Abstract
Biologic and targeted synthetic disease-modifying antirheumatic drugs (ts/bDMARDs) play a pivotal role in the treatment of rheumatoid arthritis (RA), psoriatic arthritis (PsA), and ankylosing spondylitis (AS). Persistence of therapy provides an index of a drug’s overall effectiveness. The objective of the study was to identify factors associated with discontinuation of ts/bDMARDs in a real-world dataset. The study population comprised patients diagnosed with RA, PsA, and AS included in the BIOBADASER registry for whom follow-up data were available until November 2019. Patient features and treatment data were included in the analysis. The Kaplan–Meier method was used to study survival of the different drugs according to the reason for discontinuation. Factors associated with discontinuation were studied using Cox regression models and bivariate and multivariate analyses. P values of less than 0.05 were regarded as statistically significant. The study population comprised 4,752 patients who received a total of 8,377 drugs, of which 4,411 (52.65%) were discontinued. The Kaplan–Meier curves showed that survival for first-line treatment was greater in all 3 groups (p < 0.001). Patients with RA had a greater risk of discontinuation if they were younger (HR, 0.99; 95% CI 0.99–1.00), if they were receiving anti-TNFα agents (HR, 0.61; 95% CI 0.54–0.70), and if they had more comorbid conditions (HR, 1.09; 95% CI 1.00–1.17). Patients with PsA had a higher risk if they were women (HR, 1.36; 95% CI 1.15–1.62) and if they were receiving other ts/bDMARDs (HR, 1.29; 95% CI 1.05–1.59). In patients with AS, risk increased with age (HR, 1.01; 95% CI 1.00–1.02), as did the number of comorbid conditions (HR, 1.27; 95% CI 1.12–1.45). The factors that most affected discontinuation of ts/bDMARDs were line of treatment, age, type of drug, sex, comorbidity and the year of initiation of treatment. The association with these factors differed with each disease, except for first-line treatment, which was associated with a lower risk of discontinuation in all 3 diseases.
Similar content being viewed by others
Introduction
Prescription of biologic disease-modifying antirheumatic drugs (bDMARDs) and targeted synthetic DMARDs (tsDMARDs) to patients with rheumatoid arthritis (RA), psoriatic arthritis (PsA), and ankylosing spondylitis (AS) has increased considerably in recent decades. The advantages of these agents include long-term efficacy and a favorable safety profile1,2. Persistence, or survival, of ts/bDMARDs gives us a general idea about their effectiveness, safety, and tolerability3.
Various studies have analyzed drug persistence. One real-world study on factors associated with persistence of golimumab found that the retention rate of this drug was increased when it was used as the first-line biologic or concomitantly with methotrexate4. In the TOCERRA registry, the retention rate and efficacy of tocilizumab in monotherapy or in combination (second or successive line of treatment) were higher than and similar to, respectively, those of anti-TNFα agents combined with methotrexate5. The various analyses of anti-TNFα agents performed in the BIOBADASER registry have investigated changes in discontinuation patterns in recent years6, age as a predictor of the reason for discontinuation7, better persistence of anti-TNFα agents in AS than in RA8, survival of anti-TNFα agents after failure of a first anti-TNFα agent9, and safety and retention of drugs prescribed off-label10. Other studies have analyzed factors that may affect persistence of biologics, including age, sex, underlying disease, comorbidity, and line of treatment11,12.
Nevertheless, few studies have included a general evaluation of how patient clinical factors and the type of treatment affect the discontinuation rates of various drugs in different diseases. Thus, the objectives of the present study were to evaluate how the patient’s characteristics and the type of drug affect discontinuation in each of the 3 groups of patients (RA, AS, PsA) and to investigate the impact of new drugs with novel mechanisms of action in recent years.
