Abstract
AntiTNF-α biosimilars are broadly available for the treatment of inflammatory arthritis. There are a lot of data concerning the maintenance of clinical efficacy after switching from originators to biosimilars; therefore, such a transition is increasingly encouraged both in the US and Europe. However, there are reports about flares and adverse events (AE) as a non-medical switch remains controversial due to ethical and clinical implications (efficacy, safety, tolerability). The aim of our work was to evaluate the disease activity trend after switching from etanercept originator (oETA-Enbrel) to its biosimilar (bETA-SP4/Benepali) in a cohort of patients in Turin, Piedmont, Italy. In this area, the switch to biosimilars is stalwartly encouraged. We switched 87 patients who were in a clinical state of stability from oETA to bETA: 48 patients were affected by Rheumatoid Arthritis (RA),26 by Psoriatic Arthritis (PsA) and 13 by Ankylosing Spondylitis (AS).We evaluated VAS-pain, Global-Health, CRP, number of swollen and tender joints, Disease Activity Score on 28 joints (DAS28) for RA, Disease Activity in Psoriatic Arthritis (DAPSA) for PsA, Health Assessment Questionnaire (HAQ) and Health Assessment Questionnaire for the spondyloarthropathies (HAQ-S),Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) for AS patients. 11/85 patients (12.6%) stopped treatment after switching to biosimilar etanercept. No difference was found between oETA and bETA in terms of efficacy. However, some arthritis flare and AE were reported. Our data regarding maintenance of efficacy and percentage of discontinuation were in line with the existing literature.
Similar content being viewed by others
Introduction
Biologics are target-specific, highly effective drugs approved for many pathologic conditions such as inflammatory arthritis, psoriasis, Crohn disease, uveitis, osteoporosis, cancer, HIV, multiple sclerosis and others. In particular, over the last 20 years many advances allowed drugs to actually modify the natural history of rheumatic diseases such as Rheumatoid Arthritis, SpondyloArthritis (including Ankylosing Spondylitis), Psoriatic Arthritis, Reactive Arthritis, and more recently Systemic Lupus Erythematosus, thanks to their effectiveness in reducing disease activity, joint pain, swelling and damage progression. The high cost of Biological Disease Modifying Anti-Rheumatic Drugs (bDMARDs) in the world is the main factor limiting its prescription as a first line of therapy despite the best efficacy and tolerability.
Despite their cost, the three main anti-TNF alpha originators (Humira, Enbrel and Remicade) were amongst the top 20 drugs of the world ranking (which accounted for the total global prescription drug market in the last years); the annual growth for Humira in 2016 (the first product of the ranking) was 15%, accounting for a $16-billion-sale worldwide, which could also be due to its numerous indications1,2,3,4,5,6,7,8.
Biologics are derived from living cells crossing a complex biotechnological process. The intrinsic nature of these proteins makes it almost impossible to replicate an exact copy (generic) of a biological drug; therefore, biosimilars are products similar to the original drug in the active substance, but not identical for differences in its manufacturing process, including methods used to purify and stabilize cellular lines, which influence post-translational modifications of the proteins (such as glycosylation, etc.)9,10,11.
To date, the patents for 3 anti TNF-alpha (Remicade/Infliximab, Enbrel/Etanercept, Humira/Adalimumab) and one anti B-cells (Mabthera/Rituximab) have expired, thus allowing many biosimilars to be available in the world for the treatment of inflammatory joint diseases.
Several randomized, double-blind, controlled clinical trials versus placebo demonstrated the efficacy and safety of the switch from biologic originators to the biosimilar of infliximab, etanercept and adalimumab as many experiences from trials and real world data are available12,13,14,15,16,17,18,19,20, thus having been approved for the same indications by FDA (including extrapolated suggestions as well)21.
However, despite the considerable saving, such shifts to biosimilar drugs are still being debated, principally over their ethical implications. Since the drugs are similar but not identical, the main issues are related to the adverse events and the lack of efficacy, which cannot be excluded. This also implies that biosimilars could theoretically work better than originators, but the variability in effectiveness for a single patient remains an unpredictable datum before effecting the switch.
