Abstract
A previous randomized, controlled trial had demonstrated that complete intraureteral stent placement (CIU-SP) was superior to conventional stent placement (C-SP) in terms of improvement of stent-related urinary symptoms. However, it is unclear as to which subdomain symptom and cohort could benefit the most from CIU-SP compared to C-SP in urinary symptoms while considering the baseline urinary status. To determine this, a post-hoc analysis was performed using data from a previous study (CIU-SP group, n = 39; C-SP group, n = 41). We assessed the mean changes in the International Prostate Symptom Score (I-PSS) and the Overactive Bladder Symptom Score (OABSS) from baseline to day 14. Statistical comparison between the two groups was performed using analysis of covariance with adjustment of baseline urinary status as a covariate. Among 80 patients, the total I-PSS was significantly lower in the CIU-SP group than in the C-SP group in the cohort with mild urinary symptoms (P = 0.005), but not in those with moderate/severe symptoms (P = 0.521). The CIU-SP group showed significantly improved I-PSS and OABSS daytime frequencies, with the highest t statistic (2.47 and 2.10, respectively) among subdomains of both symptom scores compared with the C-SP group (both P < 0.001). In multivariate regression analysis, the stent placement method (CIU-SP vs. C-SP) was independently associated with the I-PSS daytime frequency on day 14 (P = 0.017). This study suggests that CIU-SP significantly improved stent-related daytime frequency compared with C-SP, and it may benefit especially those patients who have mild urinary symptoms before the placement of ureteral stents.
Similar content being viewed by others
Introduction
Ureteral stents are an effective medical device for improving urinary passage obstruction mainly due to ureteral stricture and stone impaction1. These stents are also useful for preserving patency in ureteral injury during the wound healing process2. In the case of ureteroscopic lithotripsy (URS), ureteral stents are commonly placed with the intention of preventing urinary obstruction due to ureteral edema at the surgical site. This leads to a reduction in the risk of postoperative urinary infection3. However, despite such advantages, this process causes ureteral stent discomfort, such as body pain and impairment of urinary symptoms, and affects the patient’s quality of life (QoL)4. Most of these symptoms have been considered to be attributed to ureteral spasm or contact of the distal end of the stent on the bladder wall5. However, there is no uniform consensus on how to improve stent-related urinary discomfort.
Recently, our research group conducted a prospective, randomized, controlled trial to evaluate the efficacy of complete intra-ureteral stent placement (CIU-SP) vs. conventional stent placement (C-SP) in patients with an indwelling loop type of ureteral stent after URS6. The main concept of CIU-SP was omitting the distal end of ureteral stents to reduce irritation on the trigonal area of the bladder. We found that CIU-SP significantly reduced stent-related pain (as a primary outcome measure) and lower urinary tract symptoms (LUTS) (as secondary outcome measures) compared with C-SP on postoperative days 3 and 14. With regard to urinary symptoms, these should be affected not only by URS or the ureteral stent placement procedure, but are also largely affected by baseline LUTS status7,8,9. To appropriately assess the efficacy of CIU-SP in stent-related urinary symptoms To appropriately assess the efficacy of CIU-SP in ameliorating stent-related urinary symptoms and identify the subgroup that could benefit from CIU-SP compared to C-SP, we performed a post-hoc analysis to compare the mean changes in urinary symptom scores from baseline to day 14 between the two stent placement groups, with adjustment of the baseline urinary status as a covariable10. Furthermore, we examined the most relevant subdomain in urinary symptom scores that is associated with improvement by CIU-SP vs. C-SP.
Results
Patients’ characteristics
Overall, the mean total International Prostate Symptom Score (I-PSS) and Overactive Bladder Symptom Score (OABSS) were 9.57 ± 7.83 (categorized as moderate symptoms11) and 3.11 ± 2.31 (categorized as mild symptoms12), respectively. Of 80 patients, 41 (51.2%; CIU-SP: 21, C-SP: 20) and 39 (48.8%; CIU-SP: 18, C-SP: 21) had mild and moderate/severe I-PSS, respectively; and 67 (83.8%; CIU-SP: 30, C-SP: 37) had mild symptoms, and 13 (16.2%; CIU-SP: 9, C-SP: 4) had mild and moderate/severe OABSS, respectively. Baseline characteristics between the two groups were well balanced, except for OABSS urgency (P = 0.024; Table 1).