Patients and methods
Study design and setting: BIOBADASER 3.0
The present study was a real-world multicenter prospective study of patients with RA, PsA, or AS initiating a bDMARD or tsDMARD, with follow-up data until November 2019. Information was obtained from BIOBADASER III, a national prospective registry of patients with rheumatic diseases treated with bDMARDs, including biosimilars, and tsDMARDs, either with approved or off-label indications.
Phase III of this registry was initiated at the end of 2015. Patients from the previous phase were able to continue if they gave their written informed consent for their data to be collected again in this phase. The objectives and methodology of the register’s successive phases have been described elsewhere13. The registry protocol and relevant materials are available at http://biobadaser.ser.es.
In brief, BIOBADASER 3.0 recruits patients from 28 large public hospitals throughout Spain, with an estimated national coverage of bDMARD and tsDMARD treatment in RA of nearly 25%. Patients included in the registry are followed-up prospectively and evaluated when an adverse event (AE) occurs or treatment with ts/bDMARDs is changed. Evaluations are performed to assess lack of efficacy (switching to a different drug) or toxicity (discontinuation or dose reduction) at least once a year. The full database is monitored online to assess its consistency and quality; additionally, a random sample of patients (mean per center = 12) is selected and audited in situ at all 28 centers annually.
Population
This nested cohort comprised patients from the BIODASER registry diagnosed with RA, PsA, or AS according to the criteria of their treating rheumatologist who were prescribed a ts/bDMARD.
Outcome variables
The data included in this analysis were as follows: (1) patient data, including sex, date of birth, diagnosis, and date of diagnosis, (2) baseline status, including comorbidities (Charlson index) and risk factors (body mass index [BMI]); and (3) data on treatment, including time on biologic treatment, line of treatment, and type of biologics (according to the therapeutic target).
Discontinuation of treatment was defined as the interruption of treatment for a period ≥ 3 months. Temporary discontinuations (i.e., those lasting < 3 months) were not included.
Statistical analysis
Descriptive results are presented as mean and standard deviation or as numbers and percentages, as appropriate. Kaplan–Meier analysis was used to study survival of ts/bDMARDs, and various analyses were performed according to the reason for discontinuation. Factors associated with discontinuation were studied using Cox regression models. Bivariate and multivariate analyses were performed to study those factors. Statistical significance was set at p < 0.05.
All analyses were performed using Stata version 13.1 (Stata Corp., College Station, TX, USA, 2013).
All procedures and materials complied with the principles of the Declaration of Helsinki and with Spanish regulations on data protection and research. Ethical approval was granted by the Hospital Clinic of Barcelona Ethics Committee (one of the participating centers) acting as a reference committee (approval code FER-ADA-2015–01). All patients signed informed consent before register inclusion.
Results
We included 4,752 patients (2,381 in the RA group 1,121 in the AS group, and 1,250 in the PsA group). Table 1 shows the characteristics of the patients analyzed. Women were more common in the RA group (79.71%) and men in the AS group (69.94%), whereas the sex distribution was even in the PsA group (51.12% women). Most patients (55.68%) had been diagnosed more than 5 years before initiation of their ts/bDMARD. Mean age at initiation of therapy was 51.62 ± 12.94 years. Most patients were overweight or obese (64.94%). The mean age-adjusted Charlson comorbidity index was 2.13 ± 1.45. The 4,752 patients received a total of 8,377 ts /bDMARDs with anti-TNFα drugs predominating in the 3 groups (63.47%). Etanercept was the most frequently used drug (20.01%), followed by adalimumab (18.04%). Of the 8,377 treatments included, 2,719 (32.46%) were started before or during the year 2014 and 5,658 (67.54%) after the year 2014. A total of 4,411 drugs were discontinued (52.65%), mainly owing to lack of efficacy or loss of efficacy (49.13%) or because of AEs (23.31%). The most frequently indicated concomitant drugs in the 3 groups were methotrexate (60.35%) and corticosteroids (62.9%).
