Abstract
In this study, we describe a new in vitro tick feeding system that facilitates the study of ticks and tick-borne pathogens. To optimize the system, we used Dermacentor andersoni and Anaplasma marginale as a tick-pathogen interaction model. Ticks were fed on bovine blood containing 10-fold dilutions of the pathogen to determine the effect of dose on tick infection rate. After feeding on infected blood, ticks were transferred to uninfected blood to stimulate bacterial replication within the tick vector. During stimulation feeding, blood samples were collected daily to determine if infected ticks secreted viable A. marginale. The results demonstrated similar attachment rates between the first and second tick feeding. Tick midgut and salivary glands were infected with A. marginale. However, salivary gland infection rates decreased as the percentage of parasitized erythrocytes decreased during tick acquisition feeding. Bacteria recovered from the in vitro system were able to infect a naïve bovine host. Using the highly transmissible A. marginale St. Maries strain, we demonstrated that the artificial tick feeding system is a suitable tool to study tick-pathogen interactions and that A. marginale tick salivary gland infection is dose dependent. This work demonstrates the utility of an artificial tick feeding system to directly study the association between the number of acquired pathogens and transmissibility by ticks.
Similar content being viewed by others
Introduction
Tick-borne diseases caused by bacteria, protozoa, and viruses are responsible for a significant burden in human beings and animals1,2,3,4,5,6. Recent reports indicate that ticks transmit the majority of emerging arthropod-borne pathogens7,8,9,10. However, our ability to prevent transmission of tick-borne pathogens is limited. In human beings, the most effective measures rely on tick avoidance. In livestock, prevention of tick-borne pathogen transmission currently depends on the use of acaracides. Unfortunately, acaracides are toxic and, in some regions, losing efficacy11,12.
Understanding the key events at the tick-pathogen interface is the foundation for developing strategies to control tick-borne diseases. Currently, such studies require the use of an infected mammal to rear infected ticks13,14, thus limiting our ability to tightly control the delivery of the pathogen and accompanying blood meal to the tick vectors. To address this limitation, we have developed a novel in vitro tick feeding system. To demonstrate the efficacy of the in vitro tick feeding system for controlled pathogen delivery to tick vectors we used Anaplasma marginale, which causes bovine anaplasmosis15, and one of its natural vectors, Demarcentor andersoni16,17,18. Anaplasma marginale, an obligate intracellular pathogen in the family Anaplasmataceae, serves as a robust model for acquisition and transmission due to our in-depth understanding of the life cycle of this pathogen within its tick vector13,14,15,19.
In the case of the A. marginale transmission model, the male tick takes multiple blood meals and is responsible for transmission, this is called intrastadial transmission because of its occurrence within the adult life stage. In order to complete an infection cycle within the male tick, A. marginale must overcome two colonization and replication barriers, first within the midgut and then within the salivary glands13,20. During the initial feed, termed the acquisition feed, the pathogen enters and replicates in the tick midgut13,14,21,22,23. When the tick ingests a second blood meal, termed transmission feed, the bacteria transit to and replicate in the salivary glands13,14,21,22,23. The bacteria are subsequently released into the new host with the tick saliva during the transmission feed.
In this study, using the in vitro tick feeding system, we first determined if A. marginale could successfully complete its life cycle within D. andersoni by demonstrating tick midgut and salivary gland infection and the secretion of viable organisms from the tick salivary glands during the transmission feed. Secondly, four A. marginale doses were delivered concurrently to four different groups of ticks in order to determine the effect of dose on tick infection rates and the number of bacteria in tick midgut and salivary glands.
Results
Tick attachment
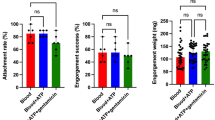
For acquisition feeding, separate feeders containing up to 120 adult male D. andersoni ticks were exposed to 10-fold differences in the percentage of parasitized erythrocytes (PPE) from 106 to 109 A. marginale per ml of blood (Table 1). The tick attachment rates ranged from 71% to 84% (Table 2). For transmission feeding, 40 to 47 adult male ticks from each group and 10 uninfected female ticks per group were used. The attachment rates for the second feeding ranged from 92% to 96% (Table 2). There were no differences in the tick attachment rates between the four treatment groups during acquisition or transmission feeding (p > 0.35). Figure 1 illustrates ticks attached to the silicone membrane during acquisition (Fig. 1A) or transmission feeding (Fig. 1B).
Tick acquisition of A. marginale
Ticks acquired A. marginale from the in vitro tick feeding system. Tick midgut infection rates ranged from 80% to 100% (Table 3), with no differences among the four treatment groups (p > 0.76). Tick salivary gland infection rates were 72% in group 1 that received 109 A. marginale/ml, 4% in group 2, that received 108 A. marginale/ml of blood, and 0% in ticks exposed to lower doses, groups 3 and 4 (Table 3). The infection rate in group 1 was different from the other groups (p < 0.01).