Despite the fact that extrapolation of indications are debated (especially for the treatment of inflammatory bowel diseases since the mechanisms of action might differ from indications22), the use of biosimilars appears to be regulated worldwide by local guidelines if safety and effectiveness are demonstrated in clinical trials for at least one indication. Moreover, data concerning immunogenicity, in at least one clinical trial comparing the development of anti-drug antibodies in patients previously exposed to the originator, are required by regulatory agencies before the approval of the biosimilar11,23,24,25,26,27.
A small survey conducted in the UK showed an agreement regarding the switch if the treatment "works as well as my existing" (40.4%), and 27.3% hoped “that someone who couldn’t otherwise get on to biologic treatment would benefit”28.
Other data showed that the cost saved by switching patients to biosimilars could enable more efficient allocations of health system resources thus improving patient care. The availability of biosimilars is an opportunity to reduce the price of biological therapies that is the main (and sometimes the only) limiting factor in many countries, as demonstrated in several studies29,30,31,32,33 such elements could allow an early access to biological treatments for the patients.
Moreover, the switch is an incentive for the originator pharmaceutical companies not only in reducing prices but also to invest in researching and developing new drugs21,34.
The price of biosimilars is 15–75% lower than the originator; since the intrinsic properties of biosimilar drugs (that are not generic drugs but bioequivalent), the interchangeability is a medical decision in almost all countries in Western Europe and in the USA. Therefore, this shift is not to be made by pharmacists or by others in order to prevent an automatic substitution10,35.
In literature, several studies and real-world data analysed the short-term impact of the switch from the anti-TNF originator to its biosimilar suggesting that there is a good maintenance of efficacy and safety; however, there are many reports of discontinuation due both lack of efficacy or adverse events. The percentage of interruption varies between 4 and 18%17,19,36,37,38,39,40,41,42,43,44,45,46. A Dutch study on 192 patients showed the highest percentage of discontinuation (24%); a sub-analysis of its data verified that the interruption was mainly related to subjective features such as tender joints and patient global assessment rather than objective variables. This phenomenon could be due to a nocebo effect43,47 and would require further investigations.
Besides, more recent data from the extension of observation in DANBIO registry confirmed that a certain percentage of switches failed due to patients factors rather than elements related to drug effects48.
However, a critical review emphasized the unbalanced cohort and results, asserting that, as of today, there is no study that properly follows FDA guidelines which state that randomised double-blind trials should be included in order to ensure: (a) the homogeneity of treatment groups and control bias; (b) an adequate control with measurement of different outcomes; (c) a proper statistical powering and an appropriate statistical analysis with a well-established evaluation of immunogenicity-related outcomes; (d) an adequate follow-up and assessments of individual patient-level outcomes to support the switch definitively49,50.
Reasons to switch, regional guidelines and aim
In Italy, the Italian competent authority for drugs (AIFA-Agenzia Italiana del Farmaco) published a position papers in 2018 about biosimilars and switching. Although AIFA leaves the final decision to the rheumatologist, it also encourages physicians to strongly consider literature data about the safety and efficacy of the switch, reminding the physicians of their role and responsibility in the economic sustainability of the health system51.
In Italy, biological drugs are fully refunded by the health system. After the authorization of the EMA, AIFA issues a decree in order to establish the class and the price of the drug; consequently, the marketing authorisation is granted. As soon as this decree is issued, each Italian region inductees an auction for the award of a public contract between the different producers; the winner of this auction is then permitted to sell their drug in the aforementioned region.
In Piedmont, SB4/Benepali won the auction vs Enbrel in 2017 with a significant price difference.
In our Region, after the AIFA approval for the reimbursement, a commission including members of the Regional Pharmaceutical Service and rheumatologists was established in order to produce a regional guidance on prescribing drugs for naïve patients and in case of switch. The prescription of biosimilars is highly recommended for naïve patients that require a specific target therapy, whilst the switch from originators to biosimilars is encouraged for all the patients treated with the originator; however, some peculiar exceptions are established: patients with history of allergy and/or particularly hypersensitive skin, off-label prescriptions, psychological reasons, active disease that requires a different treatment in the short term, paediatric patients, pregnancy52,53.
