Abstract
The distinctive properties of strongly correlated oxides provide a variety of possibilities for modulating the properties of 2D transition metal dichalcogenides semiconductors; which represent a new class of superior optical and optoelectronic interfacing semiconductors. We report a novel approach to scaling-up molybdenum disulfide (MoS2) by combining the techniques of chemical and physical vapor deposition (CVD and PVD) and interfacing with a thin layer of monoclinic VO2. MoWO3/VO2/MoS2 photodetectors were manufactured at different sputtering times by depositing molybdenum oxide layers using a PVD technique on p-type silicon substrates followed by a sulphurization process in the CVD chamber. The high quality and the excellent structural and absorption properties of MoWO3/VO2/MoS2/Si with MoS2 deposited for 60 s enables its use as an efficient UV photodetector. The electronically coupled monoclinic VO2 layer on MoS2/Si causes a redshift and intensive MoS2 Raman peaks. Interestingly, the incorporation of VO2 dramatically changes the ratio between A-exciton (ground state exciton) and trion photoluminescence intensities of VO2/(30 s)MoS2/Si from < 1 to > 1. By increasing the deposition time of MoS2 from 60 to 180 s, the relative intensity of the B-exciton/A-exciton increases, whereas the lowest ratio at deposition time of 60 s refers to the high quality and low defect densities of the VO2/(60 s)MoS2/Si structure. Both the VO2/(60 s)MoS2/Si trion and A-exciton peaks have higher intensities compared with (60 s) MoS2/Si structure. The MoWO3/VO2/(60 s)MoS2/Si photodetector displays the highest photocurrent gain of 1.6, 4.32 × 108 Jones detectivity, and ~ 1.0 × 1010 quantum efficiency at 365 nm. Moreover, the surface roughness and grains mapping are studied and a low semiconducting-metallic phase transition is observed at ~ 40 °C.
Similar content being viewed by others
Introduction
The current experiments of integrating 2D TMDCs into nano-electronic devices such as MoS2, WS2, and black phosphorous still have challenges like low carrier mobility and low photoluminescence (PL) efficiency which limit their further applications in optoelectronics. It is believed that in 2D TMDCs, the Van der Waals (VDWs) interlayer forces are weak and little defects can enhance the intrinsic phonon scattering and lead to better electrical conduction. Charge traps in 2D TMDCs are found to have a direct relationship with the carrier mobility and the output resistances of the electronic devices1. Therefore, in order to enhance the Raman intensity, PL intensity, and the charge carrier mobility of the optoelectronic devices, either by an interfacing functional channel material or a functional substrate that modulates the device output in multiple ways, many functional oxides have been used to offer unique properties such as piezoelectricity, strong polarization, and spin injection.
Chemical vapor deposition (CVD) is widely thought to be the most common method for preparing MoS2. In which MoO3 and sulfur powders are utilized with some inert gases through two or three-zone quartz tubes. The substrate can be maintained at downstream gas flow to grow MoS22,3,4. Nevertheless, this growth technique has several disadvantages through which non-homogenous, tiny flakes, and 2D MoS2 in the micro/nanoscale is formed. These disadvantages restrict its application in the industrial optoelectronic devices. On the other hand, methods such as exfoliation approaches, "top-down," have been used to prepare 2D sheets of graphene, MoS2, etc. This method including different forms such as micromechanical exfoliation5, sonication-assisted liquid-exfoliation6,7,8, shear exfoliation9,10 and chemical exfoliation11,12. Micromechanical exfoliation still has some disadvantages such as low quality, small-scale production, and high amount of defects13. Another drawback is that the exfoliated MoS2 must be transferred to a new substratum that handles its scaling and mass production14. Nonetheless, for the next generation of optoelectronics and quantum computers, the demand for wafer-scale and homogeneous 2D materials such as MoS2 has increased in recent years. Mainly, these methods including atomic layer deposition (ALD)14,15, pulsed laser deposition (PLD)16,17, thermal evaporation18,19, and magnetron sputtering techniques20,21,22. Magnetron sputtering was commonly employed at low cost and with easy control in large-scale for commercial manufacturing.
Strongly correlated oxides are a wide range of materials where the associated electronic, magnetic properties, and spin are strongly correlated with each other. The local spin density approximation (LSDA) has been used to determine the energy band structure of many kinds of materials. However, the LSDA fails to describe the electronic structure of some materials in which the interaction among the electrons is strong (e–e interaction) such as strongly correlated electron systems23. Meanwhile, the dominant role of the Coulomb repulsion forces between the electrons in VO2 and V2O3 systems is opening the insulating gap24,25. However, when strongly correlated oxides interface with 2D TMDCs, multi-functions such as bandgap, charge transfer, energy transfer, and strain can be tuned26,27. Among them, vanadium dioxide (VO2); an archetypal strongly correlated functional oxide that exhibits a metal–insulator transition (MIT) above room temperature. At ambient pressure, below the transition temperature (\({T}_{c}\)), VO2 has a monoclinic (M1 phase), with space group \({P2}_{1}/c(\#14)\)28 and lattice constant of \(a\approx 5.75\mathrm{ \AA },\mathrm{ b}\approx 4.53\mathrm{ \AA },\mathrm{ c}\approx 5.38\mathrm{ \AA },\upbeta ={122.6}^{\mathrm{o}}\)29,30. Above the \({T}_{c}\), VO2 adopts a tetragonal rutile (R) structure with space group \({P4}_{2}mnm(\#136)\) and a lattice constants \(a=b\approx 4.55\mathrm{ \AA },\mathrm{ c}\approx 2.85\mathrm{ \AA }\)30.
Due to the superior physical properties at the interface between MoS2 and VO2 layer, the manufacture of MoS2/VO2 heterostructure for optoelectronic devices has received considerable attention. However, for optical and optoelectronic products, account should be taken of the manufacturing process of homogenous and scaled-up MoS2. Oliva et al. reported the design of Van der Waals MoS2/VO2 photodetetctor31. Nevertheless, in that report, the manufacturing process of MoS2 includes several steps related to the micromechanical exfoliation process. The photo-excited carriers transfer in MoS2 and VO2 was studied, whereas the CVD powder vaporization technique was used for the growth of small flakes of MoS232. However, for the easy production of scalable and homogenous MoS2-based industrial applications, controllability and optimization of deposition time and growth parameters are highly required. Here, we report the preparation, characterization, and optoelectronic application of MoWO3/VO2/MoS2/Si (p–n–n–p) structure towards 365 nm photodetector at room temperature. Different sputtering times of Mo-O have been controlled followed by sulfurization process and interfacing with a monoclinic VO2 layer. Figure 1a shows a schematic diagram of the proposed MoWO3/VO2/MoS2/Si (p–n–n–p) photodetector. Note that most of the previously reported studies were trying to enhance the Raman, PL, optoelectronic of VO2–MoS2 by controlling the device temperature. However, these attempts did not relay for low temperature-phase transition optoelectronic devices. Here, this study focuses on the surface coupling and strain-induced optoelectronic modulation on a few-layer VO2/MoS2/Si heterostructure. Figure 1b shows the band alignment of the optimized p–n–n–p-type structure with different Mo-O sputtering times to control the MoS2 bandgap33. The nanostructured thin layer of Mo0.2W0.8O3 was deposited on the surface of VO2 as a protective and anti-reflection layer34. The optical anti-reflective layer (Mo0.2W0.8O3) is used to improve the responsivity of the photodetector and strongly eliminate the optical interference to minimize its undesired effects. Numerous applications including photodiodes35, image sensor36, and semiconductor photodetectors37 have recorded the uses of the antireflective coating. The structural and morphological characteristics are also studied here. Raman, PL, electrical, optoelectronic characterization of strongly correlated oxide (VO2), and 2D VDW heterostructure (MoS2-Si) will be discussed.