Changes in urinary symptom scores from baseline to day 14 due to stent placement
The mean changes in the I-PSS and OABSS in all patients are shown in Fig. 1 and Table 2. The total mean I-PSS, I-PSS voiding symptom subscore (Q1 + Q3 + Q5 + Q6), I-PSS storage symptom subscore (Q2 + Q4 + Q7), and total OABSS on day 14 were significantly increased compared with baseline (all P < 0.05; Fig. 1A). Although there was no significant difference between the voiding and storage symptom subscores (P = 0.884, Fig. 1A and Table 2), the storage symptom subscore was independently associated with deterioration of the I-PSS QoL index due to stent placement (β = 0.382, standard error [SE] = 0.042, t statistic = 5.682, P < 0.001; Fig. 1B). Analysis of the correlation between baseline symptom scores and the mean change in scores from the baseline revealed that lower baseline scores were significantly correlated with worse urinary symptom outcome in both I-PSS (|r| = 0.44, P < 0.001; Fig. 1C) and OABSS (|r| = 0.41, P < 0.001; Fig. 1D).
(A) Mean ± standard error changes from baseline to day 14 in all patients. *P < 0.05 (day 14 vs. baseline) using the paired t test. N.S. no significant difference using the unpaired t test. (B) Correlation analysis between International Prostate Symptom Score (I-PSS) voiding/storage symptom subscores and the I-PSS quality of life (QoL) index. ***P < 0.001 using multiple linear regression analysis. Explanatory variables: I-PSS voiding and storage symptom subscores (continuous); response variable: I-PSS QoL index (continuous). Correlation analysis between baseline total urinary symptom scores and mean change in total urinary symptom scores from the baseline; (C) I-PSS and (D) OABSS. The correlations between data were evaluated using Spearman’s rank correlation test.
Comparison of changes in urinary symptom scores from baseline to day 14 between CIU-SP and C-SP using analysis of covariance (ANCOVA) with adjustment of baseline urinary status
The mean total I-PSS was significantly lower in the CIU-SP group than in the C-SP group in the overall patient population (2.84 ± 1.09 vs. 5.80 ± 1.50, P = 0.031; Fig. 2A) and in patients with mild symptoms (3.90 ± 5.58 vs. 9.05 ± 6.49, P < 0.001; Fig. 2B), but not in patients with moderate/severe symptoms (Fig. 2C). There was no significant difference in the total OABSS between the groups in the overall patient population and in the subcategories of patients (all, P > 0.05; Fig. 2D‒F). With regard to the subscores, the I-PSS daytime frequency (0.56 ± 0.23 vs. 0.97 ± 0.27, P = 0.049), I-PSS storage symptom subscore (1.05 ± 0.46 vs. 2.43 ± 0.54, P = 0.038), and I-PSS QoL index (0.51 ± 0.35 vs. 0.95 ± 0.37, P = 0.040) were significantly lower in the CIU-SP group than in the C-SP group (Table 2). Although the OABSS daytime frequency tended to be lower in the CIU-SP group than in the C-SP group (0.12 ± 0.08 vs. 0.24 ± 0.12, P = 0.087), there were no significant differences in the OABSS subscores between the groups (Table 2).
Mean ± standard error changes in the total urinary symptom scores, total International Prostate Symptom Score (IPSS; A‒C), and Overactive Bladder Symptom Score (OABSS; D‒F), from baseline to day 14. (A) All patients, (B) patients with mild symptoms, (C) patients with moderate/severe symptoms, (D) all patients, (E) patients with mild symptoms, and (F) patients with moderate/severe symptoms. Analysis of covariance was used for statistical analysis of complete intraureteral stent placement vs. conventional stent placement. *P < 0.05, N.S. no significant difference.
