for psychiatric disorders, as assessed by two well-trained and experienced graduate students from the School of Psychology. The participants reported no history of head trauma. On the scanning day, no serious physical illness or use of medication (including antidepressant drugs) that would interfere with brain function was detected. Notably, SLIM data has been used in several scientific studies54,55,56.
In this study, the Beck Depression Inventory (BDI) was used to screen depressive symptoms in this cohort. Among all the participants, StD group comprised 59 young adults (29 males/30 females, mean age 20.12 ± 1.39 years), who exhibited either mild (score of 14–18) or moderate (score of 19–29) depressive symptoms, with a mean score of 17.52 ± 3.43. The control group comprised of 59 age-matched and gender-matched subjects with a mean BDI score of 3.48 ± 1.92 (28 male/31 females, mean age: 19.95 ± 1.42), who were selected from the same database. In addition, state and trait anxiety were also assessed based on the State and Trait Anxiety Inventory57. The demographic and clinical characteristics of the participants are summarized in Table1.
Data acquisition
Imaging data was acquired on a Siemens 3-T MAGNETOM TrioTim System (Siemens, Erlangen, Germany) at West China Hospital of Sichuan University, Chengdu, China. The resting-state BOLD signals were acquired as an 8-min scan of 242 contiguous frames. Data acquisition parameters were as follows: slices = 32; repetition time/echo time = 2000/30 ms; flip angle = 90°; field of view (FOV) = 220 × 220 mm; thickness/slice gap = 3/1 mm; and voxel size = 3.4 × 3.4 × 4 mm3. T1 weighted anatomical images were collected, with a recorded repetition time of 1900 ms, an echo time of 2.52 ms, an inversion time of 900 ms, a flip angle of 90°, FOV = 256 × 256, 176 slices with a thickness of 1.0 mm, and a voxel size = 1 × 1 × 1 mm3.
Image preprocessing
Resting state fMRI data was preprocessed using Data Processing & Analysis for (Resting‐State) Brain Imaging (DPABI, https://rfmri.org/dpabi) software58 with following steps: discarding the first 10 volumes; slice timing correction; head motion correction; spatially normalized to the Montreal Neurological Institute (MNI) template with the voxel size into 3 mm × 3 mm × 3 mm; spatially smoothing with a 6 mm full width half maximum (FWHM) Gaussian kernel to improve the signal to noise ratio; regressing out the linear trend signal, 24 head motion parameters and the first 5 principal components from a combined white matter/cerebrospinal fluid signals mask with CompCor method59; and band-pass filtering (0.01–0.1 Hz) to remove spurious fluctuations in functional connectivity. In addition, scrubbing was performed when Power frame displacement (FD) was found to be greater than 0.5 at a specific time-point. The time points before and after each time-point with FD > 0.5 were scrubbed using each of this time-point with FD > 0.5 as a regressor60. Because global signal regression could increase the number of negative functional connectivity and the global signal might be beneficial for understanding clinical populations61,62, the global signal was not used as a nuisance regressor in the preprocessing steps.
Seed based functional connectivity
Functional connectivity analysis for each subject was carried out in DPARSF by applying a seed-based approach. Seeds were derived from the automated anatomical labeling atlas from Wake Forest University PickAtlas toolbox (https://www.nitrc.org/projects/wfu_pickatlas). To address the hemisphere effect, analysis of functional connectivity for the left and right amygdala was performed separately, as previously suggested63. Figure 1 shows coronal view of the left and right amygdala ROIs as applied to each of the subject.
The averaged time course was obtained from the seed, and the correlation analysis was performed in a voxel-wise manner to generate the functional connectivity map. The correlation coefficient maps were transformed into Fisher’s Z maps using Fisher's R-to-Z transformation to improve the normality. To investigate functional connectivity of the amygdala at group level, individual Fisher’s Z functional connectivity maps obtained from the functional connectivity analysis in DPARSF were used in the second-level analysis; this was performed by between-group voxel-wise t-tests using DPABI. A correlation analysis between BDI scores and functional connectivity of the amygdala at each voxel was conducted to examine the relationship between depression severity and functional connectivity within the StD group. A threshold of voxel-wise p < 0.001 and cluster-level p < 0.05 family-wise error correction were used for all rs-FC analyses, which is recommended by previous studies64,65.
Ethical approval
This study was approved by the Research Ethics Committee of the Brain Imaging Center of Southwest University.
Informed consent
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000. Informed consent was obtained from all participants for being included in the study.
References
Organization, W. H. Depression and other common mental disorders: global health estimates (World Health Organization, Geneva, 2017).
Adam, D. Mental health: on the spectrum. Nat. News 496, 416 (2013).
