Abstract
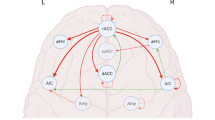
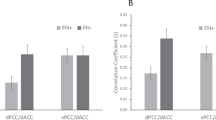
Neural markers of pathophysiological processes underlying the dimension of subsyndromal-syndromal-level depression severity can provide objective, biologically informed targets for novel interventions to help prevent the onset of depressive and other affective disorders in individuals with subsyndromal symptoms, and prevent worsening symptom severity in those with these disorders. Greater functional connectivity (FC) among the central executive network (CEN), supporting emotional regulation (ER) subcomponent processes such as working memory (WM), the default mode network (DMN), supporting self-related information processing, and the salience network (SN), is thought to interfere with cognitive functioning and predispose to depressive disorders. We examined in young adults (1) relationships among activity and FC in these networks and current depression severity, using a paradigm designed to examine WM and ER capacity in n = 90, age = 21.7 (2.0); (2) the extent to which these relationships were specific to depression versus mania/hypomania; (3) whether findings in a first, “discovery” sample could be replicated in a second, independent, “test” sample of young adults n = 96, age = 21.6 (2.1); and (4) whether such relationships also predicted depression at up to 12 months post scan and/or mania/hypomania severity in (n = 61, including participants from both samples, age = 21.6 (2.1)). We also examined the extent to which there were common depression- and anxiety-related findings, given that depression and anxiety are highly comorbid. In the discovery sample, current depression severity was robustly predicted by greater activity and greater positive functional connectivity among the CEN, DMN, and SN during working memory and emotional regulation tasks (all ps < 0.05 qFDR). These findings were specific to depression, replicated in the independent sample, and predicted future depression severity. Similar neural marker–anxiety relationships were shown, with robust DMN–SN FC relationships. These data help provide objective, neural marker targets to better guide and monitor early interventions in young adults at risk for, or those with established, depressive and other affective disorders.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
US Dept of Health and Human Services. Substance Abuse and Mental Health Services Administration. Results from the 2006 National Survey on Drug Use and Health: National Findings (Office of Applied Studies, NSDUH Series H-32, DHHS Publication No. SMA 07-4293). Rockville, MD; 2007.
Benasi G, Fava GA, Guidi J. Prodromal symptoms in depression: a systematic review. Psychother Psychosom. 2021;90:365–72.
Insel TR. The NIMH research domain criteria (RDoC) project: precision medicine for psychiatry. Am J Psychiatry. 2014;171:395–7.
Groeschel S, Vollmer B, King M, Connelly A. Developmental changes in cerebral grey and white matter volume from infancy to adulthood. Int J Dev Neurosci. 2010;28:481–9.
Østby Y, Tamnes CK, Fjell AM, Westlye LT, Due-Tønnessen P, Walhovd KB. Heterogeneity in subcortical brain development: a structural magnetic resonance imaging study of brain maturation from 8 to 30 years. J Neurosci. 2009;29:11772–82.
Tamnes CK, Østby Y, Fjell AM, Westlye LT, Due-Tønnessen P, Walhovd KB. Brain maturation in adolescence and young adulthood: regional age-related changes in cortical thickness and white matter volume and microstructure. Cereb Cortex. 2010;20:534–48.
Rose E, Ebmeier K. Pattern of impaired working memory during major depression. J Affect Disord. 2006;90:149–61.
Darke S. Anxiety and working memory capacity. Cognition Emot. 1988;2:145–54.
Thompson JM, Gray JM, Hughes JH, Watson S, Young AH, Nicol Ferrier I. Impaired working memory monitoring in euthymic bipolar patients. Bipolar Disord. 2007;9:478–89.
Townsend J, Altshuler LL. Emotion processing and regulation in bipolar disorder: a review. Bipolar Disord. 2012;14:326–39.
Joormann J, Stanton CH. Examining emotion regulation in depression: a review and future directions. Behav Res Ther. 2016;86:35–49.
Cisler JM, Olatunji BO, Feldner MT, Forsyth JP. Emotion regulation and the anxiety disorders: an integrative review. J Psychopathol Behav Assess. 2010;32:68–82.
Phillips ML, Ladouceur CD, Drevets WC. A neural model of voluntary and automatic emotion regulation: implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Mol Psychiatry. 2008;13:833–57.