Correlations between the stent placement method and urinary symptom subscores on day 14
To identify the subdomain of the I-PSS or OABSS that was the most strongly associated with the stent placement method (CIU-SP vs. C-SP), simple linear regression analysis was performed. The I-PSS incomplete emptying (SE = 0.38, t statistic = 2.18, P < 0.001), I-PSS daytime frequency (β = 0.89, SE = 0.38, t statistic = 2.47, P < 0.001), and OABSS daytime frequency (β = 0.28, SE = 0.14, t statistic = 2.10, P = 0.039) were significantly correlated with the stent placement method (Fig. 3). Daytime frequency showed the highest t statistic among the I-PSS and OABSS subdomains (2.47 and 2.10, respectively) (Fig. 3).
Correlations between stent placement methods and urinary symptom subscores on postoperative day 14. (A) International Prostate Symptom Score (IPSS) and (B) overactive Bladder Symptom Score (OABSS). *P < 0.05 using simple linear regression analysis. Explanatory variable: stent placement method (category: complete intraureteral stent placement vs. conventional stent placement); response variable: each urinary symptom subdomain (continuous).
Multivariate analysis of clinical factors associated with daytime frequency of the I-PSS or OABSS on day 14
According to the results from simple regression analysis described above, multiple regression analysis was performed to investigate the relationships between potential clinical variables and I-PSS/OABSS daytime frequency. Body mass index (β = − 0.160, SE = 0.045, t statistic = − 3.456, P = 5.859 × 10−4) and stent placement method (β = 0.853, SE = 0.349, t statistic = 2.402, P = 0.017) were significantly associated with the I-PSS daytime frequency (Table 3). No factors were significantly correlated with the OABSS daytime frequency, with only a tendency for the stent placement method (β = 0.257, SE = 0.141, t statistic = 1.859, P = 0.072) (Table 3).
Discussion
In the present study, we focused on stent-related urinary symptoms in patients with an indwelling ureteral stent after URS. The I-PSS storage symptom subscore was correlated with the I-PSS QoL index rather than its voiding symptom subscore. A novel placement technique, CIU-SP, was significantly superior to C-SP in terms of improving the total I-PSS score, daytime frequency, storage symptom subscore, and QoL index. Patients having mild urinary symptoms at the baseline could more benefit from CIU-SP than from C-SP. Daytime frequency in the I-PSS and OABSS was the most relevant subdomain that was improved by CIU-SP compared with C-SP. Finally, the stent placement method (CIU-SP vs. C-SP) was an independent clinical factor for predicting improvement of the I-PSS daytime frequency.
In contrast to our previous report6, this post-hoc analysis had several strengths for accurately evaluating stent-related LUTS for the following reasons: (1) measuring the mean change in urinary symptoms from baseline to day 14; (2) applying analysis of covariance for adjusting baseline covariates; (3) selecting day 14 as the final evaluation date for minimizing the confounding effect of discomfort of the URS procedure13; and (4) examining details regarding stent-related LUTS using the validated LUTS-specific indicators11,12. Therefore, our results have added new evidence on management of improving stent-related urinary discomfort and QoL.
In the field of LUTS research, storage-related symptoms are the most clinically bothersome and affect patients’ QoL in those with benign prostatic hyperplasia (BPH)/LUTS or overactive bladder (OAB)14,15,16,17. Charles et al. found that nocturia and daytime frequency were the primary and secondary chief complaints, respectively, based on the American Urological Association Symptom Index in 1240 men with BPH15. McVary et al. suggested that the symptom of bother and QoL were affected two-fold by storage I-PSS questions (Q2, Q4, Q7) vs. questions on voiding symptoms (Q1, Q3, Q5, Q6)16. OAB, namely a storage symptom disorder, affected patients’ mental health, work productivity, and health-related QoL, regardless of sex17.
In the present study, we found that patients’ QoL was affected approximately three-fold by a storage symptom compared with a voiding symptom, despite the fact that both symptoms equally occurred because of stent placement. Similar to the approach for BPH/LUTS or OAB, management of storage symptoms is important in controlling ureteral stent-related urinary discomfort. Physical or chemical stimulation on the bladder wall causes release of chemicals, including ATP, acetylcholine, prostaglandins, and nitric oxide, which modulate the activity of either afferent nerves or muscular components of the bladder wall18. According to such underlying mechanism(s), CIU-SP may be a reasonable placement method because of not presenting the distal end of the stent. This can prevent iatrogenic physical irritation on the bladder wall. Notably, in our study, CIU-SP significantly reduced daytime frequency among urinary symptom subdomains compared with C-SP, whereas the rate of nocturia was equal in both groups. Based on the mechanism(s) mentioned above, these findings are easy to understand because the absence of a distal tip of the stent might reduce physical stimulus on the bladder during daytime activities.