The regional recommendations refer exclusively to Etanercept and do not preclude in any way the possibility of prescribing the most suitable bDMARD or tsDMARD for the individual patient.
The aim of this work is to evaluate the disease activity trend after switching from etanercept originator Enbrel (oETA) to its biosimilar SB4/Benepali (bETA) in a population admitted to Città della Scienza e della Salute Hospital in Turin, Piedmont, Italy.
Considering that no changes in clinical outcomes were expected, and according to Regional recommendations, we properly discussed about these elements with every patient. In addition, the informed consent was binding in the physician’s final choice as the shared decision between rheumatologists and patients was mandatory54,55.
Materials and methods
We selected patients with clinical diagnosis of Rheumatoid Arthritis (RA), Psoriatic Arthritis (PsA) and Ankylosing Spondylitis (AS) who were admitted to the Rheumatology Unit of the University Hospital of Turin, Italy. The patients had been treated with oETA Enbrel® and switched to bETA Benepali®. As suggested by a Regional document, patients off-label, pregnancy and paediatric, patients with history of allergy, patients not in remission nor in low disease activity, patients with psychological reasons that forbid a change were excluded. As per EULAR guidelines54, we also excluded patients that refused the switch. At the time of the switch, every patient was informed about biosimilar properties, literature data and the possibility to return to originator if necessary. Almost all patients accepted the switch.
Sample analysis was stratified by age, sex, duration of disease and concomitant therapy. The disease activity was evaluated during the year before the introduction of the bETA, and then the trend of the disease activity was evaluated in the following 12 months during oETA treatment. Patients that stopped therapy for any reason has been evaluated only until the bETA interruption. It was also examined whether some baseline characteristics, such as the duration of treatment with oETA, concomitant therapy (conventional synthetic DMARDs and glucocorticoids) and disease activity, could influence the response to biosimilars.
Statistical analysis was performed using SPSS software (version 17.0, Windows 10 Pro build 1803) and MINI-TAB (version 14.0, Windows 10 Pro build 1803). Descriptive statistics will be provided for the clinical and laboratory demographic characteristics of the cases. In order to evaluate the presence of statistically significant differences between the parameters considered, the χ2 test for parametric variables was used. The comparison between groups was performed using the Kruskal–Wallis test and the U-Mann–Whitney test. All the performed tests were bilateral and the level of significance was set at 5% (with a 95% confidence interval).
Multivariable analysis with logistic regression was performed in order to analyze the association of interruption therapy with disease activity, concomitant therapy and oETN duration (age, gender and disease duration were already normalized at the beginning).
Results
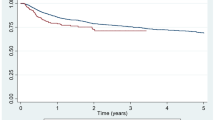
87/107 patients were included (37 male, 50 female) with a median age of 63.0 years old (IQ range 52.2–83.0); the patients were divided by pathology (RA, PsA and AS) while analyzing BMI, ACPA and RF positivity, treatment lines, duration of disease, duration of therapy with oETA and concomitant therapies (csDMARDs) (see Table 1). Patients treated with csDMARDs took Methotrexate in 96% of cases, with a dose between 10 and 15 mg per week. The comparisons of the progress of disease activity were evaluated for the different pathologies (RA, PsA and AS) with their respective clinimetric indices (DAS28, DAPSA, BASDAI, see Table 2). Data analysis showed there are no significant differences in clinimetric parameters after the switch from originator drugs to biosimilars. 11/85 patients (12.6%) stopped the treatment after switch to biosimilar drugs (bETA) due to lack of efficacy (LOE), subjective features (SF) and adverse event (AE); amongst these patients, 5 were affected by RA (3 LOE, 1 SF, 1 AE), 5 patients were affected by PsA (3 LOE, 1 SF, 1 AE), and 1 patient was affected by AS (1 LOE) (Table 3). The AE were not serious: one RA patient showed psoriasis whilst the second one experimented cutaneous rash and diffuse itch. Furthermore, a univariate analysis was performed in order to verify a possible correlation between the interruption of the therapy with bETA and the disease activity at the onset (RA p: 0.231; PsA p: 0.545; AS p: 0.823), the concomitant therapy (RA p: 0.555; PsA p: 0.623; AS p: 0.213) and the duration of oETA treatment (RA p: 0.426; PsA p: 0.676; AS p: 0.522).
