Abstract
Whether newer P2Y12 inhibitors are more efficacious and safer than clopidogrel and whether there is a superior one remain uncertain. We compared the effect of P2Y12 inhibitors on clinical outcomes in patients with acute coronary syndrome (ACS). Randomized controlled trials comparing clopidogrel, prasugrel, ticagrelor, or cangrelor, in combination with aspirin were searched. Sixteen trials with altogether 77,896 patients were included. Compared to clopidogrel, cardiovascular mortality was reduced with prasugrel (OR 0.85, 95% CI 0.75–0.97) and ticagrelor (0.82, 0.73–0.93). Myocardial infarction (0.75, 0.63–0.89) and major adverse cardiovascular events (0.80, 0.69–0.94) were reduced by prasugrel. Stent thrombosis was reduced by prasugrel (0.49, 0.38–0.63), ticagrelor (0.72, 0.57–0.90), and cangrelor (0.59, 0.43–0.81). It was reduced more by prasugrel than ticagrelor (0.69, 0.51–0.93). There were more major bleeds with prasugrel (1.24, 1.05–1.48). Thrombolysis in Myocardial Infarction (TIMI) major bleeding was increased with prasugrel compared to clopidogrel (1.36, 1.11–1.66) and ticagrelor (1.33, 1.06–1.67). TIMI minor bleeding was increased with prasugrel (1.44, 1.16–1.77) and cangrelor (1.47, 1.01–2.16) compared to clopidogrel while it was increased with prasugrel compared to ticagrelor (1.32, 1.01–1.72). Prasugrel is preferable to those ACS patients at low bleeding risk to reduce cardiovascular events whereas ticagrelor is a relatively safe antiplatelet drug of choice for most patients.
Similar content being viewed by others
Introduction
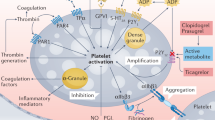
Clopidogrel is a commonly used P2Y12 inhibitor recommended for the standard treatment and secondary prevention of ischemic events in acute coronary syndrome (ACS) patients1,2. As a prodrug, its limitations such as low bioavailability, delayed onset of action, variability in patient responsiveness, and irreversible antiplatelet effect3,4,5 led to the development of more potent, newer P2Y12 inhibitors. Prasugrel, a third-generation thienopyridine with irreversible inhibition of P2Y12 receptors, has been observed to provide a better clinical efficacy than clopidogrel, but at the expense of an increased risk of major bleeding6. Ticagrelor, a cyclopentyl triazolo-pyrimidine, is a direct-acting and reversible P2Y12 inhibitor7,8,9 showing benefits in reducing ischemic events, which makes it a promising option for the treatment of ACS patients. These advantages, however, are accompanied by a numerically higher risk of major bleeding8. Cangrelor is an intravenous, fast- and direct-acting blocker of P2Y12 receptor10,11,12,13. It was reported to reduce ischemic events, especially myocardial infarction (MI) and stent thrombosis, without a significant increase in severe bleeding13,14.
The efficacy and safety of newer P2Y12 inhibitors in ACS influenced the recommendations in the clinical guidelines. It must be recognized that the greater and more rapid inhibition of platelet aggregation may be counterbalanced by the higher risk of bleeding complications6,8,15. In the 2016 American College of Cardiology (ACC)/American Heart Association (AHA) guideline on dual antiplatelet therapy (DAPT), ticagrelor was recommended over clopidogrel as a maintenance therapy in patients with non-ST-segment elevation (NSTE)-ACS or ST-segment elevation myocardial infarction (STEMI) receiving DAPT after percutaneous coronary intervention (PCI), while prasugrel was recommended for those patients at low bleeding risk and without prior stroke or transient ischemic attack (Class IIa)16. The 2017 European Society of Cardiology (ESC)/European Association for Cardio-Thoracic Surgery (EACTS) guideline recommended ticagrelor for ACS patients at moderate to high ischemic risk regardless of the initial treatment, but prasugrel for ACS patients undergoing PCI if there is no excess fatal bleeding or other contraindications (Class I)17.
Although there have been several randomized controlled trials (RCTs) comparing the efficacy and safety of newer P2Y12 inhibitors with that of clopidogrel in patients with ACS, there were no head-to-head trials between newer P2Y12 inhibitors until the report of the two recent trials directly comparing prasugrel with ticagrelor18,19. It is necessary to update the efficacy and safety profiles of P2Y12 inhibitors in ACS patients, particularly to assess the comparative effectiveness among newer P2Y12 inhibitors. Because direct evidence is limited and inadequately powered, indirect comparisons among newer P2Y12 inhibitors should also be sought as supportive evidence. Therefore, we performed a network meta-analysis to compare the effect of P2Y12 inhibitors on cardiovascular and bleeding events in patients with ACS in order to optimize therapy in clinical practice.