Results and discussion
Structural and composition
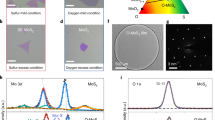
The crystal structure of vanadium oxide and molybdenum oxide was calculated in detail to understand the structural analysis of the deposited films. Figure 2a shows the XRD pattern of the deposited VO2 thin film. XRD result of VO2 shows a monoclinic phase with a JCPDS card number of [#96-153-0871] with a space group of C12/m1(12). The inserted crystal structure and visualizing 3D data are obtained using VESTA crystal software. Different diffraction peaks are observed at 2θ = 15.45°, 20.49°, 31.28°, 47.69°, and 62.32°. The unit cell parameters are a = 12.03000 Å, b = 3.69300 Å, c = 6.42000 Å while the angle is β = 106.100°. However, the Wyckoff position of metal atoms are (2a): (0, 0, 0) and (4f): (0.25, 0.25, 0.25) and oxygen atoms are (4i): (0.175, 0, 0.25), (0.175, 0.5, 0.25) and (8j): (0.075, 0.25, 0.75). The calculated amount of O:V in the VO2 compound indicating that more oxygen vacancies. Oxygen deficient in vanadium oxide (VO2−δ) has reported many times to stabilize the metallic state of VO238, decrease the semiconductor–metal phase transition (SMT)39 as reported here and to narrow the bandgap in the monoclinic phase40. Oxygen vacancies are acting as an electron donor with n-type conductivity which can change the electron orbital occupancy, band structures, and contribute to high photocurrent gain when staking with MoS241.
XRD crystal structure of (a) monoclinic VO2 and (b) Rietveld structure refinement XRD pattern of molybdenum oxide (in our case MoO2 and MoO3). The inserted 3D crystal structures are obtained using VESTA 3D visualization program (Model VESTA 3; https://jp-minerals.org/vesta/en/).
The preparation of molybdenum oxide was achieved using the Mo target at sputtering temperature of 400 °C. Figure 2b shows the XRD Rietveld refinement of Mo-O bonding in molybdenum oxide. The calculated R-factors were found to be Rwp = 7.52, Rexp = 3.26, and χ = 25.31. The Refinement analysis shows that MoO3 and MoO2 crystal phases are contributed. The distribution of atoms in unit cells of MoO2 and MoO3 was plotted with the VESTA program and shown in Fig. 2b. The amounts of Mo in MoO2 and MoO3 were calculated as 1.837 and 1, respectively. While the amounts of O2 in MoO2 and MoO3 were 3.836 and 3.012, respectively. The calculation of the crystal structure was showed that 9.46% as MoO2 phase and 54% as MoO3 phase. Moreover, the unit cell parameters of the MoO2 structure are a = 9.788, b = 8.604, c = 4.714 Å. While for MoO3 structure are a = 15.309, b = 3.719, c = 3.976 Å.
Raman characterization
The physics behind interfacing structures such as 2D semiconductors and correlated oxides should receive high attention. The importance of these structures can be highlighted by controlling the band alignment of the 2D materials such as MoS2. Moreover, controlling the carrier mobility, coupling, and strain effect (as reported in the current work)42. Raman spectra of the deposited multilayer structure MoWO3/VO2/MoS2 on p-type Si substrate are depicted in Fig. 3. The full range (200–1700 cm−1) Raman spectra of the deposited structures are shown in Fig. 3a and the magnified ranges of the full range spectra are shown in Fig. 3b–e. The Raman peaks of VO2 at RT are shown in Fig. 3a,b,d which confirm its monoclinic phase. Whereas, Fig. 3c shows the two characteristic peaks of MoS2 of \({E}_{2g}^{1}\) (385 cm−1) and \({A}_{1g}\) (405 cm−1) originate from their in-plane and out-of-plane phonon characteristics43. The peaks positions, intensities, distance between \({E}_{2g}^{1}\) and \({A}_{1g}\) position at different sputtering time of MoS2 are summarized in Table 1 and Fig. 4.
Many authors tried to study the effect of VO2 in contact with transition metal dichalcogenides semiconductors (TMDs) layers such as MoS2 and WS231,32. While in our current study, we tried to study the matching behavior of 50 nm thin VO2 layer onto the surface of MoS2/Si heterojunction. Additionally, the current work showed that different thicknesses of Mo-O structure have a direct effect on Raman, PL, electrical and optoelectronic characterization of MoS2 peaks (position and intensity). It is seen from Raman spectra that with incorporated the monoclinic VO2 layer, there is a redshift and an increase in the intensity peaks as in Fig. 4a–d. These observations showed that MoS2 and VO2 were electronically coupled, similar results observed before32. The observed shift in Raman modes may be attributed to the presence of compressive strain induced by implementing the VO2 layer, similar results observed when interfacing MoS2 with PMN-PT44. Generally, compression stress exerted on VDWs structure decreases the lattice constant44,45 and consequently increases the film crystallinity and photocurrent as reported here.
It is known that the intensity of Raman peaks is referring to high crystallinity effects. In most cases, Raman scattering is sensitive to the degree of crystallinity in a sample. Typically a crystalline material yields a spectrum with very sharp, intense Raman peaks, whilst an amorphous material will show less intense Raman peaks46,47,48. Table 1 and Fig. 4 show the Raman intensities of the corresponding thin films. These results show high attention to the applications of enhancing the Raman signal/intensity. The difference between peak positions of \({E}_{2g}^{1}\) and \({A}_{1g}\) in the presence and absence of VO2 layer are deposited in Table 1. It shows a decrease with increasing the MoS2 layer thickness from 30 to 240 s for both MoS2/Si and VO2/MoS2/Si structures, which may contribute to a decrease in the film layers and enhance the band gap49 as seen from Table 1. However, the film deposited at 180 s is out of this base. Meanwhile, the differences between the intensities of the peaks show an enhancement in the peak intensity with increasing the MoS2 film thickness which may contribute to high crystallinity effects. In counter, the film deposited at 240 s shows a decrease in the intensity. The highest intensity was observed for VO2/MoS2/Si with 180 s, whereas the lowest intensity was attributed to 240 s film. These results are concluded that the MoS2 sputtering time of 180 s is optimized for VO2 and MoS2 optical coupling. Consequently, the observed results may highlight the importance of incorporating strongly correlated oxide through 2D VDWs MoS2 structure to control film crystallinity, surface-enhanced Raman spectroscopy (SERS) of MoS2 for better signal detection and spatial resolution50,51,52, optical coupling53,54, Plasmonic local-field enhancement53 and optoelectronic behavior51.
For more details about intensity distribution versus position, Raman mappings of \({E}_{2g}^{1}, {A}_{1g}\) and Si peaks of MoS2/Si at different sputtering time of Mo-O (30, 60, 120, 180, and 240 s) are investigated at 385, 410, and 520 cm−1, respectively, and shown in Fig. S3 (a, b, c, d, and e). The Raman mapping was realized at large area (1.6 mm × 1 mm) with 320 × 200 data point using Leica microscope at 5 × magnification to provide an evidence about the ability to scale up the MoS2 thin films. The wavelength, power and integration time of the used laser were 532 nm, 3 mW and 1 s, respectively. This analysis illustrated that 30, 60, and 120 s samples show high homogeneity over the full scale (1.6 × 1 mm2). Although, 180 s sample shows regular agglomerations of nanoparticles for both E2g and A1g positions, the 240 s sample shows irregular clusters. The scaling up of MoS2 can therefore be demonstrated until sputtering time < 180 min.