Overall, the average number of A. marginale in midguts after acquisition feeding, as detected by qPCR, reflected the number of A. marginale in the blood meal, and demonstrated that D. andersoni ticks were exposed during tick feeding (Table 3). The number of A. marginale per midgut in group 1 was 106.22 (±0.093) bacteria, which was higher than the other 3 groups (p < 0.01). The number of A. marginale per midgut was similar in group 2 with 104.87 (±0.090) and group 3 with 105.00 (±0.101) receiving 108 and 107 A. marginale/ml of blood, respectively (P > 0.99). Group 4, receiving 106 A. marginale/ml of blood, had the lowest number of bacteria per midgut at 103.94 (±0.101) which was different than groups 2 and 3 (p < 0.01).
As expected, the average number of A. marginale per salivary gland pair was, overall, comparatively low. Specifically, in group 1 there were 104.45 (±0.107) bacteria per salivary gland pair (Table 3). In group 2, a single tick was infected with 103.74 bacteria per salivary gland pair, while no A. marginale was detected in groups 3 and 4. Due to the low number of infected salivary glands after acquisition feeding, statistical comparison of infected ticks could not be conducted.
Tick transmission of A. marginale
Transmission feeding had little impact on midgut infection rates that ranged from 88% to 96% (Table 4), with no differences among the four groups (p > 0.99). The increased number of A. marginale in the midguts after transmission feeding as compared to the acquisition feeding indicated replication, which was particularly evident in group 4 ticks (Table 4), which had approximately a 10 fold increase in A. marginale numbers. The number of A. marginale per midgut in group 1 was 10 6.27 (±0.095), which was greater (p < 0.01) than in groups 2 with 105.37 (±0.091), group 3 with 105.20 (±0.091) and group 4 with 105.00 (±0.092) A. marginale per midgut.
Notably, infection rates in salivary gland pairs were higher in transmission-fed as compared to acquisition-fed ticks (Table 4). The salivary gland infection rate of transmission-fed ticks varied based on the number of A. marginale in the blood meal. Specifically, group 1 and group 2 ticks had a 72% and 60% infection rate, respectively. While groups 3 and 4 ticks had infection rates of 32% and 28%, respectively. Group 1 was different from all the other groups (p < 0.01). The number of A. marginale per salivary gland pair also increased following the transmission feed, as compared to the acquisition feed, indicating replication of A. marginale in the salivary glands (Table 4). For example, the number of A. marginale per salivary gland pair increased over 10-fold in group 1 ticks and from non-detectable to >104 in groups 3 and 4. Overall, group 1 ticks had the highest number of A. marginale per salivary gland pair at 105.90 (±0.105), which was different (p < 0.01) from the other three groups and groups 2, 3, and 4 had similar numbers of bacteria per salivary gland pair (p > 0.20) that varied from 104.20 (±0.155) to 104.83 (±0.158).
Pathogen secretion during transmission feeding
Ticks successfully transmitted A. marginale into the in vitro tick feeding system. In the first four days post attachment on the siliconized membrane, there was no evidence of A. marginale secretion into the in vitro system. Ticks exposed to higher PPE began secreting quantifiable A. marginale into the in vitro system on day five. Msp5-qPCR demonstrated the number of bacteria secreted into the in vitro system at day five and six post tick attachment were 104.3 and 103.4 organisms per ml of blood, respectively.
Viability of A. marginale secreted into the in vitro tick feeding system
Intravenous inoculation of 105.7 A. marginale from the in vitro system infected a naïve, splenectomized animal. Nested msp5-PCR detected A. marginale in the peripheral blood on day 17 post inoculation. Microscopic examination of Giemsa stained blood smears detected infected erythrocytes on day 29 post inoculation (Table 5).
Stability of A. marginale strain throughout the in vitro tick feeding system
Msp1α is a single copy gene with tandem repeats that is commonly used for strain typing of A. marginale15,24. Here we used msp1α to confirm the identity and stability of the St. Maries strain throughout the entire infection cycle including the donor calf used for acquisition feeding, midgut following acquisition feeding, salivary glands following transmission feeding, and the recipient calf. The resulting amplicon of 564 base pairs was consistent with the St. Maries strain at all stages (Fig. 2). The images were cropped from a single gel image that included molecular weight standard. The full-length blots/gels are presented in Supplementary Fig. 1. Sequencing confirmed 100% identity among all amplicons at all time points (Fig. 3).
Detection of A. marginale St. Maries strain within the vertebrate and invertebrate hosts. Calf 95079: blood donor for the in vitro tick feeding system, AF-MG: tick midgut harvested after acquisition feeding, TF-SG: tick salivary gland harvested after transmission feeding, Recovered A. marginale: Bacteria secreted into the in vitro tick feeding system, and calf 95064: recipient animal inoculated with recovered A. marginale from the in vitro tick feeding. PCR targeting msp1a used for A. marginale genotyping. The images were cropped from a single gel image that included molecular weight standard. The full-length gel is presented in Supplementary Fig. 1.