Methods
Search strategy and study selection
This network meta-analysis conforms with the reporting standards in the PRISMA statement. We searched MEDLINE, EMBASE, the Cochrane database, and ClinicalTrials.gov up to December 31, 2019 for RCTs using the terms “clopidogrel” or “prasugrel” or “ticagrelor” or “cangrelor” or “P2Y12 inhibitors” or “thienopyridine” or “antiplatelet therapy” or “DAPT” or “acute coronary syndrome” or “non-ST-elevation myocardial infarction” or “unstable angina”, and their synonyms and related keywords. Study inclusion criteria for this network meta-analysis were: (1) RCTs; (2) sample size of over 100 patients in total; (3) patients over 18 years of age with ACS; (4) allocation of different P2Y12 inhibitors in patients receiving DAPT; (5) reporting the number of cardiovascular events, bleeding events, and deaths. No language restrictions were enforced.
Data extraction
Literature review and inclusion were carried out by two investigators (YF and CKL) independently. Disagreements were resolved by consensus. For eligible studies, information about the year of publication, sample size, the maximum duration of follow-up, loading dose and maintenance dose in each treatment arm, and patient characteristics including age, gender, body mass index, ethnic group, smoking status, baseline comorbidities of cardiovascular disease were extracted. Risk of bias was assessed using the Cochrane risk of bias assessment tool. The following components were evaluated: sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome, incomplete outcome data, and free from other bias. Each component was scored as a low, high, and unclear risk of bias.
Study outcomes
The primary outcomes were cardiovascular death, MI, and stroke. Secondary outcomes were major adverse cardiovascular events (MACE) defined as a composite of cardiovascular death, MI, and stroke, definite or probable stent thrombosis, all-cause mortality, Thrombolysis In Myocardial Infarction (TIMI) major bleeding which included non-coronary artery bypass grafting (CABG)-related and CABG-related TIMI major bleeding, TIMI minor bleeding, and all major bleeding including TIMI major bleeding, PLATO (PLATelet inhibition and patient Outcomes)-defined major bleeding8 or Bleeding Academic Research Consortium (BARC) major bleeding (type 3, 4, or 5)20. For those trials reporting major bleeding with more than one definition, the priority selection criteria for analysis in all major bleeding was TIMI major bleeding over BARC major bleeding over PLATO-defined major bleeding.
Statistical analysis
The efficacy and safety of different P2Y12 inhibitors were compared at the trial-level using a frequentist approach21. Pooled random-effects odds ratios (ORs) and 95% confidence intervals (95% CIs) were the summary statistics used. A 95% CI not including 1.00 or a p-value (two-tailed) less than 0.05 was considered statistically significant. Forest plots using random- and fixed-effects models to compare relative treatment effects were generated using the statistical package netmeta version 0.9-8 (https://cran.r-project.org/web/packages/netmeta/index.html) in R version 3.3.3. P-score was computed to determine the likelihood of the P2Y12 inhibitors being the best for protecting against each outcome. Subgroup analyses were performed for oral P2Y12 inhibitors and intravenous cangrelor (vs. clopidogrel), respectively.
Loop-specific approach was used to appraise the inconsistency between estimates derived from direct and indirect evidence in the network. τ2 estimate with values of 0.04, 0.14, and 0.40 corresponded to a low, moderate and high degree of heterogeneity, respectively. I2 statistics were used to assess the presence of heterogeneity within each pairwise comparison with I2 < 25%, within 25–50%, and > 50% corresponding to mild, moderate, and severe heterogeneity, respectively. Small study effects or potential publication bias was assessed visually by funnel plots and trim-and-fill test. Egger’s test for asymmetry in funnel plots would be performed only in those direct comparison groups having ten or more studies in case of misleading results.
Random-effects Bayesian framework22 with non-informative priors was used to perform sensitivity analysis in order to check the robustness of the study findings. In addition, the consistency of inferential estimates from hierarchical modelling was evaluated with a Bayesian framework by means of Markov chain Monte Carlo simulations in order to be similar to the frequentist estimates, and these were performed with 1000 tuning iterations and 5000 simulation iterations using R statistical package gemtc version 0.8-2 (https://cran.r-project.org/web/packages/gemtc/index.html) and rjags version 4-6 (https://cran.r-project.org/web/packages/rjags/index.html) to minimize Monte Carlo error. The protocol for this network meta-analysis was registered with the PROSPERO registry (number CRD42019122170).