Photoluminescence measurements (PL)
The PL spectrum of MoS2 is strongly dependent on the number of layers of 2D VDWs structures. In other words, a strong PL peak may observe in single-layer MoS2 or WS2 film and decreasing with increasing the number of layers55. Trion is defined as a quasi-particle that can potentially carry out more information and data than electrons for which make them useful towards different applications such as optoelectronics and quantum computing56. Trions are consisting of three charged particles bound together by very weak bonding energy that makes them quickly fall apart57. It is known that the dominated peak in Figs. 5 and 6 attributed to the recombination of the photogenerated electron–hole pair, whereas the observed weaker peak at lower wavelength may be attributed to the valance band splitting due to the presence of strong spin–orbit coupling of MoS258. In the literature review, the definition of trion and exciton peak is dominated by their locations and the trion peak is located at lower energy than exciton peak59,60,61. Figure 5a and b shows the PL spectra of MoS2/Si and MoWO3/VO2/MoS2/Si structures at RT. The MoS2/Si has two peaks at ~ 679 and ~ 620 nm which corresponding to components from trion and A-exciton62. However, trion and A-exciton positions have changed by controlling the deposition time of MoS2 as seen in Table 2. Also, this table shows the position of the characteristic peaks (A-exciton, B-exciton (higher spin–orbit splitting state), and trion) in the presence and absence of the VO2 layer. Generally, it is seen that the trion peak shifted to lower energy by implementing the VO2 layer onto the surface of the MoS2/Si structure as seen in Table 2.
The A-exciton peak has a higher intensity in the case of the VO2/MoS2/Si structure than in the case of MoS2/Si structure for 30 and 60 s deposition time of MoS2 layer, Fig. 6a and b. The trion peak is enhanced and shifted to longer wavelengths by increasing the deposition time to 60 s. The increase in PL intensity refers to an enhancement in light emission efficiency and increases the density of states of the photo carriers by modifying the band structure44. At 30 s, the A-exciton peak intensity is higher for the VO2/MoS2/Si structure than the MoS2/Si structure. i.e., the incorporation of VO2 dramatically changes the ratio between photoluminescence intensities of A-exciton and trion from < 1 to > 1 for VO2/(30 s)MoS2/Si structure. However, the opposite case is observed for the trion. Meanwhile, by incorporating the VO2 layer, a blue shift in the trion peak is observed, while a redshift is observed for the A-excitons. The observed peak position of PL that shifted towards lower energy (redshift) attributed to the non-radiative electron–hole recombination effect. However, by increasing the deposition time to 60 s, the A-exciton peak have higher intensity compared with the trions peak in the case of VO2/MoS2/Si structure than MoS2/Si structure as seen in Fig. 6b. By increasing the deposition time of MoS2 from 60 to 180 s, the B-exciton/A-exciton relative intensity increases, whereas the lowest ratio at 60 s deposition time refers to the high quality and the low defects densities of VO2/(60 s) MoS2/Si structure. Moreover, a redshift was observed for the trion peak and a slight blue shift when incorporating the VO2 layer. The increase in PL intensity refers to an enhancement in light emission efficiency and increases the density of states of the carriers by modifying the band structure and consequently enhance the radiative recombination of carriers, similar results were observed in a compressively strained trilayer MoS2 sheet44,63,64. This result shows that strong coupling between VO2 and MoS2 at 60 s Mo-O deposition time was observed at room temperature. While the PL intensity with MoS2/VO2 structure has only enhanced with increasing the film temperature31,32,65, while our reported results show a dramatic enhancement in the PL intensity at RT by incorporating VO2 layer on the surface of MoS2/Si structure.
On the other hand, trion peak quenching was found in Fig. 6c,d,e; with increasing the Mo-O layer from 120, 180, and 240 s when VO2 is deposited on MoS2/Si structure. We thought that the quenching of PL spectra in Fig. 6c,d,e may be owing to the fact that MoS2 is an n-type with a close Fermi level to the conduction band. However, the deposited VO2 layer at thicker Mo-O layer (120, 180, and 240 s) may shift Fermi level to the mid-band gap by drawback the electron coupling of VO2 and MoS266,67. Similar results observed using back-gating with SiO2/Si66, dopants molecule like F4-TCNQ, metal-centered Phthalocyanine molecules on the surface of monolayered TMD55,68. It is interesting to note that in all Fig. 6a–e, A-excitons have higher intensities when incorporated VO2 layer. This result draws high attention for enhancing A-exciton peak intensity and raises strong spin–orbit coupling by incorporating the monoclinic VO2 thin layer. Moreover, the B-exciton peak was observed in the MoS2/Si structure at 606 nm (2.04 eV), but it did not appear in the VO2/MoS2/Si structure as seen in the insets of Fig. 6b–e. It is known that the PL spectra of MoS2, surprisingly, increases with decreasing layer thickness62. However, the origin of PL spectrum in MoS2 arises from the direct excitonic electronic transitions which shows higher radiative recombination rate than nanocrystals69. Therefore, the enhanced photoluminescence with increasing the deposition time of Mo-O has to be attributed to a dramatically slower electronic relaxation factor \({\kappa }_{relax}\) as in Eq. 1, suggesting a substantial change in electronic structure of MoS2 when going from the short to longer deposition time of Mo-O as seen in Fig. 6.
where \({\kappa }_{rad}, {\kappa }_{defect}, {\kappa }_{relax}, and\,\,{ \eta }_{Lum}\) are representing the rates of radiative recombination, defect trapping, and electron relaxation, Luminescence quantum efficiency within the conduction and valence bands, respectively.
It is concluded that by depositing the VO2 layer on MoS2/Si structure, both trion and exciton peaks get shifted as seen in Table 2. It is implemented that the presence of the VO2 layer on the surface of the MoS2/Si structure results in a redshift through trion peaks, while a blue shift for A-exciton. The peak position of PL for trion is shifted towards lower energy due to the occurred non-radiative electron–hole recombination. However, 30 s sample is out of this rule with a blue and redshift in the trion and A-exciton peak on the VO2 layer, respectively.
Surface topography and grain boundary mapping
AFM has been used to investigate the surface topography, roughness, grain, and grain boundary mapping. Surface topography and parameters such as average roughness Ra(nm), root mean square or standard deviation of the height value Rq(nm), height different or peak-to-valley (Rpv), ten-point height (Rz), skewness (Rsk) and kurtosis (Rku) as well as fractal and grain analysis were inspected by the XEI software. Figure 7a,d,g,j,m shows a 2D surface topography, Fig. 7b,e,h,k,n shows the 3D visualization, and Fig. 7c,f,I,l,o shows the grain boundary mapping of the prepared MoWO3/VO2/MoS2/Si thin film with sputtering time of 30, 60, 120, 180, and 240 s, respectively. The films that deposited at short deposition time show higher uniformity, while with increasing the deposition time a small clusters of different sizes less than 100 nm have been observed. The average roughness values of the prepared thin films have summarized in Table 3 and show that 30 s and 180 s thin films have the lowest and highest Ra value of 3.28 and 48.0 nm, respectively. It seems that with increasing the deposition time of Mo-O, the accumulated nanoparticles show bigger sizes, consequently higher roughness factors. The calculation of the grain and grain boundaries of interfacing thin films are important parameters that provided information about the nature of interfaces between two layers. Figure 7c,f,I,l,o shows the grain size and grain boundary distribution maps of the prepared thin films. The SEM images of VO2, MoO3, Mo0.2W0.8O3, and MoS2/Si thin films are presented in Figures S1 and S2 and discussed in supplementary information. Large scale MoS2 thin films have been studied in our previous work by combining CVD and sputtering technique70. SEM images (Fig. S2, supplementary information) are provided to illustrate a homogenous and approximately uniform nanoparticles distribution. The SEM images in Fig. S2 (supplementary information) are provided to illustrate the homogeneity and approximate uniform surface distribution of nanoparticles, particularly for sputtering time < 180 s. Also, the AFM images of the MoWO3/VO2/MoS2-Si thin films, Fig. 7, show almost uniformly distributed grains for sputtering time up to 120 s. Besides the presented grain/grain boundaries mapping in Fig. 7, the scanning area of the AFM images was selected to be 5 µm × 5 µm to provide evidence about the ability to scale up the MoS2 thin films.