Furthermore, we also found a negative correlation between the degree of the urinary status at baseline and the change in urinary symptoms after ureteral stent placement. Indeed, patients who had mild urinary symptoms could benefit from CIU-SP, whereas those with moderate/severe symptoms showed little impact, regardless of placement techniques. Thus, the clinical significance of CIU-SP demonstrated in our previous study6 could be attributed to the inclusion of more than half of the patients with favorable urinary symptoms before the URS surgery in the study. In other words, patients who have moderate or more urinary symptoms could tolerate even conventional stent placement. This information can be applied in daily clinical practice as well as in clinical trial protocols associated with ureteral stent placement.
To date, many studies have attempted to alleviate stent-related discomfort by agents, such as alpha-1 blockers, anticholinergics, and beta-3 adrenergic receptor agonists13,19,20,21,22. These results regarding improvement of urinary symptoms are conflicting, and negative results have been found in several randomized, controlled trials13,20,21,22. We believe that trials using medication should consider not only improvement of stent-related irritation, but also urinary symptoms that the patient has23. Nevertheless, most ureteral stent studies did not take into account the change from baseline or baseline LUTS status13,19,20,21,22. Therefore, actual results of previous studies may change if considering these factors for statistical analysis. Unlike trials of medications, we focused on examining the pure effect of stent placement methods on urinary symptoms, and found that CIU-SP was a better placement technique than C-SP. Unfortunately, even when using CIU-SP, some patients still suffer from stent-related urinary symptoms. Therefore, further investigations are required to determine the mechanism(s) that are associated with these symptoms (i.e., extraction string, intravesical inflammation, or irradiation pain), for providing a more comfortable method for patients who require ureteral stent placement.
We acknowledge several limitations that should be interpreted with caution. First, this post-hoc study was not intended to be designed when performing the primary study. Therefore, there was a lack of calculation of required sample size for this study. Second, we used only the loop type of stents instead of double j stents, which are commonly used. Although this study could not determine the best management for using double j stents, we believe that our findings could be useful for developing a new stent design that can reduce irritation of the bladder wall. Finally, as we previously mentioned6, we did not assess the safety of CIU-SP in patients with distal ureteral stones. Therefore, further studies including patients with distal ureteral stones are required in the future.
Conclusion
This study highlights the asymmetrical relationship between ureteral stent-related storage and voiding LUTS for patients’ urinary QoL. CIU-SP may be a more beneficial strategy for patients who have mild urinary symptoms at baseline than for those who have moderate/severe symptoms. Furthermore, CIU-SP might help reduce ureteral stent-related storage symptoms compared with C-SP, especially in terms of improving daytime frequency.
Methods
Participants and study design
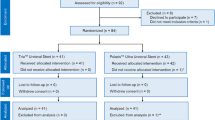
This was a post-hoc analysis that used the same data of a previous prospective, single-blind, randomized, clinical trial, which was registered at the University Hospital Medical Information Network (UMIN00017067)6. This study was approved by the ethics board of Kansai Medical University (IRB No. 2016503), and all patients provided written informed consent. This trial strictly followed the 2010 Consolidated Standards of Reporting Trials (CONSORT) statement guidelines24. Details of this trial, and inclusion and exclusion criteria were described previously6. Briefly, patients who were aged > 20 years who underwent unilateral URS with planned ureteral stent insertion were included. However, patients who had concomitant use of alpha-1 blockers, anticholinergics, corticosteroids, calcium channel blockers, and analgesics, distal ureteral stones, and preoperative ureteral stenting were excluded. Patients were equally randomized in a 1:1 ratio into the CIU-SP and C-SP groups. The modified intention-to-treat population, except for those without follow-up, sufficient clinical data, or those who withdrew consent after randomization, was used for analysis (Supplementary Figure 1).
