Abstract
Clinical impact of fine particulate matter (PM2.5) air pollution on incident atrial fibrillation (AF) had not been well studied. We used integrated machine learning (ML) to build several incident AF prediction models that include average hourly measurements of PM2.5 for the 432,587 subjects of Korean general population. We compared these incident AF prediction models using c-index, net reclassification improvement index (NRI), and integrated discrimination improvement index (IDI). ML using the boosted ensemble method exhibited a higher c-index (0.845 [0.837–0.853]) than existing traditional regression models using CHA2DS2-VASc (0.654 [0.646–0.661]), CHADS2 (0.652 [0.646–0.657]), or HATCH (0.669 [0.661–0.676]) scores (each p < 0.001) for predicting incident AF. As feature selection algorithms identified PM2.5 as a highly important variable, we applied PM2.5 for predicting incident AF and constructed scoring systems. The prediction performances significantly increased compared with models without PM2.5 (c-indices: boosted ensemble ML, 0.954 [0.949–0.959]; PM-CHA2DS2-VASc, 0.859 [0.848–0.870]; PM-CHADS2, 0.823 [0.810–0.836]; or PM-HATCH score, 0.849 [0.837–0.860]; each interaction, p < 0.001; NRI and IDI were also positive). ML combining readily available clinical variables and PM2.5 data was found to predict incident AF better than models without PM2.5 or even established risk prediction approaches in the general population exposed to high air pollution levels.
Similar content being viewed by others
Introduction
Although atrial fibrillation (AF) often leads to complications such as stroke in patients without an awareness of such preexisting diseases, electrocardiogram screening is not sufficient to detect AF in the general population1,2. Some scoring systems for predicting incident AF have been introduced, including the CHADS23, CHA2DS2-VASc4, and HATCH5 scores; however, their prediction accuracies are not sufficient for wide application. Although epidemiological studies have suggested that an elevated level of ambient particulate matter < 2.5 μm in aerodynamic diameter (PM2.5) is consistently associated with adverse cardiac events6 and arrhythmias7, including AF8, the role of PM2.5 on incident AF remains to be investigated. Recently, data-driven analyses using machine learning (ML) methods have been introduced to identify some blood biomarkers that are risk factors of AF prevalence (not incidence)9, and they were considered non-inferior to traditional analyses9,10. However, it was not clear whether these data-driven approaches could find correlations between PM2.5 and incident AF, or if they could predict incident AF better than traditional analysis in clinical practice.
Although some studies from Western countries did not show a correlation between short-term exposure to PM2.5 and incident AF11,12, the air pollution levels in those areas were much lower than the levels in Asian countries; therefore, the effect sizes could be low in those studies. Our previous study, performed in the general population of an Asian country, showed correlations between PM2.5 exposure and increased AF incidence8. Thus far, the identification of AF risk factors had been hypothesis driven and most studies performed analyses based on the selection of several cardiovascular risk factors. To perform a data-driven analysis for revealing AF risk factors, we used 27 readily available parameters including PM2.5 level in the Korean general population. All subjects without a history of previous AF were included in our population to identify the risk factors for incident AF. We also analyzed already revealed clinical risk factors to determine which risk factors best predict incident AF in this population. We investigated the robust risk factors for incident AF by using both the traditional regression method and the ML algorithm.
Methods
In this nationwide cohort study, we investigated the relationship between long-term exposure to PM2.5 and incident AF by using ML methods. The study protocol adhered to the ethical guidelines of the 1975 Declaration of Helsinki. The protocol was approved by the Institutional Review Board of Yonsei University College of Medicine, which waived the need for informed consent.
Data source
The National Health Insurance Service (NHIS) of Korea is a single-payer program that is mandatory for all residents of South Korea. Among the entire South Korean population (about 47 million people in 2002), 96.6% are registered in the NHIS. About 70% of the entire cohort had undergone a national health examination. This retrospective study used the NHIS-based National Sample Cohort (NSC) 2002–2013 dataset, comprising a random sample of 1,025,340 subjects, which amounted to 2.2% of the entire Korean population under the NHIS in 2002 (46,605,433). This sample cohort was extracted through probability sampling from all beneficiaries of the NHIS and the National Medical Aid in 2002 based on the entirety of the national cohort data. Systematic sampling was performed in each of the 1476 strata based on age, sex, eligibility status, socioeconomic status, and income level, with the sample size proportionate to the cohort size of the strata. The representativeness of the sample has been previously examined by comparing the sample to the entire Korean population13.