Results
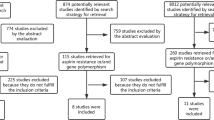
A summary of the screening and selection process is described in the PRISMA flowchart (Supplementary Fig. S1). Twenty trials fulfilled the inclusion criteria6,8,10,11,13,15,18,19,23,24,25,26,27,28,29,30,31,32,33,34. However, doubling loading or maintenance dose of clopidogrel was used in the Thrombocytes And IndividuaLization of ORal antiplatelet therapy in percutaneous coronary intervention (TAILOR)32 and Xiong et al.33 trials; two doses of ticagrelor therapy rather than different P2Y12 inhibitors were compared in the Prevention of Cardiovascular Events in Patients with Prior Heart Attack Using Ticagrelor Compared to Placebo on a Background of Aspirin–Thrombolysis In Myocardial Infarction 54 (PEGASUS-TIMI 54) trial15; ticagrelor was compared with placebo rather than the other P2Y12 inhibitors in the Effect of Ticagrelor on Health Outcomes in Diabetes Mellitus Patients Intervention Study (THEMIS) trial34, so these trials were excluded. Sixteen two-armed trials with altogether 77,896 patients were eligible for this network meta-analysis6,8,10,11,13,18,19,23,24,25,26,27,28,29,30,31. There are seven trials comparing ticagrelor vs. clopidogrel (n = 24,629)8,23,24,25,26,27,28, four trials comparing prasugrel vs. clopidogrel (n = 23,118)6,29,30,31, three trials comparing cangrelor vs. clopidogrel (n = 24,901)10,11,13, two trials comparing ticagrelor vs. prasugrel (n = 5248)18,19. In total, 41,844 patients were randomized to newer P2Y12 inhibitors while 36,052 patients were randomized to clopidogrel. The loading dose of clopidogrel used in the comparison between ticagrelor vs. clopidogrel and prasugrel vs. clopidogrel was 300 or 600 mg, followed by 75 mg clopidogrel for maintaining treatment. Cangrelor was administered intravenously as a bolus (30 μg/kg of cangrelor or matching placebo), followed by an infusion (4 μg/kg per min of cangrelor or matching placebo), while 600 mg clopidogrel was given at the end of the infusion in the cangrelor groups; 300–600 mg clopidogrel was given to the comparator groups with different timings in the three cangrelor trials included10,11,13. For each outcome of interest, there were six theoretical comparisons (Fig. 1).
Network profile for the studies comparing different P2Y12 inhibitors involved in DAPT. Each line represents a pair of direct comparison between different P2Y12 inhibitors. The width of the lines is proportional to the number of trials comparing every pair of treatments, and the size of every circle is proportional to the number of randomly assigned participants (sample size).
The main characteristics of the included trials are shown in Table 1. They all had a low risk of bias assessed using the components recommended by the Cochrane Collaboration (Supplementary Table S1 and Table S2 online). Baseline characteristics of patients enrolled in the sixteen trials are summarized in Supplementary Table S3 online. All included trials reported the frequencies of MACE, MI, and all-cause mortality. Fourteen trials reported the frequency of stroke6,8,10,11,18,19,23,24,25,27,28,29,30,31; 13 trials reported cardiovascular mortality6,8,13,18,19,23,24,25,26,27,28,30,31; nine trials reported the frequency of definite or probable stent thrombosis6,8,10,12,13,18,19,25,31. All the sixteen trials reported major bleeding events. Twelve trials reported TIMI major bleeding6,8,10,12,13,18,23,25,28,29,30,31 while three trials reported CABG-related TIMI major bleeding6,8,28 and seven trials reported non-CABG-related TIMI major bleeding6,8,13,28,29,30,31. Eleven trials reported TIMI minor bleeding6,8,10,11,13,23,25,28,29,30,31. Four trials reported BARC major bleeding18,19,26,28, in which the ISAR-REACT 519 and the Safety and the Efficacy of Ticagrelor for Coronary Stenting Post Thrombolysis (SETFAST)26 trials reported BARC bleeding only without reporting TIMI bleeding. Four trials reported PLATO-defined major bleeding8,24,27,28 in which the Phase the International Study of Ticagrelor and Clinical Outcomes in Asian ACS Patients (PHILO)24 and Wang et al.27. trials did not report TIMI major bleeding. It should be noted that the definition of MACE varied across different trials (Supplementary Table S4 online). The definition of this composite outcome used in this network meta-analysis was the same as that in the original trial.
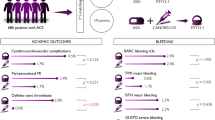
Our results showed that when compared with clopidogrel, cardiovascular mortality was reduced with both prasugrel (p = 0.015) and ticagrelor (p = 0.002) (Fig. 2A). MI (p = 0.001) and MACE (p = 0.006) were reduced by prasugrel only (Fig. 2B,D). There were fewer definite or probable stent thromboses with prasugrel (p < 0.001), ticagrelor (p = 0.003), and cangrelor (p = 0.001), respectively (Fig. 2E). In addition, there were significantly fewer definite or probable stent thromboses with prasugrel than ticagrelor (p = 0.014) (Table 2). No significant difference was found between clopidogrel, ticagrelor, prasugrel, and cangrelor with respect to the risk of stroke and all-cause death (Table 2, Fig. 2C,F).
Forest plots assessing the effects of different P2Y12 inhibitors relative to clopidogrel. (A) Cardiovascular mortality. (B) Myocardial infarction. (C) Stroke. (D) MACE. (E) Definite or probable stent thrombosis. (F) All-cause mortality. MACE = major adverse cardiovascular events. Square markers indicate odds ratios for cardiovascular outcomes comparing different P2Y12 inhibitors to clopidogrel. The horizontal lines indicate 95% confidence intervals.
In contrast, there were more all major bleeds with prasugrel (p = 0.012) when compared to clopidogrel. The risk of TIMI major bleeding was significantly increased with prasugrel when compared to clopidogrel (p = 0.003) and ticagrelor (p = 0.014). Compared to clopidogrel, the risk of TIMI minor bleeding was significantly increased with prasugrel (p < 0.001) and cangrelor (p = 0.046). There were also more TIMI minor bleeds with prasugrel than ticagrelor (p = 0.043). Moreover, an increase in the risk of non-CABG-related TIMI major bleeding was found with prasugrel (p = 0.033) and ticagrelor (p = 0.025) when compared with clopidogrel. The risk of CABG-related bleeding was increased with prasugrel when compared to clopidogrel (p = 0.030) and ticagrelor (p = 0.014) (Table 3).