Moreover, we performed the multivariable analysis with logistic regression to verify a possible correlation between the interruption of therapy with bETA, confounders and exposure variables (Table 4).
Discussion
Biosimilars drugs are similar to the originator in terms of quality, safety and effectiveness but there are many open questions about ethical implications.
The main concerns are those regarding the switch from the originator to its biosimilar product; the main doubts cover non-medical substitutions which could be performed for situations not related to drug's efficacy nor tolerability nor other medical reasons10,56.
The European Medical Agency (EMA) leaves the authority about interchangeability or substitution to each national agency.
Despite the lack of European guidelines, there is an ever-growing practical experience that demonstrates the safety and clinical effectiveness of biosimilars, as well as the savings generated from their introduction in clinical practice39,57,58,59. Therefore, further expensive trials could be avoided to demonstrate an already existing knowledge.
A large difference exists between Western Europe and Eastern Europe. In the latter, access to expensive drugs is limited so the automatic substitution is in some cases allowed, and in many other cases regulated by the law60.
The Italian competent authority for drugs (AIFA-Agenzia Italiana del Farmaco) published two position papers about biosimilars and switching. The aim of the documents was to provide health professionals and patients clear and validated information about biosimilars, including the role of biosimilars in the economic sustainability of the National Health Service. Even if the final decision about the switch is entrusted to the physician (after a proper informed consent given by patients) AIFA sustains the interchangeability of biosimilars and emphasizes the physician role in economic sustainability of the health system52.
The analysis of our real-life data in those patients who agreed to switch, confirms what has already emerged from clinical trials and real-world data in the literature. In particular, there were no statistically significant differences in disease activity after the switch to bETA and during the follow up (1 year after the switch). Furthermore, no correlations emerged between the interruption of treatment with bETA and the variables analysed. The adverse events were not serious. Amongst real-life data reports61,62,63,64,65,66,67, we collected data for up to 12 months of follow-up. However, this descriptive study has some limitations since it includes no data about pharmacokinetics or evaluation of anti-drug antibodies, from neither the originator nor the biosimilar. Finally, the nocebo effect was not investigated with psychometric measures.
In addition to this, 20 patients were excluded (21.4% of the sample); despite this element may seem to limit the study, it should be taken into consideration that 12 of these patients were carrying out therapies as per off label dose reductions (thus not being comparable with the rest), whilst 4 had psychological reasons (which are likely to be comprised inside the Nocebo effect) and 4 of them were paediatric patients. So, it can be concluded that there is an equal balance of negative and positive aspects that make this data loss less significant.
In conclusion, in our population, no difference has been observed with regard to efficacy and safety after the switch from originator to biosimilar, and no predictors of non-response to switch therapy are currently highlighted. In our opinion, the switch could be considered safe. The physician–patient cooperation play a key role for a successful switch; therefore, it is critical to ensure that patients are informed about all the relevant information related to the switch as well as the respect for patients decisions and to make a strict follow-up to check any AE immediately.
It is also fundamental to share clinical reports to improve real life data.
References
Urquhart, L. Top product forecasts for 2019. Nat. Rev. Drug Discov. 18, 91 (2019).
Terry, M. Drum Roll, Please! Top 10 Bestselling Drugs in the U.S. BioSpace. https://www.biospace.com/article/drumroll-please-top-10-bestselling-drugs-in-the-u-s-/ (2018).