2D AFM images (a, d, g, j, and m), 3D AFM images (b, e, h, k, and n), and the grain/grain boundary mapping (c, f, I, l, and o) of the prepared MoWO3/VO2/MoS2-Si thin film with sputtering time of 30, 60, 120, 180, and 240 s, respectively. XEI software was used for image analysis and processing. [XEI PSIA, Version 1.5, https://parksystems.com/102-products/park-xe-bio].
Temperature-resistance measurement (T-R)
The phase transition of the prepared VO2 thin film has been performed using a four-probe measurement system connected to a heating stage ranging from RT to 78 °C. We investigated the influence of the 50 nm VO2 thin layer on Raman, PL, and optoelectronic measurements of a few-layers MoS2 with different sputtering times of Mo-O layer. So, the electrical semiconductor–metal phase change of VO2 has been tested as depicted in Fig. 8. In our case, the VO2 phase transition temperature was calculated to be 40 °C by controlling the sputtering condition (high vacuum and long-time annealing temperature) which may affect the lattice-strain and oxygen vacancy concentrations of VO271,72,73. The reason for the low semiconductor-metallic phase transition may owing to the high concentration of oxygen vacancies. Oxygen deficient in vanadium oxide (VO2−δ) has reported many times to stabilize its metallic state10, decrease the semiconductor–metal transition (SMT)11 and narrowing the bandgap of the monoclinic VO2 phase12. Oxygen vacancies are electron donors with n-type conductivity which can change the electron orbital occupancy and band structures and contribute to the high photocurrent generation when staking by MoS213. To check these coupling effects between theses layer, UV-optoelectronic measurements have been carried out.
Electric and optoelectronic properties
This section discusses the electric characterization of MoS2/Si heterostructures before and after depositing the VO2 layer under dark and UV conditions. In order to investigate the I–V and photoresponse of the prepared devices, we measured the I–V curve under dark and upon UV light illumination by applying a sweep voltage from − 5 to + 5 V for different sputtering times of Mo-O as shown in Fig. 9. This figure shows the electrical and optoelectronic properties of MoWO3/VO2/MoS2/Si thin film with different thicknesses of the MoS2 layer. Figure 9a–e shows the current–voltage (I–V) curves under dark and UV illumination at MoS2 deposition time of 30, 60, 120, 180, and 240 s, respectively. The observed photocurrent in this figure is larger than that reported for previously proposed MoS photodetector with lateral contacts arrangement31. The back Al and front Pd-Au contacts may paly important rule in that because these contacts allow the vertical electron transport in the heterostructure photodetector besides the lateral electron transportation and consequently 2D conductivity measurements31,37. The vertical electron transport offers a high density of active edge sites37. Also, our optimized heterojunction photodetector does not have a high-resistance layer like the SiO2 layer that was previously used and obstruct the vertical electron transport31. The back Al contact has been used for better collecting signals. Al metal makes Ohmic contact type with p-Si, which is also observed when probed on two contact pads on the same side, however, noble metals such as Ag, Au, etc. make Schottky contact with p-Si. On the other hand, the Au–Pd front contact was built in the anti-reflection Mo0.2W0.8O3 layer, in which the formed Schottky barrier height and width could be controlled by the current passing through the metal–semiconductor contact. Under dark conditions and as predicted by Basyooni et al., a non-linear I–V curve was obtained indicating that a good double-Schottky contact behavior was formed between the front Pd-Au contact and the film surface34. The position of asymmetric metal contacts can provide an integrated potential gradient assigned to the work function difference of asymmetric electrodes as previously stated for various applications, such as gas sensors34 and photodetector74, which leads to enhanced device performance as reported here. For instance, Casalino et al. used an asymmetric Al–Si–Cu (metal–semiconductor–metal) structure-based Si photodetector75. Moreover, several studies used asymmetric metal contacts for the photodetector application to control the dark current70,74.
Figure S4 shows the linear and semi-logarithmic scale current–voltage characteristics of MoS2/Si device without VO2 layer under the dark condition with different sputtering times of MoS2 layer; 30, 60, 120, 180, and 240 s. The positive part shows an increase in the associated dark current with increasing the sputtering time from 30 to 60 s. While at 120 s, a jump in the forward dark current is observed due to the related folding-effects in MoS2. Folding effect decreases the interlayer coupling and enhances the photoluminescence emission yield of A- and B-exciton peaks as seen in Fig. 5. Whereas instability measurements were observed at the negative bias part. The large increase in the negative dark current for MoS2(60 s) may be attributed to the release of charges that were trapped on MoS2 's surface at the interface trap sites (oxygen sites). The highest reverse dark current, which suggests the lowest potential barrier, was observed at 60 s. This may be ascribed to the values of the optical band gaps as shown in our previous study, whereas the 60 s MoS2-Si thin film displayed optical band gaps of 1.75 and 2.01 eV33. By increasing the sputtering time the reverse dark current decreases and almost becomes identical for sputtering time ≥ 180 s, as shown in Fig. S4(a,b).
Figure 10a–e shows the log-current curves under dark and UV illumination of 30, 60, 120, 180, and 240 s conditions, respectively. It is clearly seen that UV illumination shifts the logarithmic I–V curve towards the negative voltage region. This behavior may address the induced strain effects from the VO2 layer or unidirectional charge transport mechanism from the top to bottom layer due to the different electron concentrations76. Interestingly, it seems that the VO2 layer enhances the positive and negative current. Meanwhile, the dark current obtained after depositing the VO2 layer on the surface of MoS2/Si is about 2–3 folds’ improvement over pure MoS2/Si device for 30 and 60 s samples, as in Fig. 10a,b. The observed higher value of photocurrent under UV illumination is also attributed to enhancement though the band-to-band excitation in the VO2/MoS2/Si region. Moreover, carrier recombination and tunneling across the device junction may be addressed as a reason for the enhancement I–V under UV illumination. Nevertheless, fast response and recovery times, high responsivity, high reliability, and low signal-to-noise ratio are important characteristics for detector applications77,78, which is discussed below in detail.
Transient response
Figure 11a–e shows the ON–OFF time-resolved photoresponses of MoWO3/VO2/MoS2/Si devices with varying MoS2 sputtering time (30, 60, 120, 180, and 240 s) in dark and under UV illumination. These optoelectronic transient/dynamic curves were measured with a switching time of 5 s, a bias voltage of 1 V, and UV illumination of 365 nm. Also, the optical modulations (photocurrent-time characteristics@ 1 V) under dark and UV illuminations of Mo0.2W0.8O3/VO2/MoS2/Si devices are presented in Figure S5 (supplementary information). Note that the ON/OFF and OFF/ON transitions of the UV light source are repeated many times for each 5 s at a bias voltage of 1 V. The time-resolved photoresponse curves in Fig. 11 show different sputtering time dependence. The curves at 30 and 120 s show increasing on/off behaviors, whereas the curves at 60, 180, and 240 s show stable on/off behaviors. The increasing on/off behaviors in Fig. 11a,c may come from some organic trap states that accumulated during the CVD sulfurization process. Wile, the stable on/off behaviors in Fig. 11b,d,e can be attributed to the high stability, high quality, and low density of the defects.
The response/raise time was measured when the light source turned on, while the recovery/decay time was measured when the light turned off as shown in Fig. 12a. The response and recovery times have been estimated from the ON/OFF dynamic photoresponses at different sputtering times, Fig. 11. The combined sputtering and CVD deposition process of MoWO3/VO2/MoS2/Si UV photodetector device shows symmetrical response and recovery time which not exceed 0.25 s using the selected wavelength (365 nm) and bias (1 V), as shown in Fig. 12b. Consequently, our proposed photodetector is considered more efficient than the previously tested photodetector by Ang et al.78.