Study cohort
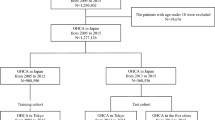
From the NHIS-NSC, adults (n = 506,805) older than 18 years who had undergone a national health examination at least once between 2009 and 2013 were selected13. Each subject was followed from the day of the health examination to December 31, 2013, and screened from January 2002 to assess the medical history (2002–2008: disease-free baseline period). Each diagnosis, based on the 10th revision of the International Classification of Disease codes, was defined as the first occurrence during at least two different days of outpatient hospital visits or on the first hospital admission (Supplementary Table 1)14,15. The following exclusion criteria were applied (Fig. 1): (i) age < 18 years; (ii) a diagnosis of AF before undergoing a health examination; (iii) valvular AF, such as mitral valve stenosis or prosthetic valve disease; (iv) change of residence to another ZIP code in 2009–2013; and (v) missing data on residential ZIP code or in questionnaires for smoking status and alcohol intake completed during the health examinations. A final population of 432,587 subjects was included in the analysis (Fig. 1). For the purpose of analysis, the subjects were divided chronologically in an approximately 7:3 ratio for a conventional discovery-validation approach.
Study cohort and included subjects from the National Health Insurance Service National Sample Cohort (NHIS-NSC; overall general population). We randomly divided the population into the discovery (n = 302,811) and validation (n = 129,776) cohorts. In the discovery cohort, model construction and training were performed. Model fitting and performance measures were performed in the validation cohort. AF atrial fibrillation.
Air pollution measurements
During the study period, PM2.5 levels, temperature, and humidity were measured hourly at the 313 sites of the Korean Nationwide Meteorological Observatory by the Korean Department of Environmental Protection. The entire Korean peninsula is divided into 256 residential ZIP codes including 74 metropolitan areas (average 73 km2). To assess long-term PM2.5 exposure effects, the nearest monitoring facility of each residence was identified and used to assess the average annual pollutant levels for each study subject16. The geographically based long-term average PM2.5 level during the study period was measured hourly by the monitoring facilities16, and 256 residential ZIP codes were matched with the nearest monitoring facilities. Meteorological variables (temperature and humidity) were included as geographically based long-term averages of the hourly measured temperature and humidity for each subject during the follow-up period. The long-term average (during the total study period for each subject) PM2.5 levels and meteorological measurements (temperature and humidity) were calculated from these hourly measurements at each site. The Korean National Ambient Air Quality Standards (NAAQS) and PM2.5 measurement methods are described in Supplementary Table 2.
Primary outcome
The primary outcome was the incidence of AF according to the PM2.5 level. AF was diagnosed on the basis of hospital admission or at least two outpatient visits for AF17,18. The cohort was followed up to the time of an AF incident, the time of disqualification from the NHIS (death or immigration), or the end of the study (December 31, 2013).
Machine learning
Twenty-six readily available clinical parameters and PM2.5 data were used for variable selection. Several supervised ML classifiers included support vector machine (SVM), decision tree, random forest, Naïve Bayes, deep neural network, and extreme gradient boosting models. Our SVM model was used to differentiate patients whether they developed new-onset AF or not by a computed hyper-plane (the optimal cost and gamma parameters were found with radial kernel) which separates these categories most effectively19. Our decision tree model constructed with a recursive tree structure using computationally selected parameters can differentiate features step by step by creating appropriate splits (recursive partitioning). And it was combined by ensembled algorithms to construct better prediction models such as gradient boosting (500 iterations using root mean square error as evaluating metric) and random forest (ten decision trees were combined to construct the best model)20. As mathematical models, artificial neural network systems mimic human neural networks which can be trained to discriminate different patterns of diseases, and we selected the three-layered deep neural network model using Tensorflow backend with Keras framework21. The entire ML process consisted of ML with automated feature selection by information gain attributive ranking algorithm22, model constructing with a boosted ensemble algorithm, and tenfold cross-validation to reduce overfitting23.