P-score ranked prasugrel having the highest likelihood for reducing MI (95.5%), stroke (68.7%), MACE (88.6%), and definite or probable stent thrombosis (99.6%). Ticagrelor had the highest rank probabilities for protecting against cardiovascular death (79.3%) while cangrelor had the highest rank probabilities for protecting against all-cause death (78.8%). Although clopidogrel had the lowest likelihood in reducing all the cardiovascular outcomes above, it was ranked the best in reducing all major bleeding (80.9%), TIMI major bleeding (72.8%) and TIMI minor bleeding (94.4%) among all the P2Y12 inhibitors assessed (Supplementary Table S5 online).
There was not a high degree of heterogeneity among studies (Supplementary Table S6 online). Significant heterogeneity in the pairwise meta-analysis was found for MACE (I2 = 53%, p = 0.0001) in the comparison between ticagrelor vs. clopidogrel, which was due to the PHILO trial24. Excluding it could reduce I2 to 39% (p = 0.15) (Supplementary Table S7 online). There was also significant heterogeneity in the comparison between cangrelor vs. clopidogrel for MACE (I2 = 65%, p = 0.06) and MI (I2 = 67%, p = 0.05), driven by the Cangrelor versus Standard Therapy to Achieve Optimal Management of Platelet Inhibition (CHAMPION) PCI trial10. I2 could be reduced to 0% after excluding it (p = 0.48 and p = 0.36, respectively) (Supplementary Table S8 and Table S9 online). There was no significant inconsistency between direct and indirect comparisons in any of the outcomes assessed (Supplementary Tables S10–S17 online). Using fixed-effects instead of random-effects models, or Bayesian instead of frequentist analysis showed similar results (Supplementary Table S18 and Table S19 online). Inspection of the funnel plots did not reveal any significant publication bias or small study effects (Supplementary Figs. S2–S10 and Supplementary Table S20 online).
Subgroup analysis of oral P2Y12 inhibitors and intravenous cangrelor showed consistent results with our main analysis. Interestingly, the effect of ticagrelor on reducing all-cause mortality became significant (OR 0.86, 95% CI 0.74–0.99, p = 0.041) when compared to clopidogrel (Supplementary Table S21 and Supplementary Fig. S11 online).
Discussion
Our network meta-analysis included the most recent RCTs to compare the efficacy and safety of newer P2Y12 inhibitors with clopidogrel in ACS patients, especially aiming to compare different newer P2Y12 inhibitors through both direct and indirect evidence. There were three main findings that provided new and further evidence on the efficacy and safety profiles of newer P2Y12 inhibitors. First, prasugrel was more beneficial in reducing MACE, MI and definite or probable stent thrombosis but resulted in a significantly higher risk of major and minor bleeding. Secondly, ticagrelor reduced definite or probable stent thrombosis and cardiovascular mortality without increasing the risk of bleeding. Finally, cangrelor reduced definite or probable stent thrombosis without additional cardiovascular benefits but caused more TIMI minor bleeds than clopidogrel.
Our findings are consistent with previous network meta-analyses35,36,37,38,39 and meta-analyses40,41,42. Newer P2Y12 inhibitors are significantly more effective than clopidogrel in reducing cardiovascular events and cardiovascular deaths in patients with ACS or undergoing PCI. However, previous studies neither compare individual P2Y12 inhibitors simultaneously nor include cangrelor for analysis. Our network meta-analysis, which includes the latest evidence, in particular, the two direct trials comparing prasugrel and ticagrelor, provides an important update on the efficacy and safety profiles of P2Y12 inhibitors that are already widely used.
Direct evidence showed that prasugrel numerically reduced cardiovascular events when compared to clopidogrel. The biggest trial comparing prasugrel and clopidogrel, the Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition with Prasugrel–Thrombolysis in Myocardial Infarction (TRITON-TIMI) 386, however, was the only trial that reported significant reduction of MACE, MI and stent thrombosis with prasugrel. These benefits were confirmed in our network meta-analysis with greater statistical power.
Whether prasugrel increases bleeding events and whether this harmful effect counterbalances its cardiovascular benefits is controversial. The significantly increased risk of non-CABG-related TIMI major bleeding (p = 0.03) with prasugrel, including life-threatening bleeding (p = 0.01) and fatal bleeding (p = 0.002) was observed in ACS patients in TRITON-TIMI 386 but not in the other three prasugrel vs. clopidogrel trials included in this network meta-analysis29,30,31. Besides, the significant excess in non-CABG-related TIMI bleeding was not seen in STEMI patients undergoing PCI43,44. Contradictory findings in some observational studies reported the lower incidence of major bleeding with prasugrel45,46. Our network meta-analysis provided a more reliable conclusion by integrating direct and indirect evidence for analysis. The cardiovascular benefits of prasugrel were found at the expense of excessive all major bleeding and TIMI bleeding including both CABG-related and non-CABG-related TIMI major bleeding and TIMI minor bleeding. The use of prasugrel should therefore be avoided in patients at high risk of bleeding, including those with prior stroke or transient ischemic attack, those undergoing CABG or other surgeries, those with trauma or at risk from falls, those with cancer, those age 75 or older, or those who weigh less than 60 kg15.