Philippidis, A. The Top 15 Best-Selling Drugs of 2017. https://www.genengnews.com/a-lists/the-top-15-best-selling-drugs-of-2017/ (2018).
Philippidis, A. The Top 15 Best-Selling Drugs of 2016. GEN—Genetic Engineering and Biotechnology News https://www.genengnews.com/a-lists/the-top-15-best-selling-drugs-of-2016/ (2017).
Top-Selling, Top-Prescribed Drugs for 2016. https://www.medscape.com/viewarticle/886404 (2017).
Bartholow, M. Top Drugs of 2015. https://www.pharmacytimes.com/publications/issue/2016/july2016/top-drugs-of-2015 (2016).
Philippidis, A. The Top 25 Best-Selling Drugs of 2014. https://www.genengnews.com/a-lists/the-top-25-best-selling-drugs-of-2014/ (2015).
Brooks, M. Top 100 Most Prescribed, Top-Selling Drugs. https://www.medscape.com/viewarticle/829246 (2014).
Liu, L. Antibody glycosylation and its impact on the pharmacokinetics and pharmacodynamics of monoclonal antibodies and Fc-fusion proteins. J. Pharm. Sci. 104, 1866–1884 (2015).
Bridges, S. L. et al. The science behind biosimilars: Entering a new era of biologic therapy. Arthritis Rheumatol. Hoboken NJ 70, 334–344 (2018).
Guideline on similar biological medicinal products containing biotechnology-derived proteins as active substance: non-clinical and clinical issues. https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-similar-biological-medicinal-products-rev1_en.pdf EMEA/CHMP/BMWP/42832/2005 Rev1 (2014).
Cohen, S. et al. Efficacy and safety of the biosimilar ABP 501 compared with adalimumab in patients with moderate to severe rheumatoid arthritis: A randomised, double-blind, phase III equivalence study. Ann. Rheum. Dis. 76, 1679–1687 (2017).
Cohen, S. et al. SAT0171 Abp 501 biosmilar to adalimumab: Final safety, immunogenicity, and efficacy results from an open-label extension study. Ann. Rheum. Dis. 76, 834–835 (2017).
Papp, K. et al. Clinical similarity of biosimilar ABP 501 to adalimumab in the treatment of patients with moderate to severe plaque psoriasis: A randomized, double-blind, multicenter, phase III study. J. Am. Acad. Dermatol. 76, 1093–1102 (2017).
Shin, D., Lee, Y., Kim, H., Körnicke, T. & Fuhr, R. A randomized phase I comparative pharmacokinetic study comparing SB5 with reference adalimumab in healthy volunteers. J. Clin. Pharm. Ther. 42, 672–678 (2017).
Emery, P. et al. A phase III randomised, double-blind, parallel-group study comparing SB4 with etanercept reference product in patients with active rheumatoid arthritis despite methotrexate therapy. Ann. Rheum. Dis. 76, 51–57 (2017).
Griffiths, C. E. M. et al. The EGALITY study: A confirmatory, randomized, double-blind study comparing the efficacy, safety and immunogenicity of GP2015, a proposed etanercept biosimilar, vs. the originator product in patients with moderate-to-severe chronic plaque-type psoriasis. Br. J. Dermatol. 176, 928–938 (2017).
Park, W. et al. A randomised, double-blind, multicentre, parallel-group, prospective study comparing the pharmacokinetics, safety, and efficacy of CT-P13 and innovator infliximab in patients with ankylosing spondylitis: The PLANETAS study. Ann. Rheum. Dis. 72, 1605–1612 (2013).
Park, W. et al. Efficacy and safety of switching from reference infliximab to CT-P13 compared with maintenance of CT-P13 in ankylosing spondylitis: 102-week data from the PLANETAS extension study. Ann. Rheum. Dis. 76, 346–354 (2017).
Yoo, D. H. et al. A randomised, double-blind, parallel-group study to demonstrate equivalence in efficacy and safety of CT-P13 compared with innovator infliximab when coadministered with methotrexate in patients with active rheumatoid arthritis: The PLANETRA study. Ann. Rheum. Dis. 72, 1613–1620 (2013).
