Abstract
Neoehrlichia mikurensis is an emerging tick-borne intracellular pathogen causing neoehrlichiosis. Its putative morphology was described in mammalian, but not in tick cells. In this study, we aim to show the presumptive morphology of N. mikurensis in salivary glands of engorged females of Ixodes ricinus. To accomplish this, we collected I. ricinus ticks in a locality with a high N. mikurensis prevalence, allowed them to feed in the artificial in vitro feeding system, dissected salivary glands and screened them by PCR for N. mikurensis and related bacteria. Ultrathin sections of salivary glands positive for N. mikurensis but negative for other pathogens were prepared and examined by transmission electron microscopy. We observed two individual organisms strongly resembling N. mikurensis in mammalian cells as described previously. Both bacteria were of ovoid shape between 0.5–0.8 μm surrounded by the inner cytoplasmic and the rippled outer membrane separated by an irregular electron-lucent periplasmic space. Detection of N. mikurensis in salivary glands of I. ricinus suggests that this bacterium uses the “salivary pathway of transmission” to infect mammals.
Similar content being viewed by others
Introduction
Neoehrlichia mikurensis is a gram-negative intracellular bacterium in the family Anaplasmataceae, widespread among the ticks in Europe and Asia1,2. In Europe, Ixodes ricinus (Linnaeus, 1758) is the primary vector1 and rodents seem to be the main reservoir hosts3. Even though N. mikurensis was discovered in 19994, limited information is available regarding its life cycle, mechanisms of cell infiltration and morphology.
In infected humans, N. mikurensis can induce “neoehrlichiosis”. Severity and symptoms of this novel disease vary greatly, from a mild illness5 to serious infections6,7 characterized by fever and vascular events endangering mainly individuals with immune-compromising conditions2. Asymptomatic courses of infection were also reported8. Nonetheless, most cases are probably misdiagnosed or not diagnosed at all since N. mikurensis cannot be currently detected by conventional approaches, but only by PCR2. Recently, Wass et al. (2019) succeeded in cultivation of this bacterium for the first time, propagating N. mikurensis in embryonal Ixodes spp. cell lines IRE/CTVM20 and ISE69. Therefore, it is no longer necessary to use the prefix “Candidatus”.
Regarding the morphology and ultrastructure of N. mikurensis, two papers were published10,11. However, both of them showed suspected N. mikurensis in mammalian cells. Some bacterial species related to N. mikurensis, e. g. Ehrlichia chaffeensis, are known to have different morphology in mammalian and tick cells12. Therefore, our goal in this study was to show the morphology N. mikurensis in salivary glands of the tick I. ricinus.
Material and methods
Tick collection
Questing adult I. ricinus were collected in April 2018 by flagging13 from the vegetation near Kvítkův Dvůr, Český Krumlov, Czech Republic (48.8093514 N, 14.2936328E), locality identified as a N. mikurensis hotspot in our previous large-scale epidemiological study, where 13,325 questing I. ricinus ticks from 140 areas throughout the Czech Republic were analyzed for the presence of N. mikurensis14. Respecting the sex, ticks were stored in 50 ml Falcon Tubes supplied with 4–6 grass blades and transported to the laboratory.
In vitro feeding
Sampled ticks were allowed to engorge in a modified artificial in vitro feeding system15. In contrast to the original protocol, we did not use chemical stimuli and hair. The collected ticks were equally distributed into 12 feeding wells, so that each contained 9–10 females and the same number of males. The bovine blood used was collected at a local abattoir and immediately supplied with heparin (15 U/ml), Nystatin (100 U/ml), Gentamicin (5 µg/ml), Adenosine 5′-triphosphate (1 mM) and glucose (4 g/ml) (all chemicals purchased at Sigma-Aldrich, Darmstadt, Germany). Blood exchanges were performed every 11–13 h throughout the whole course of feeding, which took place for 9 days. Ticks were fed in a water bath WNB 29 (Memmert GmbH, Schwabach, Germany) set to 36 °C.