The fast response and recovery speed, indicating that electron–hole pairs could be effectively generated and separated in the proposed structure under UV illumination at room temperature. It is important to note that the fabricated device using MoS2(120 s) shows the fastest response/recovery times (0.19 s at 1 V) among the studied devices as shown in Fig. 12b. The fast response/recovery time at 120 s can indicate the fast and stable generation and separation of the electron–hole pairs. Unlike Dhyani and Das who reported rapid response for the silicon-MoS2 photodetector@ 580 nm and 3 V bias79, our measurements are performed without any external series resistance. Nevertheless, it is known that higher applied bias voltage can generate more photocurrent and consequently decrease the response and recovery time. A clear high photocurrent can be observed in the ON state at 1 V which makes the gate voltage lowers the potential barriers at the contacts, resulting in highly efficient photogenerated carrier extraction and thus increased photocurrent at a low applied voltage (1 V). The reason behind this is that the gate voltage can affect the height of the Schottky barrier between the metal contact and film surface and thus shift the Fermi level80,81. It seems that our designed photodetector did not require high bias voltage which makes it more applicable for low power photodetector technology.
Photocurrent gain (Pg) and photoresponsivity (\({\mathrm{R}}_{\uplambda }\))
The induced photocurrent Iph is given by \({I}_{ph}={I}_{Light}-{I}_{Dark}\), where \({I}_{ph}\) increases with increasing the applied voltage and the light power82. Photocurrent gain (Pg) can be defined and determined by \({P}_{g}=({I}_{photo}-{I}_{dark})/{I}_{dark}\), where \({I}_{photo}\) and \({I}_{dark}\) are photocurrent and dark current respectively77.
Also, the detector responsivity (\({R}_{\lambda }\)) can be expressed as \({R}_{\lambda }=\Delta I/(A\times P)\), where \(\Delta I\) is the difference between the photocurrent and dark current, A is the illuminated area, and P is the UV light power. Figure 13a shows the photocurrent and photocurrent gain of the tested samples under a 365 nm UV illumination source. Figure 13b demonstrates the responsivity in A/W of MoS2/Si and MoWO3/VO2/MoS2/Si photodetectors with different MoS2 sputtering time (30–240 s). In both photodetectors, the responsivity values reflect nearly linear increases by increasing the sputtering time to 180 s. MoWO3/VO2/MoS2/Si UV detector responsivity shows high values ranging from 0.3 to 4.7 A/W, which corresponds to 30 to 180 s MoS2 sputtering time. The optimized value at 180 s is higher than that reported by Li et al. (2.4 mW cm−2)83. The enhanced photoresponsivity by interfacing the VO2 layer may be owing to the film strain include stresses arising from the different thermal expansion coefficients of the VO2 and MoS2/Si film due to a high deposition/sulphurization temperatures of ~ 400/650 °C and growth stresses arising from crystal structure changes after deposition. Nevertheless, more efficient light absorption involving more e–h pairs generation, resulting in higher mobility and more detection capability.
External quantum efficiency (EQE) and detectivity (\({\mathrm{D}}^{*}\))
In order to produce the photocurrent Iph, the fraction of the extracted free charge carriers to the photo flux φin collected at a given energy Eph is called the External Quantum Efficiency, defined by84 as \(EQE=\frac{hc{R}_{\lambda }}{e\lambda }\), where h is the Planck’s constant (~ 4.135 × 10−15 eV s), e is the elementary electron charge (~ 1.602 × 10−9 C), c is the light velocity (~ 3 × 108 m/s), and λ is the excitation wavelength (365 nm). The EQE values as a function of the prepared samples are plotted in Fig. 14a, where EQE varies from 6.6 × 108 to ~ 1.0 × 1010 at 365 nm which considered higher than the mesoscopic multilayer MoS2 as reported before85. Another important figure of merit of a photodetector is the detectable signal82, referred by the specific detectivity measured in Jones, which given by \({D}^{*}=\frac{{\left(AB\right)}^{0.5}{R}_{\lambda }}{{i}_{n}} (\mathrm{cm }{\mathrm{Hz}}^\frac{1}{2}{\mathrm{ W}}^{-1})\), where A is the effective area of the d in cm2, B is the bandwidth, and in is the measured noise current. If the shot noise from the dark current is the main noise source, the specific detectivity can be simplified as \({D}^{*}=\frac{{R}_{\lambda }{A}^{0.5}}{{(2e{I}_{dark})}^{0.5}}\) ,Where e is the charge of an elementary electron86. The calculated D* for MoS2/Si and MoWO3/VO2/MoS2/Si is depicted in Fig. 14b. D* of MoS2/Si device shows a progressive increase with sputtering time from 30 to 240 s. For MoS2/Si device, the maximum D* was 0.4 × 108 Jones. While the maximum D* for the MoWO3/VO2/(60 s)MoS2/Si is ~ 4.3 × 108 Jones at RT and applied voltage of 1 V. In contrast the MoWO3/VO2/MoS2/Si with a 30 s deposition time shows D* of 0.47 × 108 Jones.
Mechanism
Now we turn to the underlying photoresponse mechanism of the VO2/MoS2/Si as a UV photodetector device. The photoresponse properties of VO2/MoS2/Si heterojunction can be understood from the energy-band alignment diagram as in Fig. 1b. Due to the free dangling bonds of the surfaces of MoS2 film, the MoS2/Si heterojunction can be affected by lattice matching. Clearly, the implanting of VO2 layer-based UV photodetector was overwhelmingly play an important role in enhancing the Raman signal/intensity, PL intensity, electrical and optoelectronic performance of MoS2/Si device. Under the zero-bias condition, an insignificant current was observed due to the high depletion layer at the n–p (MoS2-Si) junction which restricts the movement of the carriers. Under VO2 interfacing, both positive and negative current increased significantly and the photocurrent Iph of VO2/MoS2/Si film is much higher than that in MoS2-Si, which can be attributed to the more photon absorption on the top of MoWO3/VO2 layer and larger photocurrent-gain due to higher carrier mobility87,88. With increasing the amount of Mo-O (deposition time of 30, 60, 120, 180, and 240 s), the induced current is enhanced due to the more electron–hole pair generation by UV light absorption and the applied voltage shifts dramatically towards negative voltage, which indicates the continuous accumulation of electrons in the vertical VO2-MoS2 channel as seen from the logarithmic scale current. In the positive voltage region, the MoS2/Si n–p structure shows that umpteen electrons are accumulated on the MoS2 band which shifts Fermi level near the conduction band. Because 1 V is able to decrease the depletion width and the barrier height, electrons are able to overcome the barrier height through thermionic emission, resulting in a high flux of photocurrent and more efficient photocurrent extraction. It is interesting to observe that under a shorter sputtering time of Mo-O (30, then 60 s), a significant forward photocurrent was observed which did not observe before in MoS2/Si structures52,89. Meanwhile, with increasing the Mo-O content, MoO3 starts to get folded and the reverse photocurrent starts to get highlighted as seen in the semi-logarithmic scale I–V, Fig. 10.
Conclusion
In summary, the next generation of optoelectronic devices integrates the physics of light-matter interaction of 2D materials at nanoscale for light-harvesting applications and these optoelectronic devices can control the light that converts trions, excitons, and photons to electrical signals. Our approach is based on a high vacuum deposition of Mo-O compound at 400 °C, followed by a sulphurization process in a chemical vapor deposition tube. Here we study the interfacing effect of monoclinic VO2 with MoS2 film for UV optoelectronic applications. It showed that different thicknesses of the MoS2 compound have a direct effect on the Raman, PL, electrical, optoelectronics of MoS2 peaks. A redshift was observed in Raman spectra with a high electronic coupling between VO2 and MoS2 for the case of 180 s sputtering time. Photoluminescence measurements showed that the intensity of the trion peak has a higher intensity than the A-exciton peak for MoS2/Si structure. On the other hand, the opposite case was observed for the VO2/MoS2/Si device. Current–voltage, response/recovery time, external quantum efficiency, time-resolved photocurrent, and detectivity, photocurrent gain, photo-responsivity of VO2/MoS2/Si device have been demonstrated. It’s shown that the increase of the deposition time of MoS2 from 30 to 240 s enhances the photo-absorption, photo-responsivity, and external quantum efficiency of the MoWO3/VO2/MoS2/Si device due to the associated folding effects of MoO3. These results show a multiplexed photodetector fabrication technique of high reproducible and scalable process based on CVD and PVD system and draw high attention towards the interfacing effects of strongly correlated oxide films MoS2 devices.