LeMoult J, Carver CS, Johnson SL, Joormann J. Predicting change in symptoms of depression during the transition to university: the roles of BDNF and working memory capacity. Cogn Affect Behav Neurosci. 2015;15:95–103.
Barkus E. Effects of working memory training on emotion regulation: transdiagnostic review. PsyCh J. 2020;9:258–79.
Joormann J, Gotlib IH. Emotion regulation in depression: relation to cognitive inhibition. Cognition Emot. 2010;24:281–98.
Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychological Rev. 2001;108:624.
Liston C, Matalon S, Hare TA, Davidson MC, Casey B. Anterior cingulate and posterior parietal cortices are sensitive to dissociable forms of conflict in a task-switching paradigm. Neuron. 2006;50:643–53.
Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–56.
Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci USA. 2001;98:676–82.
Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proc Natl Acad Sci USA. 2001;98:4259–64.
Miller CH, Hamilton JP, Sacchet MD, Gotlib IH. Meta-analysis of functional neuroimaging of major depressive disorder in youth. JAMA Psychiatry. 2015;72:1045–53.
Dai L, Zhou H, Xu X, Zuo Z. Brain structural and functional changes in patients with major depressive disorder: a literature review. PeerJ. 2019;7:e8170.
Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn Sci. 2011;15:483–506.
Chai WJ, Abd Hamid AI, Abdullah JM. Working memory from the psychological and neurosciences perspectives: a review. Front Psychol. 2018;9:401.
Utevsky AV, Smith DV, Huettel SA. Precuneus is a functional core of the default-mode network. J Neurosci. 2014;34:932–40.
Raichle ME, Snyder AZ. A default mode of brain function: a brief history of an evolving idea. Neuroimage. 2007;37:1083–90.
Buckner RL, Andrews‐Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38.
Raichle ME. The brain’s default mode network. Annu Rev Neurosci. 2015;38:433–47.
Taylor SF, Stern ER, Gehring WJ. Neural systems for error monitoring: recent findings and theoretical perspectives. Neuroscientist. 2007;13:160–72.
Gu H, Hu Y, Chen X, He Y, Yang Y. Regional excitation-inhibition balance predicts default-mode network deactivation via functional connectivity. Neuroimage. 2019;185:388–97.
Piccoli T, Valente G, Linden DE, Re M, Esposito F, Sack AT, et al. The default mode network and the working memory network are not anti-correlated during all phases of a working memory task. PLoS One. 2015;10:e0123354.
Huang AS, Klein DN, Leung H-C. Load-related brain activation predicts spatial working memory performance in youth aged 9–12 and is associated with executive function at earlier ages. Dev Cogn Neurosci. 2016;17:1–9.
Satterthwaite TD, Wolf DH, Erus G, Ruparel K, Elliott MA, Gennatas ED, et al. Functional maturation of the executive system during adolescence. J Neurosci. 2013;33:16249–61.
Tomasi D, Ernst T, Caparelli EC, Chang L. Common deactivation patterns during working memory and visual attention tasks: an intra‐subject fMRI study at 4 Tesla. Hum Brain Mapp. 2006;27:694–705.
Zuo N, Salami A, Yang Y, Yang Z, Sui J, Jiang T. Activation‐based association profiles differentiate network roles across cognitive loads. Hum Brain Mapp. 2019;40:2800–12.
Fuentes-Claramonte P, Martín-Subero M, Salgado-Pineda P, Alonso-Lana S, Moreno-Alcázar A, Argila-Plaza I, et al. Shared and differential default-mode related patterns of activity in an autobiographical, a self-referential and an attentional task. PLoS One. 2019;14:e0209376.
Čeko M, Gracely JL, Fitzcharles M-A, Seminowicz DA, Schweinhardt P, Bushnell MC. Is a responsive default mode network required for successful working memory task performance? J Neurosci. 2015;35:11595–605.
Dedovic K, Slavich GM, Muscatell KA, Irwin MR, Eisenberger NI. Dorsal anterior cingulate cortex responses to repeated social evaluative feedback in young women with and without a history of depression. Front Behav Neurosci. 2016;10:64.
Han DH, Kim SM, Bae S, Renshaw PF, Anderson JS. A failure of suppression within the default mode network in depressed adolescents with compulsive internet game play. J Affect Disord. 2016;194:57–64.