PLATO, the biggest trial comparing ticagrelor and clopidogrel, reported a significant reduction in MACE (p < 0.001) and all-cause mortality (p = 0.010) with ticagrelor8. These benefits, however, were neither seen in the other four out of seven ticagrelor vs. clopidogrel trials included23,24,26,28 nor in this network meta-analysis. Subgroup analysis of oral P2Y12 inhibitors only suggested a lower risk of all-cause mortality but not MACE associated with ticagrelor use. The possible explanations for these inconsistent results could be due to the small sample size and the imbalance in clinical characteristics of patients, and the low number of events observed due to the short follow-up period in those trials, unlike PLATO. Our network meta-analysis found a significantly lower risk of stent thrombosis and cardiovascular death with ticagrelor. However, no significant increase in the risk of bleeding events was found, which was consistent across all the included ticagrelor vs. clopidogrel trials, supporting ticagrelor as an effective and safe antiplatelet therapy.
POPular AGE, the first RCT comparing clopidogrel vs. ticagrelor or prasugrel in NSTE-ACS patients aged 70 years and over has just released its results47. Clopidogrel was found to be better than ticagrelor for the elderly due to fewer bleeding events. Although this trial treated the 5% of the trial patients prescribed prasugrel as the ticagrelor group, including this trial for analysis did not alter our conclusions (Supplementary Table S22 online). However, this study emphasized the importance of bleeding considerations in the elderly. Clopidogrel could be an alternative strategy for those patients if bleeding risk is extremely high.
A standard-dose of ticagrelor, namely, 180 mg loading dose followed by 90 mg twice daily was used in the ticagrelor trials included for analysis. The PEGASUS-TIMI 54 trial15 evaluating two maintaining doses, namely 90 mg twice daily vs. 60 mg twice daily of ticagrelor therapy suggested an improved efficacy but a similar degree of safety (bleeding) with a low-dose ticagrelor, which therefore, might offer a more attractive cost-effective option for patients.
There have been few trials comparing prasugrel and ticagrelor. The Prasugrel versus Ticagrelor in Patients with Acute Myocardial Infarction Treated with Primary Percutaneous Coronary Intervention (PRAGUE)-1818 and the Intracoronary Stenting and Antithrombotic Regimen: Rapid Early Action for Coronary Treatment (ISAR-REACT) 519 are the only two RCTs available, but they showed inconsistent findings. PRAGUE-18 reported similar safety and efficacy between prasugrel and ticagrelor in patients with STEMI. In contrast, ISAR-REACT 5 found that the incidence of death, MI, or stroke was significantly lower with prasugrel than ticagrelor, although the incidence of major bleeding was not significantly different across the whole spectrum of presentation of ACS patients. PRAGUE-18 was criticized for the premature termination and the high incidence of switching treatment drugs to clopidogrel after discharge, resulting in the unreliable comparison of clinical outcomes with prasugrel and ticagrelor during the 1-year follow-up period. In addition, two trials lacked adequate statistical power and did not allow the conduction of pairwise meta-analysis. This network meta-analysis took advantage of the indirect evidence from the comparison between these two P2Y12 inhibitors with clopidogrel. Our findings showed similar benefits of ticagrelor and prasugrel in reducing the risk of cardiovascular death while prasugrel was better than ticagrelor in reducing the risk of all the cardiovascular events. However, ticagrelor was associated with significantly fewer TIMI major and TIMI minor bleeds than prasugrel. The greater ischemic benefits with prasugrel were offset by the excessive bleeding events. These findings further favoring ticagrelor with a better safety profile among the newer P2Y12 inhibitors supported the recommendations in the current guidelines for using ticagrelor as the preferred treatment over the other P2Y12 inhibitors for ACS patients regardless of the initial treatment strategy16,17. The upcoming trials in the future will provide more evidence assessing the comparative effects between ticagrelor and prasugrel.