Kaplan–Meier survival analysis
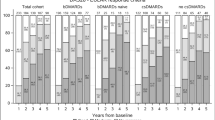
The treatment retention curves (anti-TNFα vs. other ts/bDMARDs) in the models examining discontinuation due to lack of efficacy revealed significant differences in all 3 groups of patients. Survival of other ts/bDMARDs was greater in patients with RA (p = 0.01), whereas that of anti-TNFα drugs was greater in patients with AS (p = 0.02) and PsA (< 0.001) (Fig. 1).
The treatment retention curves comparing first-line with second-line and successive treatments revealed significant differences in all 3 groups of patients (p < 0.001), with greater survival for first-line agents (Fig. 2).
Factors associated with treatment discontinuations in RA
Table 2 shows the results of the multivariate regression model in RA patients. In the model examining lack of efficacy, the factors associated with a greater risk of discontinuation were second and further lines of treatment (hazard ratio [HR], 1.85; 95% CI 1.62–2.12) and starting treatment after the year 2014 (HR, 1.19; 95% CI 1.05–1.36). A lower risk of discontinuation was observed for patients with older age at onset (HR, 0.99; 95% CI 0.99–1.00), longer time since diagnosis (HR, 0.99; 95% CI 0.98–0.99), and treatment with other ts/bDMARDs (HR, 0.61; 95% CI 0.54–0.70). In the model examining discontinuation because of AEs, a greater risk was observed for patients with older age at onset (HR, 1.01; 95% CI 1.00–1.02) and a higher Charlson comorbidity index (HR, 1.09; 95% CI 1.00–1.17). A lower risk of discontinuation was observed for patients starting treatment after the year 2014 (HR, 0.69; 95% CI 0.56–0.86).
Factors associated with treatment discontinuation in PsA
Table 3 shows the results of the multivariate regression model in patients with PsA. In the model examining lack of efficacy, a greater risk was observed in women (HR, 1.36; 95% CI 1.15–1.62), patients receiving second and further lines of treatment (HR, 1.69; 95% CI 1.41–2.03), patients who were receiving other ts/bDMARDs (HR, 1.29; 95% CI 1.05–1.59), and patients who start treatment after the year 2014 (HR, 1.29; 95% CI 1.04–1.59). In the model examining discontinuation due to AEs, women (HR, 1.92; 95% CI 1.44–2.56) and older patients (HR, 1.01; 95% CI 1.00–1.03) were at greater risk of discontinuation.
Factors associated with treatment discontinuation in AS
Table 4 shows the results of the multivariate regression model in patients with AS. In the model examining lack of efficacy, a greater risk was observed in older patients (HR, 1.01; 95% CI 1.00–1.02), second and further lines of treatment (HR, 2.17; 95% CI 1.67–2.83), concomitant treatment with methotrexate (HR, 1.33; 95% CI 1.02–1.75), and smoking (HR, 1.4; 95% CI 1.07–1.83). In the model examining discontinuation due to AEs, a greater risk was found in patients with more comorbid conditions (HR, 1.27; 95% CI 1.12–1.45).
Discussion
The present study shows that various factors affect discontinuation of treatment with bDMARDs and tsDMARDs, whether because of lack of efficacy or because of AEs. These factors do not affect the 3 rheumatic diseases studied here in the same way. Age played a role in all the groups, albeit to a different extent. Patients with RA had a greater risk of discontinuation due to lack of efficacy at an earlier age, although older patients had a greater risk of discontinuation due to AEs. The risk increased with age in patients with PsA and AS too. Anti-TNFα agents were associated with a greater risk of discontinuation in patients with RA but a lower risk in those with PsA. The risk of discontinuation was greater in women with PsA. The presence of comorbid conditions was associated with a greater risk of discontinuation in patients with RA and AS.