FDA. Biosimilars. https://www.fda.gov/drugs/therapeutic-biologics-applications-bla/biosimilars.FDA/drugs/therapeutic-biologics-applications-bla/biosimilars (2019).
Feagan, B. G. et al. The challenge of indication extrapolation for infliximab biosimilars. Biol. J. Int. Assoc. Biol. Stand. 42, 177–183 (2014).
Dörner, T. et al. The changing landscape of biosimilars in rheumatology. Ann. Rheum. Dis. 75, 974–982 (2016).
FDA. Scientific Considerations in Demonstrating Biosimilarity to a Reference Product. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/scientific-considerations-demonstrating-biosimilarity-reference-product/regulatory-information/search-fda-guidance-documents/scientific-considerations-demonstrating-biosimilarity-reference-product (2019).
Information and Submission Requirements for Biosimilar Biologic Drugs seb-pbu-2016-eng.pdf. https://www.canada.ca/content/dam/hc-sc/migration/hc-sc/dhp-mps/alt_formats/pdf/brgtherap/applic-demande/guides/seb-pbu/seb-pbu-2016-eng.pdf (2017).
Klein, A. V., Wang, J. & Bedford, P. Subsequent entry biologics (biosimilars) in Canada: approaches to interchangeability and the extrapolation of indications and uses—GaBI Journal. https://gabi-journal.net/subsequent-entry-biologics-biosimilars-in-canada-approaches-to-interchangeability-and-the-extrapolation-of-indications-and-uses.html (2014).
Nagai, S., Yanagihara, R. & Kishioka, Y. Japanese regulatory authority’s perspective on biosimilars. Lancet Oncol. 16, e101 (2015).
UKMi. Is it safe to switch to a biosimilar medicine? https://www.sps.nhs.uk/wp-content/uploads/2018/02/UKMi_QA-Biosimilars-switching_Aug-2017_FINAL.pdf (2017).
NHS England. Commissioning framework for biological medicines (including biosimilar medicines). https://www.england.nhs.uk/wp-content/uploads/2017/09/biosimilar-medicines-commissioning-framework.pdf (2017).
Deiana, S., Gabbani, T. & Annese, V. Biosimilars in inflammatory bowel disease: A review of post-marketing experience. World J. Gastroenterol. 23, 197–203 (2017).
Putrik, P. et al. In wealthier countries, patients perceive worse impact of the disease although they have lower objectively assessed disease activity: Results from the cross-sectional COMORA study. Ann. Rheum. Dis. 75, 715–720 (2016).
Putrik, P. et al. Inequities in access to biologic and synthetic DMARDs across 46 European countries. Ann. Rheum. Dis. 73, 198–206 (2014).
Putrik, P. et al. Variations in criteria regulating treatment with reimbursed biologic DMARDs across European countries. Are differences related to country’s wealth?. Ann. Rheum. Dis. 73, 2010–2021 (2014).
NHS England. Commissioning framework for biological medicines biosimilar-medicines-regulation.pdf. https://www.england.nhs.uk/wp-content/uploads/2017/09/biosimilar-medicines-commissioning-framework.pdf (2017).
Péntek, M. et al. Biological therapy in inflammatory rheumatic diseases: Issues in Central and Eastern European countries. Eur. J. Health Econ. HEPAC Health Econ. Prev. Care 15(Suppl 1), S35–S43 (2014).
IMS. The Impact of Biosimilar Competition in Europe. https://www.medicinesforeurope.com/wp-content/uploads/2017/05/IMS-Biosimilar-2017_V9.pdf (2017).
Roediger, A., Freischem, B. & Reiland, J.-B. What pricing and reimbursement policies to use for off-patent biologicals in Europe?—results from the second EBE biological medicines policy survey—GaBI Journal. https://gabi-journal.net/what-pricing-and-reimbursement-policies-to-use-for-off-patent-biologicals-in-europe-results-from-the-second-ebe-biological-medicines-policy-survey.html (2017).