On the basis of supervised ML methods to construct prediction models, we used a sequential method of feature construction and automated selection by information gain ranking to identify predictive risk factors from the various health examination parameters22. Variable selection with an entry criterion of p < 0.05 was applied, and data-driven approaches were used to identify the smallest number of variables required for each prediction model. By using each model, selected variables including PM2.5 were modeled for their association with incident AF in the discovery cohort, and subsequently evaluated in the validation cohort (Fig. 1). New-onset AF events were analyzed with the geographically based long-term average PM2.5 level during the study period for each subject. Bootstrapping and tenfold cross-validation were used to adjust the model coefficient to avoid overfitting in the discovery sample. Model accuracy was calculated in the validation sample (30% of the original dataset), and loss was calculated using binary or categorical cross-entropy. The area under the receiver operating characteristic curve or c-index was used for each constructed model, and the net reclassification improvement index (NRI) and integrated discrimination improvement index (IDI) were calculated to assess the additional discriminative ability of these models.
Networks for training and validation were constructed based on Tensorflow (version 1.10) using the Keras framework (version 2.1.6), and all statistical analyses were performed using R (version 3.5) and Python (version 3.6) software. Training and validation were performed on an Intel central processing unit Xeon Scalable Gold 6126 under two graphics processing unit (GPU) devices support (two Nvidia RTX 2080Ti GPU devices; CUDA version 9.0), and constructed models were saved for further analysis.
Statistical analyses
The baseline characteristics of subjects with and without AF in both the discovery and validation cohorts were compared. We assumed that the study subjects were exposed to ambient air pollution within their residential ZIP codes during the study period16. Individual subjects were matched with the average air pollution levels and meteorological information during the study period obtained from the nearest monitoring facilities (according to the subjects’ residential address). By using Cox proportional-hazard model regression analysis, the relationship between incident AF and PM2.5 level was analyzed using a generalized estimating equation approach with a random-effect analysis24. The proportionality of the hazard assumptions was checked with a log-minus-log graph and a test on the Schoenfeld residuals. Consequently, the test results were found to be valid for each lifestyle factor. In Cox regression analysis, the included subjects were followed from their national health examination until the development of new-onset AF, disqualification (death or immigration), or the end of the study. A two-tailed p-value of < 0.05 was considered statistically significant.
Results
Baseline characteristics
There were no significant differences in body mass index (BMI), smoking history, socioeconomic status, and follow-up duration between the groups (Table 1). Subjects who developed new-onset AF during the follow-up were older, included a higher proportion of men, were more likely to have comorbidities, and were exposed to higher average PM2.5 levels than those without AF (Table 1). Subjects with AF had higher use of antiplatelet agents, beta-blockers, and statins than those without AF.
Application of PM2.5 to traditional regression analysis improves the prediction of incident AF
The air pollution and meteorological measurements are described in detail in Supplementary Results (Supplementary Table 3). We have previously reported on the association between increased exposure to long-term average PM2.5 and increased incidence of AF8. The traditional regression analysis with a forward selection method revealed PM2.5 and the following seven clinical risk factors to be associated with incident AF: older age (odds ratio [OR] 1.06 [1.05–1.07] per year increase, p < 0.001), male sex (OR 1.57 [1.42–1.73], p < 0.001), higher BMI (OR 1.04 [1.02–1.05] per 1 kg/m2 increase, p < 0.001), higher diastolic blood pressure (OR 1.05 [1.03–1.8] per 5 mmHg increase, p = 0.013) and systolic blood pressure (OR 1.02 [1.01–1.04] per 5 mmHg increase, p = 0.034), history of previous heart failure (OR 2.20 [1.90–2.54], p < 0.001), lower serum estimated glomerular filtration rate (OR 1.04 [1.02–1.07] per 10 mL/min decrease, p < 0.001), and higher level of PM2.5 exposure (OR 1.27 [1.24–1.31] per 10 μg/m3 increase, p < 0.001). After the application of PM2.5 to the readily available clinical risk factors, the c-index of the model for predicting incident AF was improved from 0.643 (0.636–0.649) to 0.819 (0.813–0.825) (p < 0.001) (Table 2). The NRI and IDI between these models were also positive (1.069 [1.038–1.103] and 0.302 [0.294–0.322], respectively) (Table 2).