Acquisition of salivary glands
Engorged females were embedded in a wax-filled Petri dish. For each tick, the dorsal part of the cuticle covering the idiosoma was removed using Self-Opening Mini-Micro Tweezers (Excelta, Buellton, USA). Opened ticks were rinsed thoroughly with sterile Phosphate-buffered saline (PBS). Next, midgut, Malpighian tubules and ovaries were individually removed, washed and stored for an unrelated study. Remnants of the blood meal were continuously washed away by PBS during the whole course of dissection. One salivary gland of each tick was subjected to immediate DNA extraction and subsequent PCR, while the second gland was put into 3% glutaraldehyde and later processed into ultrathin sections.
DNA extraction and PCR
DNA was extracted using a NucleoSpin Tissue kit (Macherey–Nagel, Düren, Germany) following the manufacturer's protocol. After the extraction, concentration and purity were checked by an Implen NanoPhotometer P330 (Implen, Munich, Germany) and stored at − 20 °C. Conventional PCR detections of I. ricinus, N. mikurensis and related bacteria were carried out using PPP Master Mix according to the manufacturer's instructions (Top-Bio s.r.o., Vestec, Czech Republic). Primer names, sequences, annealing temperatures, amplicon lengths and sequences used for the primer design are listed in Table 1. Primers were designed with the Geneious 11.1.4 software (https://www.geneious.com). The PCR products were separated electrophoretically in 1.5% agarose gels stained with Midori Green (Elisabeth Pharmacon, Brno, Czech Republic) and visualized under UV light. Specificity of the PCR reaction was confirmed by Sanger sequencing using the same primer set as for the detections14.
Transmission electron microscopy
The salivary glands were left in 3% glutaraldehyde for 5 days. Next, glands were washed three times for 10 min in 0.1 M Millonig's Phosphate buffer and contrasted in 2% OsO4 for 60 min. After contrasting, the glands were dehydrated through a graded series of acetone at room temperature, unless specified otherwise: 30% 20 min, 50% 20 min, 70% overnight at 4 °C, 90% 30 min, and twice with 100% for 30 min. Next, samples were embedded in Epon/Durcupan (E/D) mixture according to the manufacturer's recommendations (all used chemicals were purchased from SERVA Electrophoresis GmbH, Heidelberg, Germany). This mixture was further mixed with 100% acetone 1:1 and 3:1 and samples were incubated in both solutions for 45 min. Next, samples were incubated in pure E/D solution 3 × 60 min. After this step, samples were put into gelatin capsules and left to polymerize at 60 °C for 4 days. Blocks were trimmed on Leica EM TRIM2 and sectioned (60 nm) on the ultramicrotome Leica UC 7 (both machines purchased from Leica Microsystems, Vienna, Austria). Ultrathin sections were observed in EM Philips 208 S Morgagni (FEI, Brno, Czech Republic) at an accelerating voltage of 80 kV.
Ethics approval
This study did not include any experiments in humans, vertebrates neither higher invertebrate subjects. Therefore, all experiments were conducted in compliance with relevant European Union guidelines (86/609/EEC) and with the Czech national legislation on the use of animals and protection of animals against cruelty (Animal Welfare Act No. 246/1992 Coll.). Thus, it did not require any approval of institutional animal ethical committee. All methods and experimental protocols in the study were carried out in accordance with institutional guidelines.
Results and discussion
In total, 73/118 I. ricinus females engorged in the in vitro feeding system and were dissected. DNA of N. mikurensis was detected in 7/73 salivary glands (Table 1). All amplified sequences were identical to that found on the locality of flagging (Český Krumlov) previously14, which is available in GenBank under the accession code MN151364. One of the positive glands (1/7) was found to be co-infected with Rickettsia spp. Sequencing revealed 99.82% identity with the Rickettsia helvetica on a 558-bp-long fragment of the gltA gene, which can be viewed under the accession code KU310588.1. Therefore, corresponding embedded gland was excluded from the study. None of the N. mikurensis positive salivary glands tested positive for Ehrlichia spp. (Table 1) and none was further examined for the presence of other bacteria from the order Rickettsiales.