Confirmation of A. marginale St. Maries strain identity by sequencing msp1a Calf 95079: Blood donor for the in vitro tick feeding system, AF-MG: tick midgut harvested after acquisition feeding, TF-SG: tick salivary gland harvested after transmission feeding, AM secr: Bacteria secreted into the in vitro tick feeding system, and calf 95064: Recipient animal inoculated with recovered A. marginale from the in vitro tick feeding. The start of each repeat is in boldface. Solid underlines represent the St. Maries MSP1a repeat region.
Discussion
One major limitation in understanding the tick-pathogen interface is the lack of tools to tightly control and alter the delivery of the blood meal to the tick. In the past, various membrane feeding systems used for this purpose lacked consistency in tick attachment and efficiency in acquiring pathogens25,26,27,28,29. To address these limitations, we have developed and tested an in vitro tick feeding system that can simultaneously deliver at least four different treatments. To test the ability of the system to recapitulate the life cycle of a pathogen within a tick, we used A. marginale as a model organism and completed an infection cycle including acquisition and transmission feeds. We concurrently tested the effect of dose on the tick infection rates and number of bacteria in tick midgut and salivary glands.
In vivo experiments involving tick feeding on A. marginale infected animals are typically conducted either during acute or persistent infection13,14,19,21,22. Acute infection is defined as a rising count of infected erythrocytes as detected in Giemsa stained blood smears and >108 A. marginale per ml of blood13,14. Persistent infection follows acute infection by typically 6 weeks and is defined as microscopically undetectable infected erythrocytes which corresponds to <108 A. marginale per ml of blood21,22. In our experiments, group 1 and 2 ticks received doses comparable to acute infection (109 and 108 A. marginale per ml of blood), while groups 3 and 4 ticks received doses mimicking persistent infection (107 and 106 bacteria per ml of blood).
Because the midgut is the first organ to be infected, midgut infection rates are not expected to differ markedly when comparing the acquisition and transmission fed ticks. In vivo infection rates in the Reynold’s Creek colony of D. andersoni during acute infection following a seven-day acquisition and transmission feed are typically 100%13,14,30. In our experiments, group 1 and group 2 ticks had comparable, but somewhat, lower infection rates between 88 to 100%. The infection rates in the ticks exposed to A. marginale mimicking persistent infection in group 3 and group 4 ticks were between 80–92%. The infection rates of D. andersoni fed on persistently infected animals with an average of approximately106 infected RBC/ml of blood were between 89–90%19. These somewhat lower values do not reduce the utility of the in vitro tick feeding system and may be due to some degree of increased variation in tick feeding on the artificial system as compared to cattle. Alternatively, the A. marginale doses received by the tick were calculated based on the average number of A. marginale /ml of blood for each day of tick feeding. In the artificial feeding system, the number of A. marginale are more likely to be more uniform. Thus, the differences in infection may also reflect less variation in the pathogen number in the blood meal.
The number of A. marginale in the midguts of the artificially fed ticks is overall comparable to reported values13,14,19,30,31. Little published data are available for acquisition fed ticks. In transmission fed ticks exposed to 108 A. marginale, the number of bacteria in midgut was approximately 106 organisms13,14,30. In our experiments, the ticks fed with 108 A. marginale per ml of blood had number of bacteria in midgut of approximately 105 A. marginale.
In the salivary glands, the low infection rates in all groups of ticks, except those fed on 109 A. marginale per ml of blood, following the acquisition feed is expected based on the lifecycle of A. marginale in the tick vector. Following the transmission feed, the infection rates in the salivary glands in group 1 and group 2 ticks, mimicking acute infection, was lower 72% and 60%, respectively, than the 80 to 100% reported for ticks fed on an acutely infected animal (108 A. marginale/ml of blood)13,31. However, the number of A. marginale are overall comparable13,14,30. The number of A. marginale in the salivary glands following feeding on an acutely infected animal (108 A. marginale/ml of blood) was approximately 107 A. marginale per salivary gland pair13. The ticks receiving a similar dose via artificial feeding had approximately 106 bacteria per salivary gland pair. In ticks fed on a persistently infected animal with 106 A. marginale per ml of blood, the number of pathogens in the salivary glands were 104 organisms per salivary gland pair19,32, similar to the group 4 ticks, which also had 104 A. marginale per salivary gland pair.
The effect of dose on tick infection rates and number of bacteria was only apparent in the salivary glands, indicating the salivary glands are a stronger barrier to pathogen infection as compared to the midgut. This echoes previously published data in which super-infection exclusion only occurred in D. andersoni salivary glands, but not midguts when ticks were exposed to two strains of A. marginale during sequential tick feeds33. This suggests that targeting the interaction between the pathogen and the salivary gland may lead to more effective interventions than targeting the pathogen-midgut interface.