Lee, Y. et al. The risk of developing major depression among individuals with subthreshold depression: a systematic review and meta-analysis of longitudinal cohort studies. Psychol. Med. 49, 92–102 (2019).
Rodríguez, M. R., Nuevo, R., Chatterji, S. & Ayuso-Mateos, J. L. Definitions and factors associated with subthreshold depressive conditions: a systematic review. BMC Psychiat. 12, 181 (2012).
Tuithof, M. et al. Course of subthreshold depression into a depressive disorder and its risk factors. J. Affect. Disord. 241, 206–215 (2018).
Fogel, J., Eaton, W. W. & Ford, D. E. Minor depression as a predictor of the first onset of major depressive disorder over a 15-year follow-up. Acta Psychiatr. Scand. 113, 36–43 (2006).
Beck, A. The evolution of the cognitive model of depression and its neurobiological correlates. Am. J. Psychiat. 165, 969–977 (2008).
Jones, E. B. & Sharpe, L. Cognitive bias modification: a review of meta-analyses. J. Affect. Disord. 223, 175–183 (2017).
Gotlib, I. H. et al. Coherence and specificity of information-processing biases in depression and social phobia. J. Abnorm. Psychol. 113, 386–398 (2004).
Everaert, J., Podina, I. R. & Ehw, K. A comprehensive meta-analysis of interpretation biases in depression. Clin. Psychol. Rev. 58, S0272735817303963 (2017).
Platt, B., Waters, A. M., Schulte-Koerne, G., Engelmann, L. & Salemink, E. A review of cognitive biases in youth depression: attention, interpretation and memory. Cogn. Emot. 31, 1–22 (2016).
Mennen, A. C., Norman, K. A. & Turk-Browne, N. B. Attentional bias in depression: understanding mechanisms to improve training and treatment. Curr. Opin. Psychol. 29, 266–273. https://doi.org/10.1016/j.copsyc.2019.07.036 (2019).
Clark, D. A. & Beck, A. T. Cognitive theory and therapy of anxiety and depression: convergence with neurobiological findings. Trends Cognit. Sci. 14, 418–424 (2010).
Kaiser, R. H., Andrews-Hanna, J. R., Wager, T. D. & Pizzagalli, D. A. Large-scale network dysfunction in major depressive disorder: a meta-analysis of resting-state functional connectivity. JAMA Psychiat. 72, 603–611. https://doi.org/10.1001/jamapsychiatry.2015.0071 (2015).
Gilles, P., Antonio, S. & Patrik, V. Brain mechanisms for emotional influences on perception and attention: what is magic and what is not. Biol. Psychol. 92, 492–512 (2013).
Bickart, K. C., Dickerson, B. C. & Barrett, L. F. The amygdala as a hub in brain networks that support social life. Neuropsychologia 63, 235–248. https://doi.org/10.1016/j.neuropsychologia.2014.08.013 (2014).
Price, J. L. Comparative aspects of amygdala connectivity. Ann. N. Y. Acad. Sci. 985, 50–58. https://doi.org/10.1111/j.1749-6632.2003.tb07070.x (2003).
Barbas, H., Zikopoulos, B. & Timbie, C. Sensory pathways and emotional context for action in primate prefrontal cortex. Biol. Psychiat. 69, 1133–1139. https://doi.org/10.1016/j.biopsych.2010.08.008 (2011).
Disner, S. G., Beevers, C. G., Haigh, E. A. P. & Beck, A. T. Neural mechanisms of the cognitive model of depression. Nat. Rev. Neurosci. 12, 467 (2011).
Platt, B., CohenKadosh, K. & Lau, J. Y. The role of peer rejection in adolescent depression. Depress. Anxiety 30, 809–821. https://doi.org/10.1002/da.22120 (2013).
Godlewska, B. R., Browning, M., Norbury, R., Cowen, P. J. & Harmer, C. J. Early changes in emotional processing as a marker of clinical response to SSRI treatment in depression. Transl. Psychiat. 6, e957–e957. https://doi.org/10.1038/tp.2016.130 (2016).
Young, K. D. et al. Randomized clinical trial of real-time fMRI amygdala neurofeedback for major depressive disorder: effects on symptoms and autobiographical memory recall. Am. J. Psychiat. 174, 748–755 (2017).
Han, Y. et al. Resting-state functional connectivity modulation and sustained changes after real-time functional magnetic resonance imaging neurofeedback training in depression. Brain Connect. 4, 690 (2014).
Jung, Y. H. et al. Altered amygdala resting-state functional connectivity and hemispheric asymmetry in patients with social anxiety disorder. Front Psychiat. 9, 164 (2018).