Detection of N. mikurensis in salivary glands suggests that this bacterium uses the “saliva transmission pathway”, which is generally acknowledged as a transmission route of most tick-borne pathogens16 including other members of the family Anaplasmataceae, e. g. Anaplasma marginale17. However, some other tick-borne pathogens are also transmitted by an alternative route, when the ingested blood is mixed with the bacteria present in the midgut acquired in the previous life stage and regurgitated to the host during feeding18. To the best of our knowledge, no research concerning the mechanism of infection and the pathogen distribution in tick organs has been conducted. Therefore, confirmation of the salivary transmission pathway of N. mikurensis and its distribution in individual tick organs by additional research, utilizing, for example, microscopic tracking and in situ hybridization as was shown previously19,20 is necessary.
Also, it should be noted that detection of N. mikurensis in salivary glands of I. ricinus fed on the blood treated with Gentamicin does not automatically prove its resistance to this antibiotic. It may be that N. mikurensis bacteria were not alive, but retained their morphology and genome fragments at the time of dissection. However, there are several reports of human patients with neoehrlichiosis that showed no response to Gentamicin21,22, suggesting that N. mikurensis might not be affected by this antibiotic. Thus, further research is needed to answer the question of a possible resistance of N. mikurensis to Gentamicin.
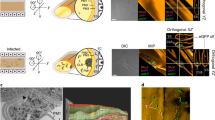
We spotted two suspected N. mikurensis organisms (Fig. 1) in one of the infected salivary glands. Both of them contained a Gram-negative type cell wall consisting of the inner cytoplasmic and the rippled outer membrane separated by an irregular periplasmic space, which is characteristic of the family Anaplasmataceae23. Both bacteria were of an ovoid shape with a diameter of 0.5–0.8 μm. These attributes correspond to the suspected N. mikurensis described previously in endothelial cells of Wister rats10 and human granulocytes11. Interestingly, the latter paper reported the finding of individual bacteria, which were also observed in our study. In contrast, Wass et al. (2019) described aggregates of N. mikurensis tightly surrounding the nucleus in ISE6 cells9, the embryo-derived I. scapularis cell lines24. Presumably, these bacteria were packed in intravacuolar microcolonies, formations of numerous organisms typical of related intracellular bacteria, e. g. Neoehrlichia lotoris25,26, Candidatus Ehrlichia khabarensis27 and Anaplasma phagocytophilum28. On the contrary to our and the two previously published papers10,11 depicting putative N. mikurensis, Wass et al. (2019) used a labelled specific DNA probe9. Hence, their findings of bacterial colonies supported by a direct hybridization-based evidence can be considered reliable. However, our findings are not necessarily in opposition, as Wass et al. (2019) mostly screened heavily infected tick cell lines during the 9th week of culture9, while we worked with naturally infected salivary glands which were acquired within 5 days post detachment. Therefore, it may be that the numbers of the present bacteria were low at this point and morulae were not spotted or developed at the time of dissection.
Transmission electron micrographs of suspected Neoehrlichia mikurensis in salivary glands of naturally infected engorged female Ixodes ricinus. Both individual organisms are surrounded by the rippled outer and the inner cytoplasmic membrane with electron-lucent periplasmic space. Scale bar: 500 nm (a), 200 nm (b).
The finding of only two putative N. mikurensis cells may be explained by different sensitivities of used methods of detection. In order to establish the level of detection of the PCR protocol, we designed gBlock covering the target area of the groEL gene plus 50 nucleotides on both ends (see Supplementary Data 1 online). We found that the detection limit of the used PCR protocol is 1–10 copies of the groEL gene. Therefore, it seems that the numbers of N. mikurensis bacteria were sufficient for the PCR detection, while it was unlikely to spot the bacteria by transmission electron microscopy, which has a detection limit of 105–106 particles/mL (viral, smaller, but comparable particles)29.