Unlike prasugrel and ticagrelor, cangrelor is not an oral drug and only used in a peri-procedural setting. It requires the administration of oral P2Y12 inhibitors, usually clopidogrel, after discontinuation of the cangrelor infusion. The cardiovascular effects of cangrelor were inconsistent in the CHAMPION studies10,11,13. Few clinical trials compared cangrelor with ticagrelor and prasugrel. In this network meta-analysis maximizing the use of direct and indirect evidence, cangrelor was found to reduce stent thrombosis but increase TIMI minor bleeding. Previous patient-level meta-analysis integrating these three studies agreed with our findings. It reported a 41% (p < 0.001) reduction in the odds of stent thrombosis and a 51% (p = 0.022) increase in the odds of TIMI minor bleeding with cangrelor, irrespective of the patient clinical presentations48. CHAMPION PCI10 and CHAMPION PLATFORM11 were given more weight to the unfavorable results of cangrelor. The definition of periprocedural MI used in these two trials did not allow for discrimination of re-infarction in patients that presented for PCI shortly after admission with a biomarker-positive ACS, leading to a decrease in the number of trial events49. Moreover, the increased TIMI minor bleeding found in our analysis but not in the CHAMPION studies could be driven by the indirect evidence and due to the increased statistical power. Although cangrelor has not been adequately studied in head-to-head comparisons with prasugrel and ticagrelor, no superiority of cangrelor over these two drugs has been identified by using indirect evidence for analysis in this network, which needs to be further confirmed in large direct comparative trials in the future. The increased speed of onset and the rapid reversibility of cangrelor offer an alternative to loading with oral P2Y12 inhibitors in the acute phase of PCI. Moreover, unlike oral P2Y12 inhibitors which need a wash-out period of 5–7 days1, cangrelor is an attractive option for those patients awaiting surgery.
Intensive inhibition of platelet aggregation is usually accompanied by an increase in bleeding. Newer P2Y12 inhibitors that result in more intensive inhibition of platelet aggregation are more likely to have a higher rate of bleeding than clopidogrel. The use of newer P2Y12 inhibitors, especially prasugrel, requires a tradeoff between decreasing ischemic risks with an increased bleeding risk for each individual patient50. In patients who are not at risk of bleeding or can tolerate therapy without increased bleeding events, and in patients for whom reducing the high risk of ischemic events outweighs the excess bleeding, prasugrel can be considered. Ticagrelor, with its better safety profile, should be considered over clopidogrel in most ACS patients. Although, current observational studies reporting inconsistent results do not confirm the superiority of newer P2Y12 inhibitors over clopidogrel in ACS patients receiving PCI51, they are prone to bias and should be interpreted with caution given the potential for confounding. Nevertheless, real-world studies are helpful in translating the findings from RCTs into practice settings in the general population.
A limitation of this network meta-analysis is the lack of patient-level data, so it was unable to adjust the analysis for the severity of presentation, the cardiovascular risk profile of patients, revascularization strategy, and use of other medications. Analysis dedicated to specific patient subgroups could help delineate appropriate therapies in different populations. Moreover, the comparative results among newer P2Y12 inhibitors were mostly driven by indirect evidence due to the scarcity of head-to-head comparative trials. Inevitably, trials included in the network meta-analysis varied in patient characteristics, comorbidities, treatment regimens, follow-up periods, and definitions of outcomes.
Conclusions
Prasugrel and ticagrelor both show greater potentials than clopidogrel in protecting against stent thrombosis and cardiovascular death in ACS patients. Prasugrel is more beneficial in reducing MACE, MI, especially in reducing definite or probable stent thrombosis among the oral P2Y12 inhibitors but at the expense of increased major and minor bleeding. Intravenous cangrelor lowers the risk of definite or probable stent thrombosis compared with clopidogrel whereas increases TIMI minor bleeding. The treatment of ACS patients should take the benefit-risk profile of each individual patient into account. Ticagrelor not increasing bleeding is therefore the antiplatelet drug of choice for most ACS patients. Prasugrel can be recommended to those ACS patients at high ischemic risk but low bleeding risk.
References
Amsterdam, E. A. et al. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 64(24), e139–e228 (2014).
O’Gara, P. T. et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 127(4), e362-425 (2013).
Yusuf, S. et al. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N. Engl. J. Med. 345, 494–502 (2001).
Gurbel, P. A., Bliden, K. P., Hiatt, B. L. & O’Connor, C. M. Clopidogrel for coronary stenting: Response variability, drug resistance, and the effect of pretreatment platelet reactivity. Circulation 107(23), 2908–2913 (2003).
Angiolillo, D. J. et al. Variability in individual responsiveness to clopidogrel: Clinical implications, management, and future perspectives. J. Am. Coll. Cardiol. 49(14), 1505–1516 (2007).
Wiviott, S. D. et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N. Engl. J. Med. 357(20), 2001–2015 (2007).
Storey, R. F. et al. Inhibitory effects of ticagrelor compared with clopidogrel on platelet function in patients with acute coronary syndromes: The PLATO (PLATelet inhibition and patient Outcomes) PLATELET substudy. J. Am. Coll. Cardiol. 56(18), 1456–1462 (2010).
Wallentin, L. et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N. Engl. J. Med. 361(11), 1045–1057 (2009).
Wallentin, L. P2Y(12) inhibitors: Differences in properties and mechanisms of action and potential consequences for clinical use. Eur. Heart. J. 30(16), 1964–1977 (2009).
Harrington, R. A. et al. Platelet inhibition with cangrelor in patients undergoing PCI. N. Engl. J. Med. 361(24), 2318–2329 (2009).
Bhatt, D. L. et al. Intravenous platelet blockade with cangrelor during PCI. N. Engl. J. Med. 361, 2330–2341 (2009).
Kastrati, A. & Ndrepepa, G. Cangrelor—A champion lost in translation?. N. Engl. J. Med. 361, 2382–2384 (2009).
Bhatt, D. L. et al. Effect of platelet inhibition with cangrelor during PCI on ischemic events. N. Engl. J. Med. 368(14), 1303–1313 (2013).