Importantly, A. marginale was detected in the blood receptacle following transmission feeding of the tick vector. Additional experiments are required to quantitate the number and viability of A. marginale secreted through time using this system. To date, A. marginale present in the salivary secretions during transmission feeding has not been quantified. Thus, there is no reference point for the number of A. marginale secreted during feeding on the artificial system. The infection of an inoculated calf confirmed viability of the organism and successful completion of an infection cycle. In a previous study, inoculation of multiple calves with residues of erythrocytes collected from artificial membrane systems during A. marginale-infected adult ticks feeding failed to infect naïve bovine hosts. Artificially fed adult ticks did acquire and transmit A. marginale as demonstrated when exposed ticks infected naïve animals, however, due to the lack of quantitative methods, it was not possible to enumerate the number of A. marginale per salivary gland pair dissected from artificially fed ticks34. In another study, D. variabilis adult ticks fed A. marginale blood via a capillary feeding system developed midgut infections. However, following a second feeding on sheep, A. marginale did not disseminate to the salivary glands as would be expected35.
In conclusion, we have demonstrated that the life cycle of A. marginale, including intrastadial transmission, as has been well defined experimentally in vivo, is recapitulated via an in vitro tick feeding system. Additionally, pathogen dose delivered to the tick has an effect on infection rates and the number of bacteria in tick salivary glands, but not in the midgut. Although A. marginale was used as the model organism in this system, it may be adapted for other tick species and pathogens. However, the efficiency of transstadial transmission, epidemiologically relevant for many tick borne pathogens, must be determined31. The use of siliconized membranes is not suitable for all studies, particularly those involving the dermal tick-pathogen-host interface. However, the in vitro tick feeding system presented here mimics the host micro-environment for tick feeding success. Thus, it may serve as an essential tool to dissect the invasion, colonization, and transmission mechanisms of pathogens and may provide a framework for reduction and refinement of animal use to study ticks and tick-borne pathogens of human and veterinary importance.
Materials and Methods
Bovine blood infected with A. marginale
For acquisition feeding, blood was collected daily from an acutely infected animal, Calf 95079, and used to infect D. andersoni ticks through the in vitro tick feeding system. Four A. marginale doses were delivered concurrently, each to a different group of ticks (Table 1).
In vitro tick feeding system
The system includes a tick feeding chamber, a digital heating power source and peristaltic pump. The feeding chamber consists of five parts assembled by threads: (1) blood heating element, (2) blood receptacle, (3) tick vessel, (4) connector, and (5) membrane frame (Fig. 4A,B). The simple assembly and disassembly of the feeding chamber allows cleaning and blood changing without interrupting tick feeding. The blood receptacle holds 20 ml of blood. The mammalian host blood temperature is mimicked through a heating element connected to a digital heating power source (Fig. 4C), that can support four independent feeders simultaneously. When blood circulation is necessary, the system can be connected to a peristaltic pump with a velocity of 70 ml per min through siliconized C-Flex Ultra tubing (Cole-Parmer, Vernon Hills, IL). The tubing is connected by two luer lock outlets on the side of the blood receptacle. Each tick feeding vessel holds approximately 100 to 150 adult ticks. Ticks are confined in the vessel by a white silk screen mesh. The entire system is placed in an incubator with a controlled temperature at 26 °C with a 14 hr light/dark photo period. Maintaining the system at a constant 26 °C provides an environment that encourages tick migration and attachment to the membrane covering the blood that is heated to 37 °C.
In vitro tick feeding system. The unassembled feeder (A) is composed of a heating lid, blood receptacle, frame to support the membrane, a connector and a tick vessel. Unassembled feeder; (B) Assembled feeder and (C) A digital heating power source with four tick feeding device with a peristaltic pump in the back.
Feeding membrane preparation
Siliconized membranes were prepared using commercially available Goldbeater’s skin (Talas, Brooklyn, NY). Five ml of each Ecoflex Supersoft 00–50 silicone components A and B (Smooth-On, Easton, PA) were mixed and thinned with 0.75 ml of hexane (Sigma-Aldrich). Goldbeater’s membranes were taped to a smooth surface and saturated with the silicone mixture to yield an approximately100 μm thick membrane. Membranes were air dried overnight at room temperature.
Animals, tick vector and pathogen
The animal use in this study (IACUC #2018-16) was approved by the Institutional Animal Care and Use Committee of the University of Idaho, Moscow, Idaho, in accordance with institutional guidelines based on the U.S. National Institutes of Health Guide for the Care and Use of Laboratory Animals. Two five-month-old Holstein calves were confirmed to be free of A. marginale as determined by the Msp5-ELISA serologic test (VMRD, Pullman, WA) and Msp5-nested PCR14,36. The specific pathogen-free Reynolds Creek colony of D. andersoni and readily transmissible St. Marie’s strain of A. marginale were used in this study13,17,21,22.