Vilgis V, Gelardi KL, Helm JL, Forbes EE, Hipwell AE, Keenan K, et al. Dorsomedial prefrontal activity to sadness predicts later emotion suppression and depression severity in adolescent girls. Child Dev. 2018;89:758–72.
Zeng C, Ross B, Xue Z, Huang X, Wu G, Liu Z, et al. Abnormal large-scale network activation present in bipolar mania and bipolar depression under resting state. Front Psychiatry. 2021;12:634299.
Roy AK, Bennett R, Posner J, Hulvershorn L, Castellanos FX, Klein RG. Altered intrinsic functional connectivity of the cingulate cortex in children with severe temper outbursts. Dev Psychopathol. 2018;30:571–9.
Fournier JC, Bertocci M, Ladouceur CD, Bonar L, Monk K, Abdul-Waalee H, et al. Neural function during emotion regulation and future depressive symptoms in youth at risk for affective disorders. Neuropsychopharmacology. 2021;46:1340–7.
Whitfield-Gabrieli S, Thermenos HW, Milanovic S, Tsuang MT, Faraone SV, McCarley RW, et al. Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proc Natl Acad Sci USA. 2009;106:1279–84.
Shapero BG, Chai XJ, Vangel M, Biederman J, Hoover CS, Whitfield-Gabrieli S, et al. Neural markers of depression risk predict the onset of depression. Psychiatry Res Neuroimaging. 2019;285:31–39.
Ho TC, Connolly CG, Blom EH, LeWinn KZ, Strigo IA, Paulus MP, et al. Emotion-dependent functional connectivity of the default mode network in adolescent depression. Biol Psychiatry. 2015;78:635–46.
Ho TC, Walker JC, Teresi GI, Kulla A, Kirshenbaum JS, Gifuni AJ, et al. Default mode and salience network alterations in suicidal and non-suicidal self-injurious thoughts and behaviors in adolescents with depression. Transl Psychiatry. 2021;11:1–14.
Woodward TS, Metzak PD, Meier B, Holroyd CB. Anterior cingulate cortex signals the requirement to break inertia when switching tasks: a study of the bivalency effect. Neuroimage. 2008;40:1311–8.
Shao J, Meng C, Tahmasian M, Brandl F, Yang Q, Luo G, et al. Common and distinct changes of default mode and salience network in schizophrenia and major depression. Brain Imaging Behav. 2018;12:1708–19.
Webb CA, Israel ES, Belleau E, Appleman L, Forbes EE, Pizzagalli DA. Mind-wandering in adolescents predicts worse affect and is linked to aberrant default mode network–salience network connectivity. J Am Acad Child Adolesc Psychiatry. 2021;60:377–87.
Guha A, Yee CM, Heller W, Miller GA. Alterations in the default mode‐salience network circuit provide a potential mechanism supporting negativity bias in depression. Psychophysiology. 2021;58:e13918.
Jiang Y, Duan M, Chen X, Chang X, He H, Li Y, et al. Common and distinct dysfunctional patterns contribute to triple network model in schizophrenia and depression: a preliminary study. Prog Neuro Psychopharmacol Biol Psychiatry. 2017;79:302–10.
Geller WN, Liu K, Warren SL. Specificity of anhedonic alterations in resting-state network connectivity and structure: a transdiagnostic approach. Psychiatry Res Neuroimaging. 2021;317:111349.
Hirschfeld RM. The comorbidity of major depression and anxiety disorders: recognition and management in primary care. Prim Care Companion J Clin Psychiatry. 2001;3:244.
Kaufman J, Charney D. Comorbidity of mood and anxiety disorders. Depression Anxiety. 2000;12:69–76.
Marek S, Tervo-Clemmens B, Calabro FJ, Montez DF, Kay BP, Hatoum AS, et al. Reproducible brain-wide association studies require thousands of individuals. Nature. 2022;603:654–60.
Tejavibulya L, Rolison M, Gao S, Liang Q, Peterson H, Dadashkarimi J, et al. Predicting the future of neuroimaging predictive models in mental health. Mol Psychiatry. 2022;27:3129–37.
Wagenmakers E-J, Sarafoglou A, Aczel B. One statistical analysis must not rule them all. Nature Publishing Group; 2022.
Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62.
Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32:50–5.
Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–35.
Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98.
Blair JR, Spreen O. Predicting premorbid IQ: a revision of the national adult reading test. Clin Neuropsychologist. 1989;3:129–36.