Intervention
For all patients, the I-PSS (mild: ≤ 7, moderate: 8–19, severe: ≥ 20)11 and the OABSS (mild: ≤ 5, moderate: 6–11, severe: ≥ 12)12 at baseline and day 14 were obtained (Supplementary Figure 1). The details of surgical intervention of this study were also described previously6. Briefly, all patients underwent unilateral URS under spinal anesthesia with or without a ureteral access sheath. After URS, a Polaris Loop Ureteral Stent (Boston Scientific, Malborough, MA, USA) with a string was inserted by the two placement methods (i.e., CIU-SP and C-SP) according to the actual ureteral length. Finally, the stent string was cut approximately 10 cm from the tip of the urethra after insertion of the urethral catheter. For discharge medication, only oral diclofenac sodium 25 mg was allowed to be used during the study period (use of antimuscarinics or alpha-blockers were prohibited). On day 14, the ureteral stent was removed at the outpatient clinic with the extraction string.
Endpoints
The primary endpoint was the mean change in the total I-PSS and OABSS from baseline to day 14. The secondary endpoints were the mean change in the I-PSS and OABSS subscores from baseline to day 14. These analyses were not part of the protocol-specified primary or secondary endpoints of the previous study6.
Statistical analysis
Continuous data are expressed as mean ± standard deviation. The Chi-square test was used to compare nominal variables, and the paired or non-paired t test was used to compare continuous variables. The correlations between data were evaluated using Spearman’s rank correlation test. For analysis of endpoints, ANCOVA was applied for comparison between the two groups (the placement group as a factor and baseline as a covariate). Simple or multiple linear regression analysis was applied to assess the association between the urinary symptom scores and potential factors, with 15 subjects per variable as the minimum required sample size25. The t statistic in linear regression analysis, which was calculated as the ratio of an estimated coefficient (β) to its standard error, was used to test the hypothesis that a coefficient is equal to zero. All statistical analyses were performed using EZR version 1.37 (Saitama Medical Center, Jichi, Japan)26. A two-sided P value of < 0.05 was considered statistically significant.
Abbreviations
- BPH:
-
Benign prostatic hyperplasia
- C-SP:
-
Conventional stent placement
- CIU-SP:
-
Complete intraureteral stent placement
- I-PSS:
-
International Prostate Symptom Score
- LUTS:
-
Lower urinary tract symptoms
- OABSS:
-
Overactive Bladder Symptom Score
- QoL:
-
Quality of life
- SE:
-
Standard error
- URS:
-
Ureteroscopy
References
Nabi, G., Cook, J., N’Dow, J. & McClinton, S. Outcomes of stenting after uncomplicated ureteroscopy: systematic review and meta-analysis. BMJ 334, 572 (2007).
Burks, F. N. & Santucci, R. A. Management of iatrogenic ureteral injury. Ther. Adv. Urol. 6, 115–124 (2014).
Ordonez, M. et al. Ureteral stent versus no ureteral stent for ureteroscopy in the management of renal and ureteral calculi. Cochrane Database Syst. Rev. 2, 012703 (2019).
Scarneciu, I., Lupu, S., Pricop, C. & Scarneciu, C. Morbidity and impact on quality of life in patients with indwelling ureteral stents: a 10-year clinical experience. Pak. J. Med. Sci. 31, 522–526 (2015).
Joshi, H. B. et al. Ureteral stent symptom questionnaire: development and validation of a multidimensional quality of life measure. J. Urol. 169, 1060–1064 (2003).
Yoshida, T. et al. Efficacy and safety of complete intraureteral stent placement versus conventional stent placement in relieving ureteral stent related symptoms: a randomized, prospective, single blind, multicenter clinical trial. J. Urol. 07(202), 164–170 (2019).
Nunes, E. V. et al. Baseline matters: the importance of covariation for baseline severity in the analysis of clinical trials. Am. J. Drug Alcohol Abuse 37, 446–452 (2011).
Hernández, A. V. et al. Subgroup analysis and covariate adjustment in randomized clinical trials of traumatic brain injury: a systematic review. Neurosurgery 57, 1244–1253 (2005) (discussion 53).