Tick acquisition feeding
Calf 95079 was inoculated intravenously with blood stabilate containing about 109 bacteria per ml. During acute infection, 100 ml of defibrinated blood was collected daily to feed ticks on the in vitro tick feeding system. Microscopic examination of Giemsa stained blood smears and Msp5-qPCR were used to assess the number of bacteria13,17. Blood was centrifuged to deplete the white blood cells and reconstituted to 30% packed cell volume with 0.2% glucose and stored at 4 °C. Prior to tick feeding in the in vitro system, blood was warmed to 37 °C. Uninfected blood was collected in the same manner of infected blood and reconstituted to 30% packed cell volume and 0.2% glucose and used to dilute the infected blood. Four in vitro tick feeding devices each containing bovine blood with 10-fold dilutions of A. marginale were used to feed the ticks. Each blood meal was changed every 8 hours. Each tick feeding device received 100 male and 20 female D. andersoni ticks. Though epidemiologically irrelevant for intrastadial transmission, the female ticks were added to the feeding chamber to stimulate the attachment of male ticks to the silicone membrane. Ticks were allowed to feed on the in vitro system for seven days to acquire A. marginale.
After acquisition feeding, the ticks were incubated at 26 °C in 94% relative humidity for five days. This interval ensured that the blood meal was completely digested. After incubation, a cohort of 25 male ticks were dissected and midgut and salivary glands harvested. Genomic DNA was extracted to confirm entry into the midgut epithelial cells and if colonization had occurred using Msp5-qPCR as previously described17.
Tick transmission feeding
Defibrinated blood was collected daily from an uninfected calf to feed ticks in the in vitro tick feeding system. Blood was centrifuged to remove the white blood cells. The blood was reconstituted to 10% packed cell volume, the minimum needed to support tick feeding, with 0.2% glucose and stored at 4 °C. Prior to feeding ticks in the in vitro system, blood was warmed to 37 °C. Four in vitro tick feeding devices, containing uninfected bovine erythrocytes, were used to feed ticks for transmission. Each feeding device contained 40 to 47 male and 10 uninfected female D. andersoni ticks. Ticks were allowed to feed for six days to stimulate A. marginale replication in the salivary glands. After transmission feeding in the in vitro system, ticks were dissected to determine A. marginale replication in the midgut and salivary glands by Msp5-qPCR17. Blood samples from the in vitro system were collected and DNA extracted to determine secretion of A. marginale into the in vitro tick feeding system.
Viability and infectivity of A. marginale through inoculation of a naïve animal
During the transmission feed, blood samples from the in vitro system were collected every 8 hours, DNA extracted and Msp5-qPCR performed to determine if A. marginale was secreted into the in vitro system during tick feeding. To evaluate infectivity, approximately 5 ml of blood recovered from the in vitro system during transmission feeding was inoculated intravenously into naïve splenectomized calf 95064. Blood samples from calf 95064 were collected daily and monitored by microscopic examination of Giemsa stained blood smears and Msp5-nested PCR37.
DNA extraction
Genomic DNA was extracted from blood by using a PureGene DNA Isolation kit following the manufacture’s guideline of (ThermoFisher Scientific, Waltham, MA). For tick midgut and salivary gland, samples were lysed in 450 μl of Tris-EDTA and sodium dodecyl sulfate (SDS) with 50 μl of Proteinase K (2 mg/ml) and incubated overnight at 55 °C. Following the incubation, 1 μl of glycogen (10 μg/ml) was added to the samples. The proteins were precipitated with ammonium acetate. Genomic DNA samples were precipitated in isopropanol, washed with 70% ethanol, and suspended in 50 μl Tris-EDTA.
TaqMan quantitative PCR
Msp5, a, highly conserved single copy gene in A. marginale, was used to quantify bacteria in all samples15,17. The primer sequences (forward 5’-CTTCCGAAGTTGTAAGTGAGGGCA-3′; reverse 5′-CTTATCGGCATGGTCGCCTAGTTT-3′) amplify a 202 bp fragment and a TaqMan probe (5′-GCCTCCGCGTCTTTCAACAATTTGGT-3′) binds between the primer sets as previously described13,17. Reactions were performed using SsoAdvanced Supermix, 50 µM each primer, 100 µM TaqMan probe, and 5 µl of template DNA. Thermocycling conditions consisted of 45 cycles of 95 °C for 3 min, melting at 95 °C for 15 sec, and annealing at 55 °C for 45 sec, with extension at 72 °C for 7 min. The assay was performed using a Biorad CFX real-time PCR detection system (Bio-Rad Laboratories, Hercules, CA). Standard curves were constructed by amplification of a serially diluted plasmid standard from 106 to 102 msp5 copies, as previously described13,17. Quantitative PCR was conducted in triplicate for each sample.