Annett M. A classification of hand preference by association analysis. Br J Psychol. 1970;61:303–21.
First MB, Williams JBW, Karg RS, Spitzer RL. Structured clinical interview for DSM-5—research version (SCID-5 for DSM-5, Research Version; SCID-5-RV). American Psychiatric Association: Arlington, VA; 2015.
SAMHSA. 2010 National Survey on Drug Use and Health. Dept. of Health and Human Services, Substance Abuse and Mental Health Services Administration, Office of Applied Studies Rockville, MD; 2011.
Ladouceur CD, Silk JS, Dahl RE, Ostapenko L, Kronhaus DM, Phillips ML. Fearful faces influence attentional control processes in anxious youth and adults. Emotion. 2009;9:855–64.
Bertocci MA, Bebko GM, Mullin BC, Langenecker SA, Ladouceur CD, Almeida JRC, et al. Abnormal anterior cingulate cortical activity during emotional n-back task performance distinguishes bipolar from unipolar depressed females. Psychol Med. 2011;42:1417–28.
Bertocci MA, Bebko G, Olino T, Fournier J, Hinze AK, Bonar L, et al. Behavioral and emotional dysregulation trajectories marked by prefrontal–amygdala function in symptomatic youth. Psychological Med. 2014;44:2603–15.
Kerestes R, Ladouceur CD, Meda S, Nathan PJ, Blumberg HP, Maloney K, et al. Abnormal prefrontal activity subserving attentional control of emotion in remitted depressed patients during a working memory task with emotional distracters. Psychological Med. 2011;42:29–40.
Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–9.
McLaren DG, Ries ML, Xu G, Johnson SC. A generalized form of context-dependent psychophysiological interactions (gPPI): a comparison to standard approaches. NeuroImage. 2012;61:1277–86.
Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron. 2005;48:175–87.
Friedman J, Hastie T, Simon N, Tibshirani R. GLMNET. 2.0-2 edn; 2014.
Picard RR, Cook RD. Cross-validation of regression models. J Am Stat Assoc. 1984;79:575–83.
Orlhac F, Eertink JJ, Cottereau A-S, Zijlstra JM, Thieblemont C, Meignan MA, et al. A guide to ComBat harmonization of imaging biomarkers in multicenter studies. J Nucl Med. 2022;63:172–9.
Bliss CI, Fisher RA. Fitting the negative binomial distribution to biological data. Biometrics. 1953;9:176–200.
NCSS. Negative binomial regression. NCSS Statistical Software; 2017.
Zhou Y, Friston KJ, Zeidman P, Chen J, Li S, Razi A. The hierarchical organization of the default, dorsal attention and salience networks in adolescents and young adults. Cereb Cortex. 2017;28:726–37.
Bartova L, Meyer BM, Diers K, Rabl U, Scharinger C, Popovic A, et al. Reduced default mode network suppression during a working memory task in remitted major depression. J Psychiatr Res. 2015;64:9–18.
Breukelaar IA, Erlinger M, Harris A, Boyce P, Hazell P, Grieve SM, et al. Investigating the neural basis of cognitive control dysfunction in mood disorders. Bipolar Disord. 2020;22:286–95.
Gärtner M, Ghisu ME, Scheidegger M, Bönke L, Fan Y, Stippl A, et al. Aberrant working memory processing in major depression: evidence from multivoxel pattern classification. Neuropsychopharmacology. 2018;43:1972–9.
Meyer BM, Rabl U, Huemer J, Bartova L, Kalcher K, Provenzano J, et al. Prefrontal networks dynamically related to recovery from major depressive disorder: a longitudinal pharmacological fMRI study. Transl Psychiatry. 2019;9:1–10.
Pomarol-Clotet E, Alonso-Lana S, Moro N, Sarro S, Bonnin MC, Goikolea JM, et al. Brain functional changes across the different phases of bipolar disorder. Br J Psychiatry. 2015;206:136–44.
Rodríguez‐Cano E, Alonso‐Lana S, Sarró S, Fernández‐Corcuera P, Goikolea JM, Vieta E, et al. Differential failure to deactivate the default mode network in unipolar and bipolar depression. Bipolar Disord. 2017;19:386–95.
Fernández-Corcuera P, Salvador R, Monté GC, Salvador Sarró S, Goikolea JM, Amann B, et al. Bipolar depressed patients show both failure to activate and failure to de-activate during performance of a working memory task. J Affect Disord. 2013;148:170–8.