In conclusion, our findings should be treated carefully, for we show 2 individual suspected N. mikurensis bacteria in salivary gland of infected engorged I. ricinus female which tested positive by PCR for this bacterium and negative for Rickettsia spp. and Ehrlichia spp. However, we do not have a direct evidence that the shown structures are truly N. mikurensis. Therefore, additional research is needed to confirm our observations. It would be beneficial if the morphology and the ultrastructure of N. mikurensis would be examined on infected tick cell lines. Still, we believe that our findings contribute to the body of knowledge of N. mikurensis in ticks.
Data availability
Data are available on request.
References
Portillo, A., Santibáñez, P., Palomar, A. M., Santibáñez, S. & Oteo, J. A. Candidatus Neoehrlichia mikurensis Europe. New Microb. New Infect. 22, 30–36 (2018).
Wennerås, C. Infections with the tick-borne bacterium Candidatus Neoehrlichia mikurensis. Clin. Microbiol. Infect. 21, 621–630 (2015).
Burri, C., Schumann, O., Schumann, C. & Gern, L. Are Apodemus spp. mice and Myodes glareolus reservoirs for Borrelia miyamotoi, Candidatus Neoehrlichia mikurensis, Rickettsia helvetica, R. monacensis and Anaplasma phagocytophilum? Ticks Tick Borne Dis. 5, 245–251 (2014).
Schouls, L. M., van de Pol, I., Rijpkema, S. G. T. & Schot, C. S. Detection and identification of Ehrlichia, Borrelia burgdorferi sensu lato, and Bartonella species in Dutch Ixodes ricinus ticks. J. Clin. Microbiol. 37, 2215–2222 (1999).
Li, H. et al. Human infection with Candidatus Neoehrlichia mikurensis China. Emerg. Infect. Dis. 18, 1636–1639 (2012).
von Loewenich, F. D. et al. Detection of ‘Candidatus Neoehrlichia mikurensis’ in two patients with severe febrile illnesses: Evidence for a European sequence variant. J. Clin. Microbiol. 48, 2630–2635 (2010).
Welinder-Olsson, C., Kjellin, E., Vaht, K., Jacobsson, S. & Wennerås, C. First case of human ‘Candidatus Neoehrlichia mikurensis’ infection in a febrile patient with chronic lymphocytic leukemia. J. Clin. Microbiol. 48, 1956–1959 (2010).
Welc-Falȩciak, R., Siński, E., Kowalec, M., Zajkowska, J. & Pancewicz, S. A. Asymptomatic ‘Candidatus Neoehrlichia mikurensis’ infections in immunocompetent humans. J. Clin. Microbiol. 52, 3072–3074 (2014).
Wass, L. et al. Cultivation of the causative agent of human neoehrlichiosis from clinical isolates identifies vascular endothelium as a target of infection. Emerg. Microb. Infect. 8, 413–425 (2019).
Kawahara, M. et al. Ultrastructure and phylogenetic analysis of ‘Candidatus Neoehrlichia mikurensis’ in the family Anaplasmataceae, isolated from wild rats and found in Ixodes ovatus ticks. Int. J. Syst. Evol. Microbiol. 54, 1837–1843 (2004).
Pekova, S. et al. Candidatus Neoehrlichia mikurensis infection identified in 2 hematooncologic patients: benefit of molecular techniques for rare pathogen detection. Diagn. Microbiol. Infect. Dis. 69, 266–270 (2011).
Dedonder, S. E., Cheng, C., Willard, L. H., Boyle, D. L. & Ganta, R. R. Transmission electron microscopy reveals distinct macrophage- and tick cell-specific morphological stages of Ehrlichia chaffeensis. PLoS ONE 7, e36749 (2012).
Tkadlec, E., Václavík, T., Kubelová, M. & Široký, P. Negative spatial covariation in abundance of two European ticks: Diverging niche preferences or biotic interaction? Ecol. Entomol. 43, 804–812 (2018).
Ondruš, J. et al. Candidatus Neoehrlichia mikurensis is widespread in questing Ixodes ricinus ticks in the Czech Republic. Ticks Tick Borne Dis. 11, 101371 (2020).
Kröber, T. & Guerin, P. M. An in vitro feeding assay to test acaricides for control of hard ticks. Pest Manag. Sci. 63, 17–22 (2007).