Risk factors including PM2.5 for incident AF and score development
Crude and multivariable-adjusted Cox regression analyses showed that heart failure, hypertension, age (≥ 75 years), diabetes, previous stroke/transient ischemic attack, previous myocardial infarction or peripheral vascular disease, male sex, chronic obstructive pulmonary disease (these are components of the CHA2DS2-VASc, CHADS2, and HATCH scores), and PM2.5 (≥ 15 μg/m3 based on the Korean annual NAAQS)25 were independent risk factors for incident AF (Table 3). According to these multivariable Cox regression analyses, PM2.5 ≥ 15 μg/m3 (HRs 1.1–1.4) was pointed as 1 compared with HRs of other clinical variables and added this to existing CHA2DS2-VASc, CHADS2, and HATCH systems, then we developed new simple PM-CHA2DS2-VASc, PM-CHADS2, and PM-HATCH scores (Table 3). The total scores ranged from 0 to 10, 0 to 7, and 0 to 8 points, respectively.
The scores showed good discrimination with c-indices of 0.859 (0.848–0.870), 0.823 (0.810–0.836), and 0.849 (0.837–0.860), respectively (Table 2). These scoring systems showed significantly better performances for predicting incident AF than each existing score (CHA2DS2-VASc, CHADS2, and HATCH), and their NRI and IDI were also positive (Table 2).
Incidence rates of AF and the PM-CHA2DS2-VASc, PM-CHADS2, and PM-HATCH scores
Supplementary Table 4 shows the annual incidence rates (%/year) and hazard ratios at each point of these scoring systems. We divided the overall population into three groups according to each score: low risk (0–1 point; 0.13%/year by PM-CHA2DS2-VASc score, 0.11%/year by PM-CHADS2 score, and 0.17%/year by PM-HATCH score), intermediate risk (2–3 points; 1.30%/year by PM-CHA2DS2-VASc score, 0.70%/year by PM-CHADS2 score, and 1.75%/year by PM-HATCH score), and high risk (≥ 4 points; 2.74%/year by PM-CHA2DS2-VASc score, 2.05%/year by PM-CHADS2 score, and 3.51%/year by PM-HATCH score). Kaplan–Meier curves showed consistent findings according to risk categories (log-rank p < 0.001, Fig. 2).
Kaplan–Meier curves for risk categories according to PM-CHA2DS2-VASc, PM-CHADS2, and PM-HATCH scores. Patients were divided into three groups as low (0–1 points), intermediate (2–3 points), and high risk (≥ 4 points) groups. We pointed as 1 if PM2.5 ≥ 15 μg/m3 based on Korean National Ambient Air Quality Standards.
Comparing ML models with the traditional regression model
To estimate the crude accuracies of the ML models for predicting incident AF, we compared the six ML models and the traditional regression analysis model with age, sex, and BMI as input variables (Table 4). The c-indices of the six ML models (SVM, decision tree, random forest, naïve Bayes, deep neural network, and extreme gradient boosting models) were 0.699 (0.688–0.710), 0.786 (0.771–0.800), 0.787 (0.772–0.801), 0.794 (0.780–0.807), 0.779 (0.768–0.790), and 0.790 (0.776–0.805), respectively, and they were all significantly better than the c-index of the traditional regression analysis model (c-index 0.604 [0.598–0.611], all p < 0.001). All NRI and IDI were also positive (Table 4). The extreme gradient boosting model showed the highest c-index for predicting incident AF among these models (Table 4).