In both the survival analysis and the multivariate regression models, first-line treatment was associated with a lower risk of discontinuation, irrespective of the underlying disease or type of treatment received. Similar findings were previously reported in the BIODASER registry in patients receiving the anti-TNFα agents available at the time9. Several studies also highlight greater persistence with first-line treatments in patients with PsA14,15. A study performed in Spanish patients confirmed the greater rates found for retention of first-line treatments in patients with RA and AS16.
In the case of patients with RA, those who were younger were at a greater risk of discontinuing treatment owing to lack of efficacy but at a lower risk of discontinuing treatment owing to AEs. In contrast, in patients with AS, the risk of discontinuation owing to lack of efficacy increased with age. One previous study reported that younger patients were more likely to discontinue treatment owing to lack of efficacy and older patients owing to AEs7. Persistence has also been shown to be greater in older patients than in younger patients11,12. The broad availability of treatment options in RA could explain in part these differences in persistence according to age. An additional factor may be that the objective of treatment is to achieve optimal control of the disease as quickly as possible in younger patients with a shorter time since diagnosis. The lower number of targets for available treatment of AS implies longer maintenance of drugs in these patients.
Patients with a shorter time since diagnosis of RA had a greater risk of discontinuing treatment owing to inefficacy. This finding is controversial, given that previous studies have demonstrated that treatment with biologics is more effective the sooner it is started17,18. It was precisely these findings that prompted the emergence of the “treat to target” strategy19. The present study did not show treatment to be less effective in patients with a shorter time since diagnosis; rather, it showed that this group had a greater risk of discontinuation. In our case, these findings could arise from the fact that patients requiring treatment with ts/bDMARDs at an early stage of their disease are usually more complex, with poor prognostic factors. Consequently, our treatment objectives were more ambitious and demanding, thus leading to more frequent changes in treatment.
The risk of discontinuation was greater in patients with RA receiving anti-TNFα. Data reported elsewhere show that the discontinuation rate for anti-TNFα gradually increased during the first year of treatment. However, the authors drew no comparisons with other groups6. Consistent with our findings, various studies of patients with RA reported a higher retention rate for other ts/bDMARDs (abatacept, tocilizumab) than for anti-TNFα drugs20,21. However, other publications showed that the discontinuation rate was lower in patients receiving anti-TNFα agents as their first-line treatment3,22.
The risk of discontinuing treatment because of AEs in patients with RA and AS was greater for those who had a higher Charlson comorbidity index. In line with these results, other studies have reported that retention of biologics was poorer in patients with a higher comorbidity index12,22,23.
Women with PsA were at greater risk of discontinuing the drug owing to both lack of efficacy and AEs. Consistent with these findings, the DANBIO registry revealed a greater persistence of anti-TNFα agents in men with PsA24. Nevertheless, results reported elsewhere did not indicate statistically significant differences in the retention of drugs according to sex or type of drug used25. These findings differ from those of the present study, where patients who received other ts/bDMARDs drugs were at greater risk of discontinuation owing to lack of efficacy. Other publications have reported a high persistence of treatment with anti-TNFα drugs in patients with PsA14,26. Nevertheless, few real-world studies compare the efficacy and retention of anti-TNFα drugs with those of other ts/bDMARDs drugs in these patients14, possibly because other ts/bDMARDs drugs are newer. Data have been published from 2 head-to-head clinical trials comparing 2 drugs with different mechanisms of action: adalimumab and secukinumab27 and adalimumab and ixekizumab28. In the first, secukinumab was not shown to be more efficacious than adalimumab in terms of the ACR20 at week 52, although retention was greater for secukinumab27. In the second, ixekizumab was equally effective as adalimumab for control of arthritis28. In the case of patients with PsA, it was observed that when treatment was discontinued because of an AE, the association with age at onset was significant, and the risk of discontinuation increased by 2% with each year of age. It was recently reported that age increased the incidence of the first AE and that this is the main risk factor for onset of AE in patients with RA, PsA, and AS29. A real-life study that compared retention of anti-TNFα agents in patients with juvenile idiopathic arthritis (< 16 years) and patients with adult-onset disease found that adults more frequently discontinued treatment owing to lack of efficacy and AEs (severe infection and neoplasms), although both groups had similar retention rates at 10 years30.