Yoo, D. H. et al. Efficacy and safety of CT-P13 (biosimilar infliximab) in patients with rheumatoid arthritis: Comparison between switching from reference infliximab to CT-P13 and continuing CT-P13 in the PLANETRA extension study. Ann. Rheum. Dis. 76, 355–363 (2017).
Smolen, J. S. et al. Safety, immunogenicity and efficacy after switching from reference infliximab to biosimilar SB2 compared with continuing reference infliximab and SB2 in patients with rheumatoid arthritis: Results of a randomised, double-blind, phase III transition study. Ann. Rheum. Dis. 77, 234–240 (2018).
Emery, P. et al. Long-term efficacy and safety in patients with rheumatoid arthritis continuing on SB4 or switching from reference etanercept to SB4. Ann. Rheum. Dis. 76, 1986–1991 (2017).
Weinblatt, M. E. et al. Switching from reference adalimumab to SB5 (adalimumab biosimilar) in patients with rheumatoid arthritis: Fifty-two-week phase III randomized study results. Arthritis Rheumatol. Hoboken NJ 70, 832–840 (2018).
Jørgensen, K. K. et al. Switching from originator infliximab to biosimilar CT-P13 compared with maintained treatment with originator infliximab (NOR-SWITCH): A 52-week, randomised, double-blind, non-inferiority trial. Lancet Lond. Engl. 389, 2304–2316 (2017).
Glintborg, B. et al. A nationwide non-medical switch from originator infliximab to biosimilar CT-P13 in 802 patients with inflammatory arthritis: 1-year clinical outcomes from the DANBIO registry. Ann. Rheum. Dis. 76, 1426–1431 (2017).
Glintborg, B. et al. FRI0190 Clinical outcomes from a nationwide non-medical switch from originator to biosimilar etanercept in patients with inflammatory arthritis after 5 months follow-up. Results from the danbio registry. Ann. Rheum. Dis. 76, 553–554 (2017).
Avouac, J. et al. Systematic switch from innovator infliximab to biosimilar infliximab in inflammatory chronic diseases in daily clinical practice: The experience of Cochin University Hospital, Paris, France. Semin. Arthritis Rheum. 47, 741–748 (2018).
Tweehuysen, L. et al. Subjective complaints as the main reason for biosimilar discontinuation after open-label transition from reference infliximab to biosimilar infliximab. Arthritis Rheumatol. Hoboken NJ 70, 60–68 (2018).
Häuser, W., Hansen, E. & Enck, P. Nocebo phenomena in medicine: Their relevance in everyday clinical practice. Dtsch. Arzteblatt Int. 109, 459–465 (2012).
McKinnon, R. A. et al. Biosimilarity and interchangeability: Principles and evidence: A systematic review. BioDrugs Clin. Immunother. Biopharm. Gene Ther. 32, 27–52 (2018).
Cohen, H. P. et al. Switching reference medicines to biosimilars: A systematic literature review of clinical outcomes. Drugs 78, 463–478 (2018).
Glintborg, B. et al. To switch or not to switch: Results of a nationwide guideline of mandatory switching from originator to biosimilar etanercept. One-year treatment outcomes in 2061 patients with inflammatory arthritis from the DANBIO registry. Ann. Rheum. Dis. 78, 192–200 (2019).
Choe, J.-Y. et al. A randomised, double-blind, phase III study comparing SB2, an infliximab biosimilar, to the infliximab reference product Remicade in patients with moderate to severe rheumatoid arthritis despite methotrexate therapy. Ann. Rheum. Dis. 76, 58–64 (2017).
AIFA. Secondo Position Paper AIFA sui Farmaci Biosimilari. https://www.aifa.gov.it/sites/default/files/pp_biosimilari_27.03.2018.pdf (2018).
Regione Piemonte. Farmaci biosimilari. https://www.regione.piemonte.it/web/sites/default/files/media/documenti/2019-03/farmaci_biosimilari_linee_indirizzo2.docx.pdf (2017).