Pomarol-Clotet E, Moro N, Sarró S, Goikolea JM, Vieta E, Amann B, et al. Failure of de-activation in the medial frontal cortex in mania: evidence for default mode network dysfunction in the disorder. World J Biol Psychiatry. 2012;13:616–26.
Balderston NL, Vytal KE, O’Connell K, Torrisi S, Letkiewicz A, Ernst M, et al. Anxiety patients show reduced working memory related dlPFC activation during safety and threat. Depression Anxiety. 2017;34:25–36.
Wang X-L, Du M-Y, Chen T-L, Chen Z-Q, Huang X-Q, Luo Y, et al. Neural correlates during working memory processing in major depressive disorder. Prog Neuro Psychopharmacol Biol Psychiatry. 2015;56:101–8.
Walter H, Wolf RC, Spitzer M, Vasic N. Increased left prefrontal activation in patients with unipolar depression: an event-related, parametric, performance-controlled fMRI study. J Affect Disord. 2007;101:175–85.
Balderston NL, Flook E, Hsiung A, Liu J, Thongarong A, Stahl S, et al. Patients with anxiety disorders rely on bilateral dlPFC activation during verbal working memory. Soc Cogn Affect Neurosci. 2020;15:1288–98.
Corballis MC. What’s left in language? Beyond the classical model. Ann N Y Acad Sci. 2015;1359:14–29.
Mundorf A, Peterburs J, Ocklenburg S. Asymmetry in the central nervous system: a clinical neuroscience perspective. Front Syst Neurosci. 2021;15:733898.
Chase HW, Fournier JC, Bertocci MA, Greenberg T, Aslam H, Stiffler R, et al. A pathway linking reward circuitry, impulsive sensation-seeking and risky decision-making in young adults: identifying neural markers for new interventions. Transl Psychiatry. 2017;7:e1096.
Edmiston EK, Fournier JC, Chase HW, Bertocci MA, Greenberg T, Aslam HA, et al. Assessing relationships among impulsive sensation seeking, reward circuitry activity, and risk for psychopathology: a functional magnetic resonance imaging replication and extension study. Biol Psychiatry Cogn Neurosci Neuroimaging. 2020;5:660–8.
Phillips ML, Swartz HA. A critical appraisal of neuroimaging studies of bipolar disorder: toward a new conceptualization of underlying neural circuitry and a road map for future research. Am J Psychiatry. 2014;171:829–43.
Ives-Deliperi VL, Howells F, Stein DJ, Meintjes EM, Horn N. The effects of mindfulness-based cognitive therapy in patients with bipolar disorder: a controlled functional MRI investigation. J Affect Disord. 2013;150:1152–7.
Chou T, Dougherty DD, Nierenberg AA, Deckersbach T. Restoration of default mode network and task positive network anti-correlation associated with mindfulness-based cognitive therapy for bipolar disorder. Psychiatry Res Neuroimaging. 2022;319:111419.
Du X, Mao Y, Ran Q, Zhang Q, Luo Q, Qiu J. Short-term group cognitive behavior therapy contributes to recovery from mild depression: evidence from functional and structural MRI. Psychiatry Res Neuroimaging. 2016;251:53–59.
Weintraub MJ, Schneck CD, Miklowitz DJ. Network analysis of mood symptoms in adolescents with or at high risk for bipolar disorder. Bipolar Disord. 2020;22:128–38.
Funding
This work was supported by R37MH100041 (to MLP) from the National Institute of Mental Health, the Pittsburgh Foundation (PI: MLP), and the Brain and Behavior Research Foundation (PI: MAB). The funding sources exerted no influence over the work.
Author information
Authors and Affiliations
Contributions
MAB, YA-A, and MLP conceived of and wrote the manuscript. MAB, YA-A, and RR completed the analyses. MAB, YA-A, RR, SI, and MLP conceived of and interpreted the statistical analysis. RS, HAA, and GB contributed substantially to the acquisition of the data. All authors made significant contributions to the manuscript and all approved the final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bertocci, M.A., Afriyie-Agyemang, Y., Rozovsky, R. et al. Altered patterns of central executive, default mode and salience network activity and connectivity are associated with current and future depression risk in two independent young adult samples. Mol Psychiatry 28, 1046–1056 (2023). https://doi.org/10.1038/s41380-022-01899-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-022-01899-8