Nested PCR
Msp5 was used as the gene target for nested PCR. External primer sets of forward (5′-GCATAGCCTCCGCGTCTTTC-3′) and reverse (5′-ACACGAAACTGTACCACTGCC-3′) were used to amplify a fragment of 525 base pairs13,17. Reactions containing 2 μl of template DNA, 1 μl of 10 µM of each primer, 10 µl of RedTaq (Sigma-Aldrich) and 6 µl of nuclease free water (Ambion) under the following conditions: one cycle at 95 °C for 4.5 min, 35 cycles of 95 °C for 30 sec, 65 °C for 1 min, 72 °C for 1 min, and extension at 72 °C for 5 min. Internal primer sets of forward (5′- TACACGTGCCCTACCGAGTTA-3′) and reverse (5′- TCCTCGCCTTGGCCCTCAGA-3′) were used to amplify a fragment of 343 base pairs13,17. Nested reactions were performed using 0.5 μl of external PCR product, 1 μl of 10 µM of each primer, and 10 µl of RedTaq (Sigma-Aldrich) under the following conditions: One cycle at 95 °C for 4.5 min, 35 cycles of 95 °C for 30 sec, 55 °C for 30 sec, 72 °C for 30 sec, and extension at 72 °C for 5 min. Following electrophoresis, amplicons were visualized on a 2% agarose gel.
Strain-specific PCR
Primer sets of forward (5′-GTGCTTATGGCAGACATTTCC-3′) and reverse (5′-CTCAACACTCGCAACCTTGG-3′) were used to amplify the 5′ end of Msp1a, which has tandem repeats used as strain markers as previously described1,14,24. PCR was performed using 1 μl of template DNA, 1 μl of 10 µM of each primer, and RedTaq (Sigma-Aldrich) under the following conditions: One cycle at 95 °C for 3 min, 35 cycles of 95 °C for 30 sec, 55 °C for 30 sec, 72 °C for 45 sec, and extension at 72 °C for 7 min. Amplicons were visualized by 1% agarose gel electrophoresis and PCR products sequenced (Eurofins Genomics, Louisville, KY).
Statistical analysis
An analysis of variance was conducted for A. marginale numbers, tick attachment, and infection rate. A mixed model was used with binomial tick attachment data where ticks were considered positive if A. marginale was detected with Msp5-qPCR and fixed effects were type of tick feeding and A. marginale dose and including a repeated effect of individual tick (SAS 9.4 GLIMMIX, SAS® Inst. Inc., Cary, NC). The mixed model for comparisons of binomial infection rate (GLIMMIX) and continuous and greater than 0 A. marginale (MIXED) included fixed effects of tissue sample, A. marginale dose, and type of tick feeding with a repeated effect of individual tick. A. marginale per salivary gland pair was transformed to log10 due to heterogeneous variances between groups. Pairwise multiple comparisons were conducted with Tukey’s test.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Palmer, G. H. et al. Stochastic transmission of multiple genotypically distinct Anaplasma marginale strains in a herd with high prevalence of Anaplasma infection. J. Clin. Microbiol 42, 5381–5384, https://doi.org/10.1128/jcm.42.11.5381-5384.2004 (2004).
Ueti, M. W., Palmer, G. H., Scoles, G. A., Kappmeyer, L. S. & Knowles, D. P. Persistently infected horses are reservoirs for intrastadial tick-borne transmission of the apicomplexan parasite Babesia equi. Infect Immun. 76, 3525–3529, https://doi.org/10.1128/iai.00251-08 (2008).
Michelitsch, A., Wernike, K., Klaus, C., Dobler, G. & Beer, M. Exploring the reservoir hosts of tick-borne encephalitis virus. Viruses 11, https://doi.org/10.3390/v11070669 (2019).
Garrison, A. R., Smith, D. R. & Golden, J. W. Animal Models for Crimean-Congo Hemorrhagic Fever Human Disease. Viruses 11, https://doi.org/10.3390/v11070590 (2019).
Palmer, G. H., Rurangirwa, F. R. & McElwain, T. F. Strain composition of the ehrlichia Anaplasma marginale within persistently infected cattle, a mammalian reservoir for tick transmission. J. Clin. Microbiol 39, 631–635, https://doi.org/10.1128/jcm.39.2.631-635.2001 (2001).
Howell, J. M., Ueti, M. W., Palmer, G. H., Scoles, G. A. & Knowles, D. P. Persistently infected calves as reservoirs for acquisition and transovarial transmission of Babesia bovis by Rhipicephalus (Boophilus) microplus. J. Clin. Microbiol 45, 3155–3159, https://doi.org/10.1128/jcm.00766-07 (2007).
McFee, R. B. Emerging Infectious Diseases - Overview. Dis Mon 64, 163–169, https://doi.org/10.1016/j.disamonth.2018.01.002 (2018).
Bloch, E. M., Kumar, S. & Krause, P. J. Persistence of Babesia microti infection in humans. Pathogens (Basel, Switzerland) 8, https://doi.org/10.3390/pathogens8030102 (2019).