Ursula, A. Biosimilars—Position Paper Updating position statement from the European League Against Rheumatism (EULAR) Standing Committee of People with Arthritis/Rheumatism in Europe (PARE) August 2018. 7.
Kay, J. et al. Consensus-based recommendations for the use of biosimilars to treat rheumatological diseases. Ann. Rheum. Dis. 77, 165–174 (2018).
PMDA. Guideline for the Quality, Safety, and Efficacy Assurance of Follow-on Biologics. https://www.pmda.go.jp/files/000153851.pdf (2009).
Gulácsi, L. et al. Biosimilars for the management of rheumatoid arthritis: Economic considerations. Expert Rev. Clin. Immunol. 11(Suppl 1), S43-52 (2015).
Gulacsi, L. et al. Biosimilars for the management of inflammatory bowel diseases: Economic considerations. Curr. Med. Chem. 26, 259–269 (2019).
La Noce, A. & Marcin, E. Switching from reference to biosimilar products: An overview of the European approach and real-world experience so far. Eur. Med. J. https://www.emjreviews.com/rheumatology/article/switching-from-reference-to-biosimilar-products-an-overview-of-the-european-approach-and-real-world-experience-so-far/ (2018).
Rémuzat, C. et al. Key drivers for market penetration of biosimilars in Europe. J. Mark. Access Health Policy 5, 1272308 (2017).
Cantini, F. & Benucci, M. Mandatory, cost-driven switching from originator etanercept to its biosimilar SB4: Possible fallout on non-medical switching. Ann. Rheum. Dis. 79, e13 (2020).
Considerations in Demonstrating Interchangeability With a Reference Product Guidance for Industry. 23 (2019).
Gentileschi, S. et al. Switch from infliximab to infliximab biosimilar: Efficacy and safety in a cohort of patients with different rheumatic diseases. Expert Opin. Biol. Ther. 16, 1311–1312 (2016).
Nikiphorou, E. et al. Clinical effectiveness of CT-P13 (Infliximab biosimilar) used as a switch from Remicade (infliximab) in patients with established rheumatic disease. Report of clinical experience based on prospective observational data. Expert Opin. Biol. Ther. 15, 1677–1683 (2015).
Codreanu, C., Šírová, K., Jarošová, K. & Batalov, A. Assessment of effectiveness and safety of biosimilar infliximab (CT-P13) in a real-life setting for treatment of patients with active rheumatoid arthritis or ankylosing spondylitis. Curr. Med. Res. Opin. 34, 1763–1769 (2018).
Abdalla, A. et al. Long-term safety and efficacy of biosimilar infliximab among patients with inflammatory arthritis switched from reference product. Open Access Rheumatol. Res. Rev. 9, 29–35 (2017).
Batticciotto, A. et al. Safety and efficacy of switching from originator to CT-P13 infliximab biosimilar in patients affected by spondyloarthritis. A 6-month observational study. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/safety-and-efficacy-of-switching-from-originator-to-ct-p13-infliximab-biosimilar-in-patients-affected-by-spondyloarthritis-a-6-month-observational-study/ (2016).
Author information
Authors and Affiliations
Contributions
E.F. offered contributions to the design of the work; M.C.D. offered contribution in data collection, review of the literature and drafting of the work; S.P. offered contributions in statistical analisys. M.P., S.S., A.L. and C.L.P. offered contributions in data collection; A.D. offered contributions in interpretation of data.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ditto, M.C., Parisi, S., Priora, M. et al. Efficacy and safety of a single switch from etanercept originator to etanercept biosimilar in a cohort of inflammatory arthritis. Sci Rep 10, 16178 (2020). https://doi.org/10.1038/s41598-020-73183-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-73183-0
This article is cited by
-
Rapid monitoring of health services use following a policy to switch patients from originator to biosimilar etanercept—a cohort study in British Columbia
BMC Rheumatology (2022)
-
Patients Retransitioning from Biosimilar TNFα Inhibitor to the Corresponding Originator After Initial Transitioning to the Biosimilar: A Systematic Review
BioDrugs (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.