Patients with RA and PsA have a greater risk of discontinuing treatment owing to inefficacy if they initiated treatment during 2015–2019 compared with those who started treatment in previous years. Given the greater availability and variety of therapeutic targets in these diseases in recent years, there is a tendency to switch treatment. In line with these findings, other studies have reported that, over the years, the tendency to make more changes in treatment is increasing22,31. In the case of discontinuation owing to adverse events, patients with RA had a lower risk of discontinuing owing to adverse events during 2015–2019 with respect to previous years. During the early years of biologics, the lack of experience may have led these agents to be discontinued quickly when an adverse event occurred. However, over the years, knowledge of the safety profile of the drugs has improved1,32,33.
Our study has both strengths and limitations. We analyzed data from a national registry with abundant real-world, clinical practice data and followed patients over a long period. We also included recent persistence data that reflect the impact of the drugs aimed at new therapeutic targets. Our main limitation is that some variables that could be of interest in this type of analysis were not collected appropriately; for example, disease activity indexes and the patient’s weight on discontinuing treatment and starting a new treatment were not homogeneously collected for all patients. The study is also limited by the way treatment with rituximab was recorded, since initiation and discontinuation of each cycle or dose of treatment are recorded separately. The reason for discontinuation of cycles of rituximab is usually recorded as “other” in most cases. Therefore, the analyses performed in the present study did not take into account some of the cases of discontinuation of rituximab where it was not specified that the reason for discontinuation was the lack of efficacy or adverse events. Nevertheless, considering the number of treatments with rituximab included in the analysis (< 5%), the impact of this observation on our findings can be considered minimal.
Conclusion
The discontinuation of ts/bDMARDs analyzed in the present study seems to be affected by various factors. First-line treatment was associated with a lower risk of discontinuing treatment in all 3 diseases studied. Age, sex, type of drug, the presence of comorbid conditions and the year of initiation of treatment were associated with differences in the retention rate of the treatments studied. The association was different for each of the diseases studied. The availability of more treatment options in some of the diseases studied may have changed trends in the use of drugs and in the factors associated with discontinuation rates.
Ethics approval
All procedures and materials complied with the principles of the Declaration of Helsinki and with Spanish regulations on data protection and research. Ethical approval was granted by the Hospital Clinic of Barcelona Ethics Committee (one of the participating centers) acting as a reference committee (approval code FER-ADA-2015–01).
Consent to participate
All patients gave their written informed consent to be included in the BIOBADASER registry. Informed consent included consent for subsequent analysis, such as the present analysis. Patients’ information was managed as anonymized aggregated data and, as approved by the Clinical Research Committee; specific informed consent for this analysis was not required.
Consent for publication
All of the authors consent to the publication of the manuscript. This manuscript has not been submitted for publication to any other journal, and the data it reports have not been published elsewhere and are not being evaluated by other journals.
Data availability
The data that support the findings of this study are available from Spanish Society of Rheumatology but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of Spanish Society of Rheumatology.
References
Sepriano, A. et al. Safety of synthetic and biological DMARDs: a systematic literature review informing the 2019 update of the EULAR recommendations for the management of rheumatoid arthritis. Ann. Rheum. Dis. 79, 760–770 (2020).
Kerschbaumer, A. et al. Efficacy of pharmacological treatment in rheumatoid arthritis: a systematic literature research informing the 2019 update of the EULAR recommendations for management of rheumatoid arthritis. Ann. Rheum. Dis. 79, 744–759 (2020).
Gomes, J. L. et al. Predictors and causes of first-line biologic agent discontinuation in rheumatoid arthritis: data from Reuma.pt. Acta Reumatol. Port. 44, 57–64 (2019).