Materials and methods
Device fabrication
Preparation of MoS2 layer on p-type Si substrate has prepared through two steps in physical vapor deposition (PVD)—Radio Frequency magnetron sputtering system, followed by chemical vapor deposition (CDV) process. Si substrates were cleaned through many steps; firstly, kept in NH4OH-H2O2 solution diluted with de-ionized (DI) water for 5 min at 75 °C, then rinsed with DI water for 5 min. After that, they left in HF (%5) solution for 5 s, then rinsed in DI water and dried with high purity N2. Immediately, the cleaned Si-substrates transferred to a 3 × 10−7 Torr RF magnetron sputtering system (VAKSIS Midas 3M1T). In-situ Ar-plasma source has activated for 10 min at a power of 100 W and low pressure of 6 × 10−3 Torr at room temperature to activate the Si surface. Mo-O thin films were grown using a 3-inch. pure molybdenum target (99.9%) utilizing Ar plasma as a carrier gas and O2 as a reactive gas. The substrate temperature was stabilized at 400 °C for more than 30 min before the deposition process with steps of 100 °C/30 min. The O2 and Ar flow rates were kept constant, whereas O2/(Ar + O2) of ¼ ratio. The deposition was carried out at 5 × 10−3 Torr and 137 W with different sputtering times of 30, 60, 120, 180, and 240 s. The system was kept to cool down normal up to the room temperature (RT), then immediately transferred to the two-zone CVD quartz chamber (MTI-OTF 1200 system) for the sulphurization process. The as-deposited molybdenum oxide (Mo-O) thin films transferred to the center of the CVD furnace and the temperature is raised to 650 °C. Sulphur powder (0.5 g) is put in a ceramic boot with 100sccm high purity Ar source. An external heating belt with a distance of 50 cm to the substrate was used to evaporate the sulphur for 22 min. Then, the system cooled down until RT with the same flow rate of Ar (100 sccm).
After forming the MoS2 layer, a thin layer of monoclinic VO2 has grown. The same sputtering system was used with a 190 W deposition power and Ar/O2 ratio was 41/2.2 sccm, while the deposition time was set to produce 50 nm film thickness. Then the samples were in-situ annealed at 400 °C for 2 h with 50 sccm Ar flow. A protective and anti-reflection thin layer of Mo0.2W0.8O3 was deposited on the surface of VO2 as optimized in our previous work34. High vacuum thermal evaporation system was used to deposit aluminum and gold–palladium that used as a back and front contacts, respectively.
Device characterization
The crystal structures were analyzed using Grazing Incidence X-ray diffraction (XRD GNR ADP PRO 2000), with CuKα (λ = 1.5405 Å) radiation source with a step of 0.01. VO2 layer was deposited at a high vacuum condition to ensure its high crystallinity and low semiconductor-metallic phase transition. Parameters such as space group, diffraction peaks, angles, Wyckoff position of vanadium (V) and oxygen (O) atoms, ratio of O:V, and oxygen vacancy concentrations were calculated from refinement analysis. The refinement calculations were done using Match and Fullprof Suite program. Moreover, the refined structures were plotted in a three-dimensional view using 3D visualization VESTA program. The surface morphology was recorded using scanning electron microscopy (SEM) TESCANMAIA3 XMU. Atomic Force Microscopy (AFM) has been used to investigate the surface topography, roughness, and grain mapping. Each sample was characterized by XE-6 AFM (Park Systems Corp., Suwon-Korea) that controlled with XEP software for data acquisition and XEI software for image analysis and processing. AFM images were obtained through a 0.5 × 0.5 μm area (x–y accessible area) at a 0.5 Hz scan rate. Measurements were taken with a non-contact mode using a PPP-NCHR silicon cantilever consisting of tip radius < 10 nm and 42 N/m force constant (Nanosensors TM, Neuchâtel-Switzerland). Raman measurements and photoluminescence (PL) spectra were carried out using Renishaw in Via Confocal Raman microscope with a 532 nm laser beam, while an incident laser power of 3 mW was chosen to acquire a single Raman spectrum. The temperature-resistance measurement of monoclinic and high crystalline nanostructure VO2 thin film has been performed using a four-probe measurement system connected to a heating stage ranging from RT to 100 °C. The electrical and optoelectronics measurements were measured using 2450 Kethley Source -Meter and 365 nm ultraviolet (UV) light lamp for optoelectronic measurements.
References
Kim, J. K. et al. Trap-mediated electronic transport properties of gate-tunable pentacene/MoS 2 p-n heterojunction diodes. Sci. Rep. 6, 36775 (2016).
Yu, J., Li, J., Zhang, W. & Chang, H. Synthesis of high quality two-dimensional materials via chemical vapor deposition. Chem. Sci. 6, 6705–6716 (2015).
Cai, Z., Liu, B., Zou, X. & Cheng, H. M. Chemical vapor deposition growth and applications of two-dimensional materials and their heterostructures. Chem. Rev. 118, 6091–6133 (2018).
Wang H et al. (2012) Large-scale 2D electronics based on single-layer MoS2 grown by chemical vapor deposition. In Technical digest—international electron devices meeting, IEDM (2012). https://doi.org/10.1109/IEDM.2012.6478980.
Sharma, D. et al. Transfer characteristics and low-frequency noise in single- and multi-layer MoS2 field-effect transistors. Appl. Phys. Lett. 107, 162102 (2015).
Nicolosi, V., Chhowalla, M., Kanatzidis, M. G., Strano, M. S. & Coleman, J. N. Liquid exfoliation of layered materials. Science 340, 1226419 (2013).
Coleman, J. N. et al. ChemInform abstract: two-dimensional nanosheets produced by liquid exfoliation of layered materials. ChemInform 42, 568 (2011).
Yu, X., Prévot, M. S. & Sivula, K. Multiflake thin film electronic devices of solution processed 2D MoS2 enabled by sonopolymer assisted exfoliation and surface modification. Chem. Mater. 26, 5892–5899 (2014).
Paton, K. R. et al. Scalable production of large quantities of defect-free few-layer graphene by shear exfoliation in liquids. Nat. Mater. 13, 624–630 (2014).
Varrla, E. et al. Large-scale production of size-controlled MoS2 nanosheets by shear exfoliation. Chem. Mater. 27, 1129–1139 (2015).
Knirsch, K. C. et al. Basal-plane functionalization of chemically exfoliated molybdenum disulfide by diazonium salts. ACS Nano 9, 6018–6030 (2015).
Eda, G. et al. Photoluminescence from chemically exfoliated MoS 2. Nano Lett. 11, 5111–5116 (2011).
Yang, X. Y. & Chauhan, N. Photoenergy and Thin Film Materials. Wiley, New York. https://doi.org/10.1002/9781119580546 (2019).
Giannazzo, F., Lara Avila, S., Eriksson, J. & Sonde, S. Integration of 2D Materials for Electronics Applications (MDPI, Basel, 2019).
Browning, R. et al. Atomic layer deposition of MoS2 thin films. Mater. Res. Exp. 2, 035006 (2015).
Loh, T. A. J. & Chua, D. H. C. Growth mechanism of pulsed laser fabricated few-layer MoS2 on metal substrates. ACS Appl. Mater. Interfaces 6, 15966–15971 (2014).