Ogden, N. H. & Gachon, P. Climate change and infectious diseases: What can we expect? Can Commun. Dis. Rep. 45, 76–80, https://doi.org/10.14745/ccdr.v45i04a01 (2019).
Bouchard, C. et al. N Increased risk of tick-borne diseases with climate and environmental changes. Can Commun. Dis. Rep. 45, 83–89, https://doi.org/10.14745/ccdr.v45i04a02 (2019).
Janadaree Bandara, K. M. U. & Parakrama Karunaratne, S. H. P. Mechanisms of acaricide resistance in the cattle tick Rhipicephalus (Boophilus) microplus in Sri Lanka. Pestic Biochem Physiol 139, 68–72, https://doi.org/10.1016/j.pestbp.2017.05.002 (2017).
Li, A. Y., Chen, A. C., Miller, R. J., Davey, R. B. & George, J. E. Acaricide resistance and synergism between permethrin and amitraz against susceptible and resistant strains of Boophilus microplus (Acari: Ixodidae). Pest Manag. Sci. 63, 882–889, https://doi.org/10.1002/ps.1417 (2007).
Ueti, M. W. et al. Identification of midgut and salivary glands as specific and distinct barriers to efficient tick-borne transmission of Anaplasma marginale. Infect Immun 75, 2959–2964, https://doi.org/10.1128/iai.00284-07 (2007).
Ueti, M. W. et al. Quantitative differences in salivary pathogen load during tick transmission underlie strain-specific variation in transmission efficiency of Anaplasma marginale. Infect Immun 77, 70–75, https://doi.org/10.1128/iai.01164-08 (2009).
Brayton, K. A. et al. Complete genome sequencing of Anaplasma marginale reveals that the surface is skewed to two superfamilies of outer membrane proteins. Proc. Natl Acad. Sci. USA 102, 844–849, https://doi.org/10.1073/pnas.0406656102 (2005).
Kocan, K. M., de la Fuente, J., Blouin, E. F., Coetzee, J. F. & Ewing, S. A. The natural history of Anaplasma marginale. Vet Parasitol 167, 95–107, https://doi.org/10.1016/j.vetpar.2009.09.012 (2010).
Scoles, G. A., Ueti, M. W., Noh, S. M., Knowles, D. P. & Palmer, G. H. Conservation of transmission phenotype of Anaplasma marginale (Rickettsiales: Anaplasmataceae) strains among Dermacentor and Rhipicephalus ticks (Acari: Ixodidae). J. Med. Entomol 44, 484–491, https://doi.org/10.1603/0022-2585(2007)44[484:cotpoa]2.0.co;2 (2007).
Eriks, I. S., Stiller, D. & Palmer, G. H. Impact of persistent Anaplasma marginale rickettsemia on tick infection and transmission. J. Clin. Microbiol 31, 2091–2096 (1993).
Futse, J. E., Ueti, M. W., Knowles, D. P. Jr. & Palmer, G. H. Transmission of Anaplasma marginale by Boophilus microplus: retention of vector competence in the absence of vector-pathogen interaction. J. Clin. Microbiol 41, 3829–3834, https://doi.org/10.1128/jcm.41.8.3829-3834.2003 (2003).
Kocan, K. M., de la Fuente, J., Blouin, E. F. & Garcia-Garcia, J. C. Anaplasma marginale (Rickettsiales: Anaplasmataceae): recent advances in defining host-pathogen adaptations of a tick-borne rickettsia. Parasitology 129(Suppl), S285–300, https://doi.org/10.1017/s0031182003004700 (2004).
Galletti, M. F., Ueti, M. W., Knowles, D. P. Jr., Brayton, K. A. & Palmer, G. H. Independence of Anaplasma marginale strains with high and low transmission efficiencies in the tick vector following simultaneous acquisition by feeding on a superinfected mammalian reservoir host. Infect Immun. 77, 1459–1464, https://doi.org/10.1128/iai.01518-08 (2009).
Leverich, C. K., Palmer, G. H., Knowles, D. P. Jr. & Brayton, K. A. Tick-borne transmission of two genetically distinct Anaplasma marginale strains following superinfection of the mammalian reservoir host. Infect Immun. 76, 4066–4070, https://doi.org/10.1128/iai.00594-08 (2008).
Noh, S. M. et al. Stability and tick transmission phenotype of gfp-transformed Anaplasma marginale through a complete in vivo infection cycle. Appl. Environ. Microbiol 77, 330–334, https://doi.org/10.1128/aem.02096-10 (2011).
Barbet, A. F. & Allred, D. R. The msp1 beta multigene family of Anaplasma marginale: nucleotide sequence analysis of an expressed copy. Infect Immun. 59, 971–976 (1991).
Waladde, S. M., Ochieng, S. A. & Gichuhi, P. M. Artificial-membrane feeding of the ixodid tick, Rhipicephalus appendiculatus, to repletion. Exp. Appl. Acarol. 11, 297–306 (1991).