Tang, S. et al. Abnormal amygdala resting-state functional connectivity in adults and adolescents with major depressive disorder: A comparative meta-analysis. EBioMedicine 36, 436–445. https://doi.org/10.1016/j.ebiom.2018.09.010 (2018).
Cole, M. W. & Schneider, W. The cognitive control network: integrated cortical regions with dissociable functions. NeuroImage 37, 343–360 (2007).
Cole, M. W. et al. Multi-task connectivity reveals flexible hubs for adaptive task control. Nat. Neurosci. 16, 1348 (2013).
Disner, S. G., Beevers, C. G., Haigh, E. A. & Beck, A. T. Neural mechanisms of the cognitive model of depression. Nat. Rev. Neurosci. 12, 467 (2011).
Beck, A. T. & Bredemeier, K. A unified model of depression: Integrating clinical, cognitive, biological, and evolutionary perspectives. Clin. Psychol. Sci. 4, 596–619 (2016).
Grahek, I., Everaert, J., Krebs, R. M. & Koster, E. H. Cognitive control in depression: toward clinical models informed by cognitive neuroscience. Clin. Psychol. Sci. 6, 464–480 (2018).
Inzlicht, M., Bartholow, B. D. & Hirsh, J. B. Emotional foundations of cognitive control. Trends Cognit. Sci. 19, 126–132 (2015).
Ochsner, K. N. & Gross, J. J. The cognitive control of emotion. Trends Cognit. Sci. 9, 242–249 (2005).
Salehinejad, M. A., Ghanavai, E., Rostami, R. & Nejati, V. Cognitive control dysfunction in emotion dysregulation and psychopathology of major depression (MD): evidence from transcranial brain stimulation of the dorsolateral prefrontal cortex (DLPFC). J. Affect. Disord. 210, 241–248 (2017).
Joormann, J. & Tanovic, E. Cognitive vulnerability to depression: examining cognitive control and emotion regulation. Curr. Opin. Psychol. 4, 86–92 (2015).
Pannekoek, J. N. et al. Aberrant resting-state functional connectivity in limbic and salience networks in treatment-naïve clinically depressed adolescents. J. Child Psychol. Psychiatry 55, 1317–1327. https://doi.org/10.1111/jcpp.12266 (2014).
36Chen, X., Feng, Z. & Jiang, J. Effect of strengthened or weakened emotional processing on cognitive control in individuals with subthreshold depression: an event-related potential study. Acta Academiae Medicinae Militaris Tertiae17 (2016).
Hwang, J. et al. Subthreshold depression is associated with impaired resting-state functional connectivity of the cognitive control network. Transl. Psychiat. 5, e683 (2015).
Li, H., Wei, D., Sun, J., Zhang, Q. & Qiu, J. Fronto-limbic alterations in negatively biased attention in young adults with subthreshold depression. Front. Psychol. 8, 1354 (2017).
Kurth, F., Zilles, K., Fox, P. T., Laird, A. R. & Eickhoff, S. B. A link between the systems: functional differentiation and integration within the human insula revealed by meta-analysis. Brain Struct. Funct. 214, 519–534 (2010).
Wager, T. D., Davidson, M. L., Hughes, B. L., Lindquist, M. A. & Ochsner, K. N. Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron 59, 1037–1050 (2008).
Tye, K. M., Stuber, G. D., de Ridder, B., Bonci, A. & Janak, P. H. Rapid strengthening of thalamo-amygdala synapses mediates cue–reward learning. Nature 453, 1253 (2008).
Sripada, R. K. et al. Altered resting-state amygdala functional connectivity in men with posttraumatic stress disorder. J. Psychiat. Neurosci. JPN 37, 241 (2012).
He, Z., Zhang, D., Muhlert, N. & Elliott, R. Neural substrates for anticipation and consumption of social and monetary incentives in depression. Soc. Cognit. Affect. Neurosci (2019).
Bechdolf, A. et al. Amygdala and insula volumes prior to illness onset in bipolar disorder: a magnetic resonance imaging study. Psychiat. Res. Neuroimaging 201, 34–39. https://doi.org/10.1016/j.pscychresns.2011.06.010 (2012).
Margulies, D. S. et al. Precuneus shares intrinsic functional architecture in humans and monkeys. Proc. Natl. Acad. Sci. 106, 20069–20074 (2009).
46Li, R. et al. developmental maturation of the Precuneus as a functional core of the default mode network. Journal of cognitive neuroscience, 1–14 (2019).
Nejad, A. B., Fossati, P. & Lemogne, C. Self-referential processing, rumination, and cortical midline structures in major depression. Front. Hum. Neurosci. 7, 666 (2013).