Validation by ML models and the application of PM2.5 for predicting incident AF
We used several ML models and performed analyses for predicting incident AF in our cohort. We used the 27 variables listed in Table 1 as input variables for the ML models and performed training using a discovery cohort of 302,811 subjects including 2444 with incident AF (0.8%) that developed over the 5-year follow-up period (Table 1). The feature selection process revealed variables that were most relevant for building the ML models (Supplementary Figure or Variable importance 10 ranking—Table 5). For random forest and extreme gradient boosting ML models, based on decision trees, the rank variable importance is determined by the selection frequency of the variables as a decision node, whereas SVM uses the sensitivity of generalization error bounds with respect to a variable and neural networks use overall weighting of the variable within the model26. PM2.5 was also highly ranked and other variables were also selected, as described in Supplementary Figure and Table 5.
After applying the tenfold cross-validation algorithm, the best ML model was the extreme gradient boosting model of the boosted ensemble algorithm with a c-index of 0.845 (0.837–0.853) (Table 2). After adding PM2.5 as an input variable, the performance of predicting incident AF was improved, with a c-index of 0.954 (0.949–0.959) with NRI of 1.277 (1.218–1.334) and IDI of 0.461 (0.438–0.485) (Table 2). For models using SVM, decision tree, random forest, naïve Bayes, and deep neural network algorithms, the performances for predicting incident AF were significantly improved after applying PM2.5, with c-indices of 0.903 (0.895–0.910), 0.931 (0.925–0.937), 0.939 (0.933–0.945), 0.894 (0.888–0.900), and 0.849 (0.834–0.865), respectively (Fig. 3 and Table 2). All NRI and IDI were also positive (Table 2).
Comparison of models for predicting incident atrial fibrillation based on the c-index. DNN deep neural network model, DT decision tree model, NB naïve Bayes model, PM2.5 particulate matter < 2.5 μm in diameter, RF random forest model, SVM support vector machine, TR1 traditional regression analysis model using clinical six variables (adjusted variables were same with that of TR1 (model 1) in Table 2) as input variables, TR2 traditional regression analysis model using clinical 12 variables (adjusted variables were same with that of TR2 (model 2) in Table 2), XGBM extreme gradient boosting model.
Discussion
There have been few studies about the correlations between PM2.5 and incident AF, and the clinical significance of PM2.5 for predicting AF incidence has not been investigated. In this study, we investigated the clinical impact of PM2.5 on predicting AF incidence by using ML methods in the general population of an Asian country affected by high air pollution levels. ML methods identified the clinically important variables for predicting incident AF, and PM2.5 was also identified as a highly ranked important variable. With the addition of the PM2.5 variable, the prediction performance significantly improved with both traditional regression analysis and ML methods. Additionally, based on traditional regression analysis, we constructed scoring systems for predicting incident AF by adding the PM2.5 variable to existing risk prediction systems: PM-CHA2DS2-VASc, PM-CHADS2, and PM-HATCH, which showed better predicting performances than the established scoring systems.
Although some studies, including our previous study8, have shown the relationships between air pollution exposure and AF development in patients with known cardiac diseases27, some studies from Western countries did not show a relationship between PM2.5 exposure and incident AF11,12. However, these studies were performed in European countries and the United States, where the air pollution levels were much lower than the levels in Asian countries. Therefore, the effect sizes could be low in those studies. In our nationwide dataset, to facilitate the data-driven analysis for revealing AF risk factors, we used 27 readily available parameters among the general population, and PM2.5 was identified as a highly ranked variable. Adding the information of PM2.5 exposure to known clinical risk factors can enable a better prediction of incident AF in the general population. Additionally, we attempted to apply this information about PM2.5 exposure for predicting incident AF in clinical practice by constructing relevant risk scores based on the Korean NAAQS, which might add some information when managing patients with AF risk factors. Further prospective studies using these new risk scoring systems will be needed whether upstream medical therapy is beneficial to prevent incident AF in the general population.
The adverse health effects related to air pollution have been studied since 199328, even for arrhythmias29. One suggested mechanism is the occurrence of myocardial repolarization abnormalities contributing to arrhythmias30 caused by systemic inflammatory cytokines produced by pulmonary inflammatory responses after inhaling particles6. Another suggested mechanism includes alteration of the cardiac autonomic nervous system that occurs with the inhalation of particles mediated by reactive oxygen species31,32, these adverse inhalation effects can be diminished in patients with chronic lung parenchymal diseases33,34.