Kaplan, S. A. et al. Solifenacin plus tamsulosin combination treatment in men with lower urinary tract symptoms and bladder outlet obstruction: a randomized controlled trial. Eur. Urol. 63, 158–165 (2013).
Colantuoni, E. & Rosenblum, M. Leveraging prognostic baseline variables to gain precision in randomized trials. Stat. Med. 34, 2602–2617 (2015).
Barry, M. J. et al. The American Urological Association symptom index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. J. Urol. 148, 1549–1557 (1992) (discussion 64).
Homma, Y. & Gotoh, M. Symptom severity and patient perceptions in overactive bladder: how are they related?. BJU Int. 104, 968–972 (2009).
Beddingfield, R. et al. Alfuzosin to relieve ureteral stent discomfort: a prospective, randomized, placebo controlled study. J. Urol. 181, 170–176 (2009).
Agarwal, A. et al. What is the most bothersome lower urinary tract symptom? Individual- and population-level perspectives for both men and women. Eur. Urol. 65, 1211–1217 (2014).
Welliver, C. et al. Analyzing why men seek treatment for lower urinary tract symptoms and factors associated with nonimprovement. Urology 86, 862–867 (2015).
McVary, K. T. et al. Use of structural equation modeling to demonstrate the differential impact of storage and voiding lower urinary tract symptoms on symptom bother and quality of life during treatment for lower urinary tract symptoms associated with benign prostatic hyperplasia. J. Urol. 196, 824–830 (2016).
Coyne, K. S. et al. The impact of overactive bladder on mental health, work productivity and health-related quality of life in the UK and Sweden: results from EpiLUTS. BJU Int. 108, 1459–1471 (2011).
Fry, C. H. & Vahabi, B. The role of the mucosa in normal and abnormal bladder function. Basic Clin. Pharmacol. Toxicol. 119(Suppl 3), 57–62 (2016).
Lamb, A. D., Vowler, S. L., Johnston, R., Dunn, N. & Wiseman, O. J. Meta-analysis showing the beneficial effect of α-blockers on ureteric stent discomfort. BJU Int. 108, 1894–1902 (2011).
Norris, R. D. et al. A prospective, randomized, double-blinded placebo-controlled comparison of extended release oxybutynin versus phenazopyridine for the management of postoperative ureteral stent discomfort. Urology 71, 792–795 (2008).
Oh, J. J. et al. Effects of naftopidil on double-J stent-related discomfort: a multicenter, randomized, double-blinded, placebo-controlled study. Sci. Rep. 06(7), 4154 (2017).
Tae, B. S. et al. Does mirabegron relieve ureteric stent-related discomfort? A prospective, randomized, multicentre study. BJU Int. 11(122), 866–872 (2018).
Kozminski, M. A., Wei, J. T., Nelson, J. & Kent, D. M. Baseline characteristics predict risk of progression and response to combined medical therapy for benign prostatic hyperplasia (BPH). BJU Int. 115, 308–316 (2015).
Moher, D. et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. Int. J. Surg. 10, 28–55 (2012).
Schmidt, F. L. The relative efficiency of regression and simple unit predictor weights in applied differential psychology. Educ. Psychol. Meas. 31, 699–714 (1971).
Kanda, Y. Investigation of the freely available easy-to-use software “EZR” for medical statistics. Bone Marrow Transpl. 48, 452–458 (2013).
Author information
Authors and Affiliations
Contributions
Study concept and design: T.Y., and T.M.; data acquisition: T.Y., T.M. and M.T.; data processing: T.M., T.Y., N.T. and M.T.; data analysis and interpretation: T.M. and T.Y.; manuscript drafting: T.M. and T.Y.; critical manuscript revision for important intellectual content: T.M., T.Y., T.M., K.N., H.K., and T.M.; statistical analysis: T.M. and T.Y.; study supervision: T.Y. and T.M.; all authors approved the final draft of this manuscript for submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Matsuzaki, T., Yoshida, T., Murota, T. et al. Complete intraureteral stent placement relieves daytime urinary frequency compared with conventional placement in patients with an indwelling ureteral stent: post-hoc analysis of a randomized, controlled trial. Sci Rep 10, 15892 (2020). https://doi.org/10.1038/s41598-020-72937-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-72937-0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.