Hernandez, M. V. et al. Factors associated with long-term retention of treatment with golimumab in a real-world setting: an analysis of the Spanish BIOBADASER registry. Rheumatol. Int. 39, 509–515 (2019).
Lauper, K. et al. Comparative effectiveness of tocilizumab versus TNF inhibitors as monotherapy or in combination with conventional synthetic disease-modifying antirheumatic drugs in patients with rheumatoid arthritis after the use of at least one biologic disease-modifying antirheumatic drug: analyses from the pan-European TOCERRA register collaboration. Ann. Rheum. Dis. 77, 1276–1282 (2018).
Gómez-Reino, J. J. et al. Change in the discontinuation pattern of tumour necrosis factor antagonists in rheumatoid arthritis over 10 years: data from the Spanish registry BIOBADASER 2.0. Ann. Rheum. Dis. 71, 382–385 (2012).
Busquets, N. et al. Age at treatment predicts reason for discontinuation of TNF antagonists: data from the BIOBADASER 2.0 registry. Rheumatology (Oxford) 50, 1999–2004 (2011).
Carmona, L. et al. Survival of TNF antagonists in spondylarthritis is better than in rheumatoid arthritis: Data from the Spanish registry BIOBADASER. Arthritis. Res. Ther. 8, R72 (2006).
Gomez-Reino, J. J. et al. Switching TNF antagonists in patients with chronic arthritis: an observational study of 488 patients over a four-year period. Arthritis. Res. Ther. 8, R29 (2006).
Carmona, L. et al. Safety and retention rate of off-label uses of TNF antagonists in rheumatic conditions: data from the Spanish registry BIOBADASER 20. Rheumatology (Oxford) 50, 85–92 (2011).
Jacob, L. et al. Persistence with biological drugs in patients treated in rheumatology practices in Germany. Rheumatol. Int 39, 525–531 (2018).
Mahlich, J. et al. Persistence with biologic agents for the treatment of rheumatoid arthritis in Japan. Patient Prefer Adherence 10, 1509–1519 (2016).
Sanchez-Piedra, C. et al. Objectives and methodology of BIOBADASER phase iii. Reumatol. Clin. 15, 229–236 (2019).
Costa, L. et al. Switching between biological treatments in psoriatic arthritis: a review of the evidence. Drugs R D 17, 509–522 (2017).
Fagerli, K. M. et al. Switching between TNF inhibitors in psoriatic arthritis: data from the NOR-DMARD study. Ann. Rheum. Dis 72, 1840–1844 (2013).
González-Fernández, M. Á. et al. Persistence of biological agents over an eight-year period in rheumatoid arthritis and spondyloarthritis patients. Farm Hosp 43, 24–30 (2019).
Aletaha, D. et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis. Rheum. 62, 2569–2581 (2010).
Taylor, P. C. et al. When the first visit to the rheumatologist is established rheumatoid arthritis. Best Pract. Res. Clin. Rheumatol. 33, 101479 (2019).
Ramiro, S. et al. Is treat-to-target really working in rheumatoid arthritis? a longitudinal analysis of a cohort of patients treated in daily practice (RA BIODAM). Ann. Rheum. Dis. 79, 453–459 (2020).
Ebina, K. et al. Drug retention and discontinuation reasons between seven biologics in patients with rheumatoid arthritis -The ANSWER cohort study. PLoS One 13, e0194130 (2018).
Johnston, S. S. et al. Comparison of biologic disease-modifying antirheumatic drug therapy persistence between biologics among rheumatoid arthritis patients switching from another biologic. Rheumatol. Ther. 2, 59–71 (2015).
Ramiro, S. et al. Discontinuation rates of biologics in patients with rheumatoid arthritis: are TNF inhibitors different from non-TNF inhibitors?. RMD Open 1, 1 (2015).