As an advanced computing technology for artificial intelligence, ML is increasingly used in cardiology to meaningfully process data that exceed the capacity of the human brain35. Unlike traditional statistical analyses, ML models can accept enormous data as input variables and can improve the prediction performances through a repetitive training process, thus offering more applicable prediction models to external datasets20,36. Everyone can access this advanced computing technology and it can rapidly construct models by automatically training process, consequently it can offer better prediction model than traditional model manually made by human35. However, ML is highly data-dependent (“garbage-in, garbage-out”) and hard to interpret, and it often develops overfitting problems35.
To the best of our knowledge, this is the first cohort study with 1,666,528 person-years of follow-up to assess the prediction performance of long-term PM2.5 exposure for incident AF. Additionally, long-term PM2.5 exposure was identified as a highly important variable for predicting incident AF by using ML methods. After adding the PM2.5 variable to established AF prediction scoring systems, the prediction performances for incident AF significantly improved.
Our study suggests that applying long-term average PM2.5 measurements in clinical practice could better predict the development of AF in patients. Additionally, ML using boosted ensemble methods can predict incident AF better, with readily available subject characteristics, than traditional regression analysis. The detailed characteristics of the subjects in this study allowed blood pressure measurements, blood test results including fasting glucose and cholesterol profiles, and smoking and alcohol intake habits to be integrated in these analyses.
On the basis of these findings, we constructed scoring systems for predicting incident AF by adding the PM2.5 variable to existing risk prediction approaches: PM-CHA2DS2-VASc, PM-CHADS2, and PM-HATCH, which showed better predicting performances than established scoring systems.
Although our findings from traditional regression analysis and novel ML methods drew similar results, there are some limitations. Although we set disease-free baseline period (7 years: 2002–2008) with excluding subjects with a previous AF history, the existence of selection bias cannot be ruled out. However, the diagnostic accuracy of AF with this manner was previously validated in our NHIS database17. Although we excluded subjects who changed residence within the study period, subjects’ air pollutant exposure or specific locations could not be fully reflected during the period. In addition, as our data were from the National Health Insurance administrative claims database, the exact hour of AF development could not be identified. Therefore, we thought that the analysis for the effects of acute exposure might draw somewhat biased results, and further investigations are needed8. Although we used previously established risk scoring systems such as CHA2DS2-VASc4, CHADS23, and HATCH5 scores, they were not originally designed for predicting incident AF. However, these scores included important clinical comorbidities affect AF development, and we also assessed the prediction performances of traditional regression analyses and ML methods using readily available clinical risk factors and it showed better prediction performances with PM2.5 than those without PM2.5 (Table 2 and Fig. 3). Our prediction models did not adjust some confounders such as echocardiographic parameters (left atrial size, left ventricular ejection fraction, and ventricular chamber size), other chronic diseases (liver diseases, dementia, and chronic systemic inflammatory diseases), and some exposure confounders (occupational aspects who usually work inside or outside) that affect AF development or air pollution exposures. As we did not investigate the associations between air pollution and myocardial repolarization or inflammatory markers, the mechanism behind the relationship of exposure to air pollution and AF remains unclear. Although statistical approaches including cross-validation to minimize overfitting were applied when constructing ML models, and they supported our main results, external validation is needed especially in Western countries where the air pollution level is low.
Conclusions
Data-driven approaches suggested long-term exposure to PM2.5 air pollution as a risk factor robustly associated with incident AF. Such ML models combining readily available clinical characteristics and PM2.5 measurements were found to predict incident AF better than traditional statistical models or even established risk prediction approaches in the Korean general population exposed to high levels of air pollution. Further external validation is warranted especially in Western countries affected by low levels of air pollution.
References
Wachter, R. et al. Holter-electrocardiogram-monitoring in patients with acute ischaemic stroke (Find-AFRANDOMISED): an open-label randomised controlled trial. Lancet Neurol. 16, 282–290 (2017).