Serrao, C. R. et al. Highly crystalline MoS2 thin films grown by pulsed laser deposition. Appl. Phys. Lett. 106, 052101 (2015).
Ma, X. & Shi, M. Thermal evaporation deposition of few-layer MoS2 films. Nano-Micro Lett. 5, 135–139 (2013).
Wu, S. et al. Vapor-solid growth of high optical quality MoS2 monolayers with near-unity valley polarization. ACS Nano 7, 2768–2772 (2013).
Pacley, S. et al. Impact of reduced graphene oxide on MoS2 grown by sulfurization of sputtered MoO3 and Mo precursor films. J. Vac. Sci. Technol. A Vac. Surf. Films 34, 041505 (2016).
Hussain, S. et al. Synthesis and characterization of large-area and continuous MoS2 atomic layers by RF magnetron sputtering. Nanoscale 8, 4340–4347 (2016).
Matsuura, K. et al. Low-carrier-density sputtered MoS2 film by vapor-phase sulfurization. J. Electron. Mater. 47, 3497–3501 (2018).
Imai, Y., Solovyev, I. & Imada, M. Electronic structure of strongly correlated systems emerging from combining path-integral renormalization group with the density-functional approach. Phys. Rev. Lett. 95, 176405 (2005).
Sass, B. et al. Structural and electronic properties of epitaxial V2O3 thin films. J. Phys. Condens. Matter 16(1), 77–87 (2004).
Rozenberg, M. J. et al. Optical conductivity in Mott-Hubbard systems. Phys. Rev. Lett. 75, 105–108 (1995).
Tongay, S. et al. Tuning interlayer coupling in large-area heterostructures with CVD-grown MoS2 and WS2 monolayers. Nano Lett. 14, 3185–3190 (2014).
Sun, Y. et al. Probing local strain at MX2-Metal boundaries with surface plasmon-enhanced Raman scattering. Nano Lett. 14, 5329–5334 (2014).
Goodenough, J. B. The two components of the crystallographic transition in VO2. J. Solid State Chem. 3, 490–500 (1971).
Budai, J. D. et al. In situ X-ray microdiffraction studies inside individual VO2 microcrystals. Acta Mater. 61, 2751–2762 (2013).
McWhan, D. B., Marezio, M., Remeika, J. P. & Dernier, P. D. X-ray diffraction study of metallic VO2. Phys. Rev. B 10, 490–495 (1974).
Oliva, N. et al. Van der Waals MoS2/VO2 heterostructure junction with tunable rectifier behavior and efficient photoresponse. Sci. Rep. 7, 14250 (2017).
Lin, Y. C. et al. Photoluminescence of monolayer transition metal dichalcogenides integrated with VO2. J. Phys. Condens. Matter 28, 504001 (2016).
Karataş, A. & Yılmaz, M. Molybdenum disulfide thin films fabrication from multi-phase molybdenum oxide using magnetron sputtering and CVD systems together. Superlattices Microstruct. 143, 106555 (2020).
Basyooni, M. A., Zaki, S. E., Ertugrul, S., Yilmaz, M. & Eker, Y. R. Fast response of CO2 room temperature gas sensor based on mixed-valence phases in molybdenum and tungsten oxide nanostructured thin films. Ceram. Int. 46, 9839–9853. https://doi.org/10.1016/j.ceramint.2019.12.259 (2020).
US7196314B2—Image sensor and pixel having an anti-reflective coating over the photodiode—Google Patents. https://patents.google.com/patent/US7196314B2/en. Accessed on 18th June 2020.
US8546742B2—Array of nanowires in a single cavity with anti-reflective coating on substrate—Google Patents. https://patents.google.com/patent/US8546742B2/en. Accessed on 2nd July 2020.
US6803249B2—Method of making an integrated photodetector in which a silicon nitride layer forms an anti-reflective film and part of multi-layer insulator within transistor structures—Google Patents. https://patents.google.com/patent/US6803249B2/en. Accessed on 2nd July 2020.
Zhang, J. et al. Evolution of structural and electrical properties of oxygen-deficient VO2 under low temperature heating process. ACS Appl. Mater. Interfaces 9, 27135–27141 (2017).
Wang, X., Wang, Z., Zhang, G. & Jiang, J. Insight into electronic and structural reorganizations for defect-induced VO2 metal-insulator transition. J. Phys. Chem. Lett. 8, 3129–3132 (2017).
Chen, L. et al. First-principles study of the effect of oxygen vacancy and strain on the phase transition temperature of VO2. RSC Adv. 6, 86872–86879 (2016).
Fan, S., Fan, L., Li, Q., Liu, J. & Ye, B. The identification of defect structures for oxygen pressure dependent VO2 crystal films. Appl. Surf. Sci. 321, 464–468 (2014).
Yuan, Z., Hou, J. & Liu, K. Interfacing 2D semiconductors with functional oxides: fundamentals, properties, and applications. Crystals 7, 265 (2017).
Zhang, X. et al. Phonon and Raman scattering of two-dimensional transition metal dichalcogenides from monolayer, multilayer to bulk material. Chem. Soc. Rev. 44, 2757–2785 (2015).
Hui, Y. Y. et al. Exceptional tunability of band energy in a compressively strained trilayer MoS2 sheet. ACS Nano 7, 7126–7131 (2013).
Manzeli, S., Allain, A., Ghadimi, A. & Kis, A. Piezoresistivity and strain-induced band gap tuning in atomically thin MoS2. Nano Lett. 15, 5330–5335 (2015).
Kotula, A. P., Snyder, C. R. & Migler, K. B. Determining conformational order and crystallinity in polycaprolactone via Raman spectroscopy. Polymer (Guildf) 117, 1–10 (2017).
Nikolaeva, G. Y. et al. Using Raman spectroscopy to determine the structure of copolymers and polymer blends. J. Phys. Conf. Ser. 826, 012002 (2017).
Samuel, A. Z. Direct estimation of polymer crystallinity with Raman spectroscopy using ratio of scattering cross-sections estimated from variable temperature measurements. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 224, 117431 (2020).
Sundaram, R. S. et al. Electroluminescence in single layer MoS2. Nano Lett. 13, 1416–1421 (2013).
Ghopry, S. A., Alamri, M. A., Goul, R., Sakidja, R. & Wu, J. Z. Extraordinary sensitivity of surface-enhanced Raman spectroscopy of molecules on MoS2 (WS2) nanodomes/graphene van der Waals heterostructure substrates. Adv. Opt. Mater. 7, 1801249 (2019).
Er, E. et al. High-yield preparation of exfoliated 1T-MoS2 with SERS activity. Chem. Mater. 31, 5725–5734 (2019).
Majee, B. P., Mishra, S., Pandey, R. K., Prakash, R. & Mishra, A. K. Multifunctional few-layer MoS2 for photodetection and surface-enhanced raman spectroscopy application with ultrasensitive and repeatable detectability. J. Phys. Chem. C 123, 18071–18078 (2019).
Jeong, H. Y. et al. Optical gain in MoS2 via coupling with nanostructured substrate: Fabry-Perot interference and plasmonic excitation. ACS Nano 10, 8192–8198 (2016).
Hu, J. Q., Shi, X. H., Wu, S. Q., Ho, K. M. & Zhu, Z. Z. Dependence of electronic and optical properties of MoS2 multilayers on the interlayer coupling and Van Hove singularity. Nanosc. Res. Lett. 14, 288 (2019).
Mouri, S., Miyauchi, Y. & Matsuda, K. Tunable photoluminescence of monolayer MoS2 via chemical doping. Nano Lett. 13, 5944–5948 (2013).
Plechinger, G. et al. Identification of excitons, trions and biexcitons in single-layer WS2. Phys. Status Solidi Rapid Res. Lett. 9, 457–461 (2015).
Kwon, H. et al. Probing trions at chemically tailored trapping defects. ACS Cent. Sci. 5, 1786–1794 (2019).