Eisen, A. et al. Cangrelor compared with clopidogrel in patients with prior myocardial infarction—Insights from the CHAMPION trials. Int. J. Cardiol. 250, 49–55 (2018).
Bonaca, M. P. et al. Long-term use of ticagrelor in patients with prior myocardial infarction. N. Engl. J. Med. 372(19), 1791–1800 (2015).
Levine, G. N. et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines: an update of the 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention, 2011 ACCF/AHA guideline for coronary artery bypass graft surgery, 2012 ACC/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease, 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction, 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes, and 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery. Circulation 134(10), e123–e155 (2016).
Valgimigli, M. et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart. J. 39(3), 213–260 (2018).
Motovska, Z. et al. 1-year outcomes of patients undergoing primary angioplasty for myocardial infarction treated with prasugrel versus ticagrelor. J. Am. Coll. Cardiol. 71(4), 371–381 (2018).
Schüpke, S. et al. Ticagrelor or prasugrel in patients with acute coronary syndromes. N. Engl. J. Med. 381(16), 1524–1534 (2019).
Mehran, R. et al. Standardized bleeding definitions for cardiovascular clinical trials: A consensus report from the Bleeding Academic Research Consortium. Circulation 123(23), 2736–2747 (2011).
Rucker, G. Network meta-analysis, electrical networks and graph theory. Res. Synth. Methods. 3(4), 312–324 (2012).
Salanti, G., Higgins, J. P., Ades, A. E. & Ioannidis, J. P. Evaluation of networks of randomized trials. Stat. Methods. Med. Res. 17, 279–301 (2008).
Cannon, C. P. et al. Safety, tolerability, and initial efficacy of AZD6140, the first reversible oral adenosine diphosphate receptor antagonist, compared with clopidogrel, in patients with non-ST-segment elevation acute coronary syndrome: Primary results of the DISPERSE-2 trial. J. Am. Coll. Cardiol. 50(19), 1844–1851 (2007).
Goto, S., Huang, C. H., Park, S. J., Emanuelsson, H. & Kimura, T. Ticagrelor vs. clopidogrel in Japanese, Korean and Taiwanese patients with acute coronary syndrome—Randomized, double-blind, phase III PHILO study. Circ. J. 79(11), 2452–2460 (2015).
Tang, X. et al. Assessment of ticagrelor versus clopidogrel treatment in patients with ST-elevation myocardial infarction undergoing primary percutaneous coronary intervention. J. Cardiovasc. Pharmacol. 68(2), 115–120 (2016).
Dehghani, P. et al. Effects of ticagrelor versus clopidogrel on platelet function in fibrinolytic-treated STEMI patients undergoing early PCI. Am. Heart. J. 192, 105–112 (2017).
Wang, H. & Wang, X. Efficacy and safety outcomes of ticagrelor compared with clopidogrel in elderly Chinese patients with acute coronary syndrome. Ther. Clin. Risk. Manag. 12, 1101–1105 (2016).
Berwanger, O. et al. Ticagrelor vs clopidogrel after fibrinolytic therapy in patients with ST-elevation myocardial infarction: A randomized clinical trial. JAMA. Cardiol. 3(5), 391–399 (2018).
Wiviott, S. D. et al. Randomized comparison of prasugrel (CS-747, LY640315), a novel thienopyridine P2Y12 antagonist, with clopidogrel in percutaneous coronary intervention: Results of the Joint Utilization of Medications to Block Platelets Optimally (JUMBO)-TIMI 26 trial. Circulation 111(25), 3366–3373 (2005).
Roe, M. T. et al. Prasugrel versus clopidogrel for acute coronary syndromes without revascularization. N. Engl. J. Med. 367(14), 1297–1309 (2012).
Saito, S. et al. Efficacy and safety of adjusted-dose prasugrel compared with clopidogrel in Japanese patients with acute coronary syndrome: The PRASFIT-ACS study. Circ. J. 78(7), 1684–1692 (2014).
Dridi, N. P. et al. Prasugrel or double-dose clopidogrel to overcome clopidogrel low-response–the TAILOR (Thrombocytes AndIndividuaLization of ORal antiplatelet therapy in percutaneous coronary intervention) randomized trial. Platelets 25(7), 506–512 (2014).
Xiong, R. et al. A randomized controlled trial to assess the efficacy and safety of doubling dose clopidogrel versus ticagrelor for the treatment of acute coronary syndrome in patients with CYP2C19*2 homozygotes. Int. J. Clin. Exp. Med. 8(8), 13310–13316 (2015).
Steg, P. G. et al. Ticagrelor in patients with stable coronary disease and diabetes. N. Engl. J. Med. 381(14), 1309–1320 (2019).
Bavishi, C., Panwar, S., Messerli, F. H. & Bangalore, S. Meta-Analysis of comparison of the newer oral P2Y12 inhibitors (prasugrel or ticagrelor) to clopidogrel in patients with non-ST-elevation acute coronary syndrome. Am. J. Cardiol. 116(5), 809–817 (2015).