Freedman, B. et al. Screening for atrial fibrillation: a report of the AF-SCREEN International Collaboration. Circulation 135, 1851–1867 (2017).
Chao, T. F. et al. CHADS2 score and risk of new-onset atrial fibrillation: a nationwide cohort study in Taiwan. Int. J. Cardiol. 168, 1360–1363 (2013).
Hsieh, C. Y., Lee, C. H., Wu, D. P. & Sung, S. F. Prediction of new-onset atrial fibrillation after first-ever ischemic stroke: a comparison of CHADS2, CHA2DS2-VASc and HATCH scores and the added value of stroke severity. Atherosclerosis 272, 73–79 (2018).
Suenari, K. et al. Usefulness of HATCH score in the prediction of new-onset atrial fibrillation for Asians. Medicine (Baltimore) 96, e5597 (2017).
Brook, R. D. et al. Particulate matter air pollution and cardiovascular disease: an update to the scientific statement from the American Heart Association. Circulation 121, 2331–2378 (2010).
Link, M. S. & Dockery, D. W. Air pollution and the triggering of cardiac arrhythmias. Curr. Opin. Cardiol. 25, 16–22 (2010).
Kim, I. S. et al. Long-term exposure of fine particulate matter air pollution and incident atrial fibrillation in the general population: a nationwide cohort study. Int. J. Cardiol. 283, 178–183 (2019).
Chua, W. et al. Data-driven discovery and validation of circulating blood-based biomarkers associated with prevalent atrial fibrillation. Eur. Heart J. 40, 1268–1276 (2019).
Krittanawong, C. et al. Deep learning for cardiovascular medicine: a practical primer. Eur. Heart J. 40, 2058–2073 (2019).
Milojevic, A. et al. Short-term effects of air pollution on a range of cardiovascular events in England and Wales: case-crossover analysis of the MINAP database, hospital admissions and mortality. Heart 100, 1093–1098 (2014).
Bunch, T. J. et al. Atrial fibrillation hospitalization is not increased with short-term elevations in exposure to fine particulate air pollution. Pacing Clin. Electrophysiol. 34, 1475–1479 (2011).
Lee, J., Lee, J. S., Park, S. H., Shin, S. A. & Kim, K. Cohort Profile: The National Health Insurance Service-National Sample Cohort (NHIS-NSC), South Korea. Int. J. Epidemiol. 46, e15 (2017).
Chimonas, M. A. & Gessner, B. D. Airborne particulate matter from primarily geologic, non-industrial sources at levels below National Ambient Air Quality Standards is associated with outpatient visits for asthma and quick-relief medication prescriptions among children less than 20 years old enrolled in Medicaid in Anchorage, Alaska. Environ. Res. 103, 397–404 (2007).
Dabass, A. et al. Systemic inflammatory markers associated with cardiovascular disease and acute and chronic exposure to fine particulate matter air pollution (PM2.5) among US NHANES adults with metabolic syndrome. Environ. Res. 161, 485–491 (2018).
Di, Q. et al. Air pollution and mortality in the medicare population. N. Engl. J. Med. 376, 2513–2522 (2017).
Kim, T. H. et al. CHA2DS2-VASc Score (Congestive Heart failure, hypertension, age >/=75 [doubled], diabetes mellitus, prior stroke or transient ischemic attack [doubled], vascular disease, age 65–74, female) for stroke in asian patients with atrial fibrillation: a Korean Nationwide Sample Cohort Study. Stroke 48, 1524–1530 (2017).
Kim, T. H. et al. CHA2DS2-VASc Score for identifying truly low-risk atrial fibrillation for stroke: a Korean Nationwide Cohort study. Stroke 48, 2984–2990 (2017).
Kim, K. A. et al. Mortality prediction of rats in acute hemorrhagic shock using machine learning techniques. Med. Biol. Eng. Comput. 51, 1059–1067 (2013).
Al’Aref, S. J. et al. Clinical applications of machine learning in cardiovascular disease and its relevance to cardiac imaging. Eur. Heart J. 40, 1975–1986 (2019).