Zhu, Z. Y., Cheng, Y. C. & Schwingenschlögl, U. Giant spin-orbit-induced spin splitting in two-dimensional transition-metal dichalcogenide semiconductors. Phys. Rev. B Condens. Matter Mater. Phys. 84, 153402 (2011).
Christopher, J. W., Goldberg, B. B. & Swan, A. K. Long tailed trions in monolayer MoS2: temperature dependent asymmetry and resulting red-shift of trion photoluminescence spectra. Sci. Rep. 7, 1–8 (2017).
Lin, Z. et al. Controllable growth of large-size crystalline MoS2 and resist-free transfer assisted with a Cu thin film. Sci. Rep. 5, 1–10 (2015).
Liu, H. & Chi, D. Dispersive growth and laser-induced rippling of large-area singlelayer MoS 2 nanosheets by CVD on c-plane sapphire substrate. Sci. Rep. 5, 1–8 (2015).
Splendiani, A. et al. Emerging photoluminescence in monolayer MoS2. Nano Lett. 10(4), 1271–1275. https://doi.org/10.1021/nl903868w (2010).
Bano, A. & Gaur, N. K. Interfacial coupling effect on electron transport in MoS2/SrTiO3 heterostructure: an ab-initio study. Sci. Rep. 8, 1–8 (2018).
Zhang, J. et al. Observation of strong interlayer coupling in MoS2/WS2 heterostructures. Adv. Mater. 28, 1950–1956 (2016).
Tongay, S. et al. Thermally driven crossover from indirect toward direct bandgap in 2D semiconductors: MoSe2 versus MoS2. Nano Lett. 12, 5576–5580 (2012).
Mak, K. F. et al. Tightly bound trions in monolayer MoS2. Nat. Mater. 12, 207–211 (2013).
Peimyoo, N. et al. Chemically driven tunable light emission of charged and neutral excitons in monolayer WS2. ACS Nano 8, 11320–11329 (2014).
Choi, J., Zhang, H. & Choi, J. H. Modulating optoelectronic properties of two-dimensional transition metal dichalcogenide semiconductors by photoinduced charge transfer. ACS Nano 10, 1671–1680 (2016).
Godefroo, S. et al. Classification and control of the origin of photoluminescence from Si nanocrystals. Nat. Nanotechnol. 3, 174–178 (2008).
Hwang, J. D. et al. Suppressing the dark current of metal-semiconductor-metal SiGe/Si heterojunction photodetector by using asymmetric structure. Thin Solid Films 515, 3837–3839 (2007).
Xu, H. Y. et al. Effects of annealing ambient on oxygen vacancies and phase transition temperature of VO2 thin films. RSC Adv. 6, 79383–79388 (2016).
Wang, L. et al. Oxygen vacancy induced room-temperature metal-insulator transition in nickelate films and its potential application in photovoltaics. ACS Appl. Mater. Interfaces 8, 9769–9776 (2016).
Zaki, S. E. et al. Role of oxygen vacancies in vanadium oxide and oxygen functional groups in graphene oxide for room temperature CO2 gas sensors. Sensors Actuators A Phys. 294, 17–24 (2019).
Jain, S. K. et al. Effect of metal contacts on a GaN/Sapphire-based MSM ultraviolet photodetector. J. Electron. Mater. 47, 6086–6090 (2018).
Casalino, M., Iodice, M., Sirleto, L., Rendina, I. & Coppola, G. Asymmetric MSM sub-bandgap all-silicon photodetector with low dark current. Opt. Exp. 21, 28072 (2013).
Reddy, M. S. P., Kim, B.-J. & Jang, J.-S. Dual detection of ultraviolet and visible lights using a DNA-CTMA/GaN photodiode with electrically different polarity. Opt. Exp. 22, 908 (2014).
Chao, C.-H., Weng, W.-J. & Wei, D.-H. Enhanced UV photodetector response and recovery times using a nonpolar ZnO sensing layer. J. Vac. Sci. Technol. A Vac. Surf. Films 34, 02D106 (2016).
Ling, Z. P. et al. Large-scale two-dimensional MoS_2 photodetectors by magnetron sputtering. Opt. Exp. 23, 13580 (2015).
Dhyani, V. & Das, S. High-speed scalable silicon-MoS2 P-N heterojunction photodetectors. Sci. Rep. 7, 44243 (2017).
Das, S., Chen, H. Y., Penumatcha, A. V. & Appenzeller, J. High performance multilayer MoS2 transistors with scandium contacts. Nano Lett. 13, 100–105 (2013).
Yang, Y., Huo, N. & Li, J. Sensitized monolayer MoS2 phototransistors with ultrahigh responsivity. J. Mater. Chem. C 5, 11614–11619 (2017).
Ko, P. J., Abderrahmane, A., Kim, N. H. & Sandhu, A. High-performance near-infrared photodetector based on nano-layered MoSe2. Semicond. Sci. Technol. 32, 065015 (2017).
Zhuo, R. et al. High-performance self-powered deep ultraviolet photodetector based on MoS2/GaN p-n heterojunction. J. Mater. Chem. C 6, 299–303 (2018).
Wu, J. M. & Chang, W. E. Ultrahigh responsivity and external quantum efficiency of an ultraviolet-light photodetector based on a single VO2 microwire. ACS Appl. Mater. Interfaces 6, 14286–14292 (2014).
Saenz, G. A., Karapetrov, G., Curtis, J. & Kaul, A. B. Ultra-high photoresponsivity in suspended metal-semiconductor-metal mesoscopic multilayer MoS2 broadband detector from UV-to-IR with low Schottky barrier contacts. Sci. Rep. 8, 1–11 (2018).
Liu, X. et al. All-printable band-edge modulated ZnO nanowire photodetectors with ultra-high detectivity. Nat. Commun. 5, 1–9 (2014).
Choi, W. et al. High-detectivity multilayer MoS2 phototransistors with spectral response from ultraviolet to infrared. Adv. Mater. 24, 5832–5836 (2012).
Dhakal, K. P. et al. Confocal absorption spectral imaging of MoS2: optical transitions depending on the atomic thickness of intrinsic and chemically doped MoS2. Nanoscale 6, 13028–13035 (2014).
Lopez-Sanchez, O. et al. Light generation and harvesting in a van der waals heterostructure. ACS Nano 8, 3042–3048 (2014).
Acknowledgements
The authors declare that they have received financial support from Scientific Research Projects Coordination (BAP)—Konya Necmettin Erbakan University (NEÜ), under Project Number: 191319007 that have appeared to influence the work reported in this paper. Moreover, the authors would like to thank the Science and Technology Research and Application Center (BITAM)—NEÜ for the continuous support through the characterizations section.
Author information
Authors and Affiliations
Contributions
M.A.B. suggested, designed and performed the whole experiments, characterizations, analysis, and writing the manuscript. S.E.Z. performed some experiments, characterization, and contributed for writing the manuscript. Y.R.E. and M.Y. contributed to writing, supervision, discussions and analysis of the data. M.S. characterized and analyzed the data, contributes to the plot of some figures, writing of the manuscript in addition to revision and editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Basyooni, M.A., Zaki, S.E., Shaban, M. et al. Efficient MoWO3/VO2/MoS2/Si UV Schottky photodetectors; MoS2 optimization and monoclinic VO2 surface modifications. Sci Rep 10, 15926 (2020). https://doi.org/10.1038/s41598-020-72990-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-72990-9
This article is cited by
-
MoS2 quantum dots and their diverse sensing applications
Emergent Materials (2024)
-
Controlled addition of Fe3O4 for enhancing photocarrier generation in MoS2 visible light photodetector
Journal of Materials Science: Materials in Electronics (2024)
-
Ultrasonic-Assisted Exfoliation Bulk-Phase of MoS2 with Chitosan/Acetic Acid Solution
JOM (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.