Iannone, F. et al. Influence of baseline modified Rheumatic Disease Comorbidity Index (mRDCI) on drug survival and effectiveness of biological treatment in patients affected with Rheumatoid arthritis, Spondyloarthritis and Psoriatic arthritis in real-world settings. Eur. J. Clin. Invest. 48, e13013 (2018).
Højgaard, P. et al. Gender differences in biologic treatment outcomes-a study of 1750 patients with psoriatic arthritis using Danish Health Care Registers. Rheumatology (Oxford) 57, 1651–1660 (2018).
Navarini, L. et al. Retention rates and identification of factors associated with anti-TNFα, anti-IL17, and anti-IL12/23R agents discontinuation in psoriatic arthritis patients: results from a real-world clinical setting. Clin. Rheumatol. 39, 2663–2670 (2020).
Fabbroni, M. et al. Drug retention rates and treatment discontinuation among anti-TNF-α agents in psoriatic arthritis and ankylosing spondylitis in clinical practice. Mediators Inflamm. 2014, 862969 (2014).
McInnes, I. B. et al. Secukinumab versus adalimumab for treatment of active psoriatic arthritis (EXCEED): a double-blind, parallel-group, randomised, active-controlled, phase 3b trial. Lancet 395, 1496–1505 (2020).
Smolen, J. S. et al. Multicentre, randomised, open-label, parallel-group study evaluating the efficacy and safety of ixekizumab versus adalimumab in patients with psoriatic arthritis naïve to biological disease-modifying antirheumatic drug: final results by week 52. Ann. Rheum. Dis. 79, 1310–1319 (2020).
Vela, P. et al. Influence of age on the occurrence of adverse events in rheumatic patients at the onset of biological treatment: data from the BIOBADASER III register. Arthritis Res. Ther. 22, 143 (2020).
Favalli, E. G. et al. Real-life 10-year retention rate of first-line anti-TNF drugs for inflammatory arthritides in adult- and juvenile-onset populations: similarities and differences. Clin. Rheumatol. 36, 1747–1755 (2017).
Desai, R. J. et al. Temporal trends in use of biologic DMARDs for rheumatoid arthritis in the united states: a cohort study of publicly and privately insured patients. J. Manag. Care Spec. Pharm. 23, 809–814 (2017).
Ramiro, S. et al. Safety of synthetic and biological DMARDs: a systematic literature review informing the 2013 update of the EULAR recommendations for management of rheumatoid arthritis. Ann Rheum Dis 73, 529–535 (2014).
Ramiro, S. et al. Safety of synthetic and biological DMARDs: a systematic literature review informing the 2016 update of the EULAR recommendations for management of rheumatoid arthritis. Ann Rheum Dis 76, 1101–1136 (2017).
Acknowledgements
The authors thank the Research Unit of the Spanish Society of Rheumatology (SER) for their support with the statistical analysis and their critical review of the manuscript. The authors thank Thomas O’Boyle for providing medical writing/editorial assistance during the preparation of the manuscript.
Funding
BIOBADASER is supported by the Spanish Agency of Medicines and Medical Devices (AEMPS), Biogen, Bristol Myers-Squibb (BMS), Celltrion Healthcare, Lilly, Merck, Novartis, Pfizer, Regeneron Pharmaceuticals, and Samsung Bioepis.
Author information
Authors and Affiliations
Contributions
AP-E, LM, and SH-P were involved in the design of the study. CS-P and FS-A contributed to data management and statistical analysis. AP-E, CS-P, LM, and SH-P drafted the manuscript, and all authors were involved in the critical review of the manuscript. All authors contributed to the discussion and interpretation of the results. The final version of the manuscript was seen and approved by all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Prior-Español, A., Sánchez-Piedra, C., Campos, J. et al. Clinical factors associated with discontinuation of ts/bDMARDs in rheumatic patients from the BIOBADASER III registry. Sci Rep 11, 11091 (2021). https://doi.org/10.1038/s41598-021-90442-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-90442-w
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.