Yoo, T. K., Kim, D. W., Choi, S. B., Oh, E. & Park, J. S. Simple scoring system and artificial neural network for knee osteoarthritis risk prediction: a cross-sectional study. PLoS ONE 11, e0148724 (2016).
Urbanowicz, R. J., Olson, R. S., Schmitt, P., Meeker, M. & Moore, J. H. Benchmarking relief-based feature selection methods for bioinformatics data mining. J. Biomed. Inform. 85, 168–188 (2018).
Molinaro, A. M., Simon, R. & Pfeiffer, R. M. Prediction error estimation: a comparison of resampling methods. Bioinformatics 21, 3301–3307 (2005).
Zeger, S. L., Liang, K. Y. & Albert, P. S. Models for longitudinal data: a generalized estimating equation approach. Biometrics 44, 1049–1060 (1988).
Annual Report of Air Quality in Korea, National Institute of Environmental Research, Republic of Korea https://library.me.go.kr/search/DetailView.Popup.ax?sid=4&cid=5618423 (2018).
Weng, S. F., Reps, J., Kai, J., Garibaldi, J. M. & Qureshi, N. Can machine-learning improve cardiovascular risk prediction using routine clinical data?. PLoS ONE 12, e0174944 (2017).
Link, M. S. et al. Acute exposure to air pollution triggers atrial fibrillation. J. Am. Coll. Cardiol. 62, 816–825 (2013).
Dockery, D. W. et al. An association between air pollution and mortality in six U.S. cities. N. Engl. J. Med. 329, 1753–1759 (1993).
Rich, D. Q. et al. Increased risk of paroxysmal atrial fibrillation episodes associated with acute increases in ambient air pollution. Environ. Health Perspect. 114, 120–123 (2006).
Kim, J. B. et al. Particulate air pollution induces arrhythmia via oxidative stress and calcium calmodulin kinase II activation. Toxicol. Appl. Pharmacol. 259, 66–73 (2012).
Schwartz, J. et al. Traffic related pollution and heart rate variability in a panel of elderly subjects. Thorax 60, 455–461 (2005).
Pope, C. A. 3rd. et al. Heart rate variability associated with particulate air pollution. Am. Heart J. 138, 890–899 (1999).
Kim, I. S. et al. Association of air pollution with increased incidence of ventricular tachyarrhythmias recorded by implantable cardioverter defibrillators: Vulnerable patients to air pollution. Int. J. Cardiol. 240, 214–220 (2017).
Kim, I. S. et al. Long-term fine particulate matter exposure and cardiovascular mortality in the general population: a nationwide cohort study. J. Cardiol. 75, 549–558 (2019).
Rajkomar, A., Dean, J. & Kohane, I. Machine learning in medicine. N. Engl. J. Med. 380, 1347–1358 (2019).
Johnson, K. W. et al. Artificial intelligence in cardiology. J. Am. Coll. Cardiol. 71, 2668–2679 (2018).
Levey, A. S. et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 150, 604–612 (2009).
Acknowledgements
This study was supported by research grants from the Korean Healthcare Technology R&D project funded by the Ministry of Health & Welfare (HI15C1200, HC19C0130), and a faculty research grant of Department of Internal Medicine, Yonsei University College of Medicine for 2019-8020-80717-4323130, a research grant from the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology (NRF-2017R1A2B3003303).
Author information
Authors and Affiliations
Contributions
All authors contributed to this paper. I.S.K. performed the data analysis and wrote the entire manuscript. P.S.Y. and E.J. performed primary data acquisition and sorting. H.J. and S.C.Y. performed data analysis and took part in constructing the study design. H.T.Y. and T.H.K. also performed data analysis and revised the manuscript. J.S.U. and J.Y.K. checked analyzed data and revised “Discussion” section. H.N.P., and M.H.L. also checked the analyzed data and revised entire manuscript. J.Y.K. and B.J. coordinated this study as corresponding authors and helped to draft the manuscript. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kim, IS., Yang, PS., Jang, E. et al. Long-term PM2.5 exposure and the clinical application of machine learning for predicting incident atrial fibrillation. Sci Rep 10, 16324 (2020). https://doi.org/10.1038/s41598-020-73537-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-73537-8
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.