Šimo, L., Kazimirova, M., Richardson, J. & Bonnet, S. I. The essential role of tick salivary glands and saliva in tick feeding and pathogen transmission. Front. Cell. Infect. Microbiol. 7, 281 (2017).
Kocan, K. M., De La Fuente, J., Blouin, E. F. & Garcia-Garcia, J. C. Anaplasma marginale (Rickettsiales: Anaplasmataceae): recent advances in defining host-pathogen adaptations of a tick-borne rickettsia. Parasitology 129, S285–S300 (2004).
Benach, J. L., Coleman, J. L., Skinner, R. A. & Rosler, E. M. Adult Ixodes dammini on rabbits: A hypothesis for the development and transmission of Borrelia burgdorferi. J. Infect. Dis. 155, 1300–1306 (1987).
Pospisilova, T. et al. Tracking of Borrelia afzelii transmission from infected Ixodes ricinus nymphs to mice. Infect. Immun. 87, e00896-e918 (2019).
Lynn, G. E. et al. Tissue distribution of the Ehrlichia muris-like agent in a tick vector. PLoS ONE 10, e0122007 (2015).
Grankvist, A. et al. Infections with the tick-borne bacterium ‘Candidatus Neoehrlichia mikurensis’ mimic noninfectious conditions in patients with B cell malignancies or autoimmune diseases. Clin. Infect. Dis. 58, 1716–1722 (2014).
Fehr, J. S. et al. Septicemia caused by tick-borne bacterial pathogen Candidatus Neoehrlichia mikurensis. Emerg. Infect. Dis. 16, 1127–1129 (2010).
Rikihisa, Y. The tribe Ehrlichieae and ehrlichial diseases. Clin. Microbiol. Rev. 4, 286–308 (1991).
Munderloh, U. G., Liu, Y., Wang, M. M., Chen, C. S. & Kurtti, T. J. Establishment, maintenance and description of cell-lines from the tick Ixodes scapularis. J. Parasitol. 80, 533–543 (1994).
Munderloh, U. G., Yabsley, M. J., Murphy, S. M., Luttrell, P. M. & Howerth, E. W. Isolation and establishment of the raccoon Ehrlichia-like agent in tick cell culture. Vector-Borne Zoonotic Dis. 7, 418–425 (2007).
Yabsley, M. J. et al. Characterization of ‘Candidatus Neoehrlichia lotoris’ (family Anaplasmataceae) from raccoons (Procyon lotor). Int. J. Syst. Evol. Microbiol. 58, 2794–2798 (2008).
Rar, V. A. et al. Molecular-genetic and ultrastructural characteristics of ‘Candidatus Ehrlichia khabarensis’, a new member of the Ehrlichia genus. Ticks Tick Borne Dis. 6, 658–667 (2015).
Munderloh, U. G. et al. Isolation of the equine granulocytic ehrlichiosis agent, Ehrlichia equi, in tick cell culture. J. Clin. Microbiol. 34, 664–670 (1996).
Roingeard, P. Viral detection by electron microscopy: Past, present and future. Biol. Cell 100, 491–501 (2008).
Acknowledgements
This research was funded by IGA VFU project 201/2019/FVHE, project RO0518 of the Czech Ministry of Agriculture and the project FIT, number CZ.02.1.01/0.0/0.0/15_003/0000495. We cordially acknowledge Josef Illek, Jarmila Cebáková, Kamil Zeman, Adam Novobilský, Josef Mašek, Kristína Zechmeisterová and Jaroslav Turánek. Also, we are grateful to Ludmila Faldíková for English corrections.
Author information
Authors and Affiliations
Contributions
J.O. designed the study, acquired funding, performed research, analyzed data and wrote the manuscript. P.K. analyzed data. O.S. and P.Š. revised the first draft of the manuscript. All authors approved the submitted version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ondruš, J., Kulich, P., Sychra, O. et al. Putative morphology of Neoehrlichia mikurensis in salivary glands of Ixodes ricinus. Sci Rep 10, 15987 (2020). https://doi.org/10.1038/s41598-020-72953-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-72953-0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.