Troughton, D. R. & Levin, M. L. Life cycles of seven ixodid tick species (Acari: Ixodidae) under standardized laboratory conditions. J. Med. Entomol 44, 732–740, https://doi.org/10.1603/0022-2585(2007)44[732:lcosit]2.0.co;2 (2007).
Kemp, D. H., Koudstaal, D., Roberts, J. A. & Kerr, J. D. Feeding of Boophilus microplus larvae on a partially defined medium through thin slices of cattle skin. Parasitology 70, 243–254 (1975).
Kuhnert, F., Diehl, P. A. & Guerin, P. M. The life-cycle of the bont tick Amblyomma hebraeum in vitro. Int. J. Parasitol 25, 887–896 (1995).
Pierce, A. E. & Pierce, M. H. A note on the cultivation of Boophilus microplus (Canestrini, 1887) (Ixodidae: Acarina) on embryonated hen egg. Aust. Vet. J. 32, 144–146 (1956).
Kocan, K. M. et al. Persistence of Anaplasma marginale (Rickettsiales: Anaplasmataceae) in male Dermacentor andersoni (Acari: Ixodidae) transferred successively from infected to susceptible calves. J. Med. Entomol. 29, 657–668, https://doi.org/10.1093/jmedent/29.4.657 (1992).
Kocan, K. M. et al. Development of Anaplasma marginale in salivary glands of male Dermacentor andersoni. Am. J. Vet. Res. 54, 107–112 (1993).
Palmer, G. H., Brown, W. C. & Rurangirwa, F. R. Antigenic variation in the persistence and transmission of the ehrlichia Anaplasma marginale. Microbes Infect 2, 167–176, https://doi.org/10.1016/s1286-4579(00)00271-9 (2000).
Noh, S. M. et al. Superinfection exclusion of the ruminant pathogen Anaplasma marginale in its tick vector is dependent on the time between exposures to the strains. Appl Environ Microbiol 82, 3217–3224, https://doi.org/10.1128/aem.00190-16 (2016).
Howarth, J. A. & Hokama, Y. Artificial feeding of adult and nymphal Dermacentor andersoni (Acari: Ixodidae) during studies on bovine anaplasmosis. J. Med. Entomol. 20, 248–256, https://doi.org/10.1093/jmedent/20.3.248 (1983).
Kocan, K. M. et al. Capillary tube feeding system for studying tick-pathogen interactions of Dermacentor variabilis (Acari: Ixodidae) and Anaplasma marginale (Rickettsiales: Anaplasmataceae). J. Med. Entomol. 42, 864–874, https://doi.org/10.1093/jmedent/42.5.864 (2005).
Chung, C. et al. Improved diagnostic performance of a commercial Anaplasma antibody competitive enzyme-linked immunosorbent assay using recombinant major surface protein 5-glutathione S-transferase fusion protein as antigen. J. Vet. Diagn. Invest. 26, 61–71, https://doi.org/10.1177/1040638713511813 (2014).
Futse, J. E., Brayton, K. A., Dark, M. J., Knowles, D. P. Jr. & Palmer, G. H. Superinfection as a driver of genomic diversification in antigenically variant pathogens. Proc. Natl Acad. Sci. USA 105, 2123–2127, https://doi.org/10.1073/pnas.0710333105 (2008).
Acknowledgements
We express our gratitude to Ralph Horn, Kathy Mason, James Allison, Lowell Kappmeyer, Jessie Ujczo and Megan Jacks for their excellent assistance. This work was supported by Agricultural Research Service CRIS project #2090-32000-038-00D.
Author information
Authors and Affiliations
Contributions
R.V., W.C.J., K.A.B., G.A.S., S.M.N., M.W.U. conceived the experiment(s), R.V., W.C.J., S.M.N. and M.W.U. conducted the experiment(s), R.V., W.C.J., M.R.M., S.M.N. and M.W.U. analyzed the results, R.V. and M.W.U. wrote the original draft - R.V., W.C.J., M.R.M., K.A.B., G.A.S., S.M.N., M.W.U. revised and edited. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Vimonish, R., Johnson, W.C., Mousel, M.R. et al. Quantitative analysis of Anaplasma marginale acquisition and transmission by Dermacentor andersoni fed in vitro. Sci Rep 10, 470 (2020). https://doi.org/10.1038/s41598-019-57390-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-57390-y
This article is cited by
-
In vitro feeding of all life stages of two-host Hyalomma excavatum and Hyalomma scupense and three-host Hyalomma dromedarii ticks
Scientific Reports (2024)
-
Kinetics of tick infection by the relapsing fever spirochete Borrelia hermsii acquired through artificial membrane feeding chambers
Scientific Reports (2022)
-
Isolation of infectious Theileria parva sporozoites secreted by infected Rhipicephalus appendiculatus ticks into an in vitro tick feeding system
Parasites & Vectors (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.