Zhang, L. et al. Meta-analysis of comparison of the newer P2Y12 inhibitors (oral preparation or intravenous) to clopidogrel in patients with acute coronary syndrome. J. Cardiovasc. Pharmacol. 69(3), 147–155 (2017).
Bellemain-Appaix, A. et al. New P2Y12 inhibitors versus clopidogrel in percutaneous coronary intervention: A meta-analysis. J. Am. Coll. Cardiol. 56(19), 1542–1551 (2010).
Tang, X. F. et al. Impact of new oral or intravenous P2Y12 inhibitors and clopidogrel on major ischemic and bleeding events in patients with coronary artery disease: A meta-analysis of randomized trials. Atherosclerosis. 233(2), 568–578 (2014).
Aradi, D., Komócsi, A., Vorobcsuk, A. & Serebruany, V. L. Impact of clopidogrel and potent P2Y 12-inhibitors on mortality and stroke in patients with acute coronary syndrome or undergoing percutaneous coronary intervention: A systematic review and meta-analysis. Thromb. Haemost. 109(1), 93–101 (2013).
Shah, R. et al. Meta-analysis of the relative efficacy and safety of oral P2Y12 inhibitors in patients with acute coronary syndrome. Am. J. Cardiol. 119(11), 1723–1728 (2017).
Chatterjee, S. et al. Comparing newer oral anti-platelets prasugrel and ticagrelor in reduction of ischemic events-evidence from a network meta-analysis. J. Thromb. Thrombolysis. 36(3), 223–232 (2013).
Rafique, A. M. et al. Optimal P2Y12 inhibitor in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention: A network meta-analysis. JACC.Cardiovasc. Interv. 9(10), 1036–1046 (2016).
Montalescot, G. et al. Prasugrel compared with clopidogrel in patients undergoing percutaneous coronary intervention for ST-elevation myocardial infarction (TRITON-TIMI 38): Double-blind, randomized controlled trial. Lancet 373(9665), 723–731 (2009).
Brener, S. J. et al. Outcomes in patients with ST-segment elevation acute myocardial infarction treated with clopidogrel versus prasugrel (from the INFUSE-AMI trial). Am. J. Cardiol. 113(9), 1457–1460 (2014).
Bacquelin, R. et al. Safety of prasugrel in real- world patients with ST-segment elevation myocardial infarction: 1-year results from a prospective observational study (Bleeding and Myocardial Infarction Study). Arch. Cardiovasc. Dis. 109(1), 31–38 (2016).
Lattuca, B. et al. One-year incidence and clinical impact of bleeding events in patients treated with prasugrel or clopidogrel after ST-segment elevation myocardial infarction. Arch. Cardiovasc. Dis. 109(5), 337–347 (2016).
Gimbel, M. et al. Clopidogrel versus ticagrelor or prasugrel in patients aged 70 years or older with non-ST-elevation acute coronary syndrome (POPular AGE): The randomized, open-label, non-inferiority trial. Lancet 395, 1374–1381 (2020).
Steg, P. G. et al. Effect of cangrelor on periprocedural outcomes in percutaneous coronary interventions: A pooled analysis of patient-level data. Lancet 382(9909), 1981–1992 (2013).
Leonardi, S. et al. Rationale and design of the cangrelor versus standard therapy to achieve optimal management of platelet Inhibition PHOENIX trial. Am. Heart. J. 163(5), 768-776.e2 (2012).
Fei, Y., Tsoi, M. F., Cheung, T. T. & Cheung, B. M. Optimal duration of dual antiplatelet therapy after drug-eluting stent implantation: Meta-analysis of randomized controlled trials. Int. J. Cardiol. 220, 895–900 (2016).
Patti, G., Micieli, G., Cimminiello, C. & Bolognese, L. The role of clopidogrel in 2020: A reappraisal. Cardiovasc. Ther. 2020, 8703627 (2020).
Author information
Authors and Affiliations
Contributions
Y.F. designed the study, performed the statistical analysis, interpreted the data and wrote the first draft. Y.F. and C.K.L. participated in the literature searching and selecting the eligible studies. Y.F. and B.M.Y.C. contributed to the data interpretation and writing of the final version of the manuscript. All authors have approved the final version of the manuscript and its conclusions. The corresponding author has full access to the data and had final responsibility for the decision to submit for publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fei, Y., Lam, C.K. & Cheung, B.M.Y. Efficacy and safety of newer P2Y12 inhibitors for acute coronary syndrome: a network meta-analysis. Sci Rep 10, 16794 (2020). https://doi.org/10.1038/s41598-020-73871-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-73871-x
This article is cited by
-
Efficacy and safety of cangrelor as compared to ticagrelor in patients with ST-elevated myocardial infarction (STEMI): a systematic review and meta-analysis
The Egyptian Heart Journal (2024)
-
Time from blood draw to multiple electrode aggregometry and association with platelet reactivity
Journal of Thrombosis and Thrombolysis (2022)
-
Clinical efficacy and safety of tirofiban combined with conventional dual antiplatelet therapy in ACS patients undergoing PCI
Scientific Reports (2021)
-
Low-dose aspirin for primary and secondary prevention of cardiovascular events in Denmark 1998–2018
Scientific Reports (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.