Sheline, Y. I. et al. The default mode network and self-referential processes in depression. Proc. Natl. Acad. Sci. 106, 1942–1947 (2009).
Baas, D., Aleman, A. & Kahn, R. S. Lateralization of amygdala activation: a systematic review of functional neuroimaging studies. Brain Res. Rev. 45, 96–103 (2004).
Wager, T. D., Phan, K. L., Liberzon, I. & Taylor, S. F. Valence, gender, and lateralization of functional brain anatomy in emotion: a meta-analysis of findings from neuroimaging. Neuroimage 19, 513–531 (2003).
Vasa, R. A. et al. Enhanced right amygdala activity in adolescents during encoding of positively valenced pictures. Dev. Cognit. Neurosci. 1, 88–99 (2011).
Ochsner, K. N. et al. Bottom-up and top-down processes in emotion generation: common and distinct neural mechanisms. Psychol. Sci. 20, 1322–1331 (2009).
Wang, J. et al. Electroconvulsive therapy selectively enhanced feedforward connectivity from fusiform face area to amygdala in major depressive disorder. Soc. Cogn. Affect. Neurosci. 12, 1983–1992 (2017).
Horien, C., Shen, X., Scheinost, D. & Constable, R. T. The individual functional connectome is unique and stable over months to years. NeuroImage 189, 676–687 (2019).
Sherbaf, F. G., Same, K., Ashraf-Ganjouei, A. & Aarabi, M. H. Altered white matter microstructure associated with mild and moderate depressive symptoms in young adults, a diffusion tensor imaging study. NeuroReport 29, 685–689 (2018).
Osamu, A. et al. Voxel-based analyses of gray/white matter volume and diffusion tensor data in major depression. Psychiat. Res. 181, 64–70 (2010).
Shek, D. T. L. The Chinese version of the State-Trait Anxiety Inventory: Its relationship to different measures of psychological well-being. J. Clin. Psychol. 49, 349–358. https://doi.org/10.1002/1097-4679(199305)49:3%3c349::aid-jclp2270490308%3e3.0.co;2-j (1993).
Amico, F. et al. Structural MRI correlates for vulnerability and resilience to major depressive disorder. J. Psychiat. Neurosci. Jpn 36, 15 (2011).
Behzadi, Y., Restom, K., Liau, J. & Liu, T. T. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. NeuroImage 37, 90–101. https://doi.org/10.1016/j.neuroimage.2007.04.042 (2007).
Power, J. D., Barnes, K. A., Snyder, A. Z., Schlaggar, B. L. & Petersen, S. E. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage 59, 2142–2154 (2012).
Bergouignan, L. et al. Can voxel based morphometry, manual segmentation and automated segmentation equally detect hippocampal volume differences in acute depression?. Neuroimage 45, 29–37. https://doi.org/10.1016/j.neuroimage.2008.11.006 (2009).
Chaney, A. et al. Effect of childhood maltreatment on brain structure in adult patients with major depressive disorder and healthy participants. J. Psychiatry Neurosci. 38, 120208 (2013).
Tang, S. et al. Abnormal amygdala resting-state functional connectivity in adults and adolescents with major depressive disorder: a comparative meta-analysis. EBioMedicine 36, 436–445 (2018).
Eklund, A., Nichols, T. E. & Knutsson, H. Cluster failure: Why fMRI inferences for spatial extent have inflated false-positive rates. Proc. Natl. Acad. Sci. U.S.A. 113, 7900–7905. https://doi.org/10.1073/pnas.1602413113 (2016).
Woo, C.-W., Krishnan, A. & Wager, T. D. Cluster-extent based thresholding in fMRI analyses: pitfalls and recommendations. NeuroImage 91, 412–419. https://doi.org/10.1016/j.neuroimage.2013.12.058 (2014).
Funding
This study was supported by Nature Science Foundation of China (ref: 31900806). The funding organizations played no further role in study design, data collection, analysis and interpretation, and paper writing.
Author information
Authors and Affiliations
Contributions
R.Z design the study. X.P., W.L, and R.Z. analyzed, and wrote the main manuscript text and C.W and N. L prepared figures 1-3. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Peng, X., Lau, W.K.W., Wang, C. et al. Impaired left amygdala resting state functional connectivity in subthreshold depression individuals. Sci Rep 10, 17207 (2020). https://doi.org/10.1038/s41598-020-74166-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-74166-x
This article is cited by
-
Association of executive function with suicidality based on resting-state functional connectivity in young adults with subthreshold depression
Scientific Reports (2023)
-
Functional activation of insula and dorsal anterior cingulate for conflict control against larger monetary loss in young adults with subthreshold depression: a preliminary study
Scientific Reports (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.