Abstract
More than 150 ginsenosides have been isolated and identified from Panax plants. Ginsenosides with different glycosylation degrees have demonstrated different chemical properties and bioactivity. In this study, we systematically cloned and characterized 46 UGT94 family UDP-glycosyltransferases (UGT94s) from a mixed Panax ginseng/callus cDNA sample with high amino acid identity. These UGT94s were found to catalyze sugar chain elongation at C3-O-Glc and/or C20-O-Glc of protopanaxadiol (PPD)-type, C20-O-Glc or C6-O-Glc of protopanaxatriol (PPT)-type or both C3-O-Glc of PPD-type and C6-O-Glc of PPT-type or C20-O-Glc of PPD-type and PPT-type ginsenosides with different efficiencies. We also cloned 26 and 51 UGT94s from individual P. ginseng and P. notoginseng plants, respectively; our characterization results suggest that there is a group of UGT94s with high amino acid identity but diverse functions or catalyzing activities even within individual plants. These UGT94s were classified into three clades of the phylogenetic tree and consistent with their catalytic function. Based on these UGT94s, we elucidated the biosynthetic pathway of a group of ginsenosides. Our present results reveal a series of UGTs involved in second sugar chain elongation of saponins in Panax plants, and provide a scientific basis for understanding the diverse evolution mechanisms of UGT94s among plants.
Similar content being viewed by others
Introduction
Plants collectively synthesize more than 200,000 natural products, a significant proportion of which are glycosylated1. Glycosylation alters the hydrophilicity, stability, and subcellular localization of natural products, and thus, their chemical properties and bioactivities2. Glycosylation of natural products in plants is catalyzed by glycosyltransferases. In the glycosylation of natural products, the sugar donors are usually uridine diphosphate (UDP)-activated monosaccharides; therefore, these glycosyltransferases are termed as UDP-glycosyltransferases (UGTs)3.
Ginsenosides, mainly comprising protopanaxadiol (PPD)- and protopanaxatriol (PPT)-type saponins, are the main bioactive constituents of Panax plants, including P. ginseng and P. notoginseng4,5,6,7. More than 150 structurally diverse ginsenosides have been isolated from Panax species; they are conjoined with one to six sugar units, and up to three at the C3 and C20 sites of PPD-type ginsenosides or the C6 and C20 sites of PPT-type ginsenosides8. The number, positions, and types of sugar units, mainly comprising glucose, xylose, arabinose, and rhamnose, contribute greatly to ginsenoside diversity8. Among most reported ginsenosides, glucose preferentially links with the dammarane skeleton, with all four sugar unit types appearing in the second unit of the sugar chain attached to the dammarane skeleton. The diversity and combination of sugar units allow ginsenosides to exhibit diverse bioactivities. Therefore, it is also of high importance for ginsenosides drug research to elucidate the mechanisms of sugar chain elongation of ginsenosides.
In previous studies, we characterized several UGTs from P. ginseng. PgUGT71A53 (UGTPg1), PgUGT74AE4 (UGTPg45), and PgUGT71A54 (UGTPg100) or PgUGT71A55 (UGTPg101) were found to control glycosylation of the first sugar unit toward the C20-OH, C3-OH, and C6-OH sites of PPD or PPT, respectively9,10,11. The biosynthetic pathways of five ginsenosides, PPD-type ginsenosides CK and Rh2 and PPT-type ginsenosides Rh1, F1, and Rg1 have been elucidated. However, most ginsenosides among Panax plants typically harbor two or more sugar units at a single position of the PPD or PPT skeleton, suggesting that more UGTs are responsible for sugar chain elongation of ginsenosides; these UGTs remain uncharacterized. Although a purified UGT from P. notoginseng has been characterized to catalyze Rd to produce Rb1 by sugar chain elongation, its encoding gene was unknown at that time12. We previously characterized PgUGT94Q2 (UGTPg29) as the first sequenced Panax UGT to exhibit sugar chain elongation activity (sugar–sugar UGT). PgUGT94Q2 has subsequently been demonstrated to belong to the UGT94 family and to transfer a glucose moiety to the C3-O-Glc site of Rh2 to produce Rg310,13. However, the sugar–sugar UGTs responsible for transferring a glucose unit to the C6-O-Glc of PPT-type ginsenosides and C20-O-Glc of PPD- and PPT-type ginsenosides, as well as transferring a xylose, arabinose, or rhamnose unit to the C3, C6, or C20 glucose moiety of Panax plants, have not yet been reported.
Large quantities of low-molecular-weight natural products have been linked with two to four sugar units at a single position in plants. Accordingly, sugar–sugar UGTs, which are responsible for transferring a sugar group to an existing sugar moiety in natural products, may be diverse14. However, only a limited number of sugar–sugar UGTs have been characterized, mainly from the UGT94, UGT73, UGT79, and UGT91 families14,15,16,17,18,19,20,21,22,23. These sugar–sugar UGTs could add a glucosyl/glucuronosyl/xylose/rhamnosyl/galactosyl molecule to an existing sugar moiety in natural products, including flavonoids and terpenoids. It has been reported that some UGTs from the UGT91 family (UGT91H4 and UGT91H9) can transfer a rhamnosyl or glucosyl moiety to the third position of the sugar chain22,23. In the past few decades, research has been focused on cloning and characterizing UGTs that glycosylate the OH and/or COOH group of natural products. Therefore, more effort should be devoted to mining and characterizing sugar–sugar UGTs14.
In this study, we systematically cloned and characterized a series of UGT94s with high amino acid identity from P. ginseng and P. notoginseng; these UGT94s are sugar–sugar UGTs being responsible for second sugar chain elongation in ginsenosides. Our results elucidated possible biosynthetic pathways of a group of ginsenosides in Panax plants, and contributed to the elucidation of a ginsenoside biosynthetic pathway network, as well as the discovery of sugar–sugar UGTs involved in saponin biosynthesis in other plants. The cloning and characterization of these UGT94s provides a basis for setting up a platform to synthesize a series of ginsenosides from glucose using microbial cell factories.
Results and discussion
Cloning and functional characterization of P. ginseng UGT genes with high amino acid identity to PgUGT94Q2
In one of our previous studies, the UDP-glycosyltransferase PgUGT94Q2 was cloned and characterized to selectively transfer a glucose moiety to the C3-O-Glc of Rh2 to form Rg310. Some of the UGT fragments (ORFs) in our established Panax cDNA database were found to be aligned to PgUGT94Q2 with high amino acid identity (> 75%)10. Four pairs of full-length primers were designed based on these fragments and applied to clone UGT genes using a mixed cDNA sample of a P. ginseng plant and callus as the template. In total, 303 clones were picked up and sequenced (Table S1). The deduced amino acids of the 303 UGTs were all predicted to belong to the UGT94 family24, and were assigned into 46 individual UGT units (Table S1). The deduced amino acids of the 46 PgUGT94s shared high amino acid identity, ranging from 85.87% to 99.78% (Table S2).
Of the 46 PgUGT94s, 11 could be cloned by two or more pairs of primers, among which PgUGT94Q2 and PgUGT94Q5-V1 were cloned by the four pairs of primers. Multiple clones were detected for 19 PgUGT94s, whereas only one clone was detected for the remaining 27. In total, the deduced amino acid sequences of 84 and 49 clones were identical to those of PgUGT94Q2 and PgUGT94Q5-V1, respectively (Table S1), demonstrating that they could be the major UGT94 units in P. ginseng.
For the in vitro enzymatic functional assay, the PPD-type saponins CK, Rh2, and Rd, as well as the PPT-type saponins Rh1 and F1, were selected as putative sugar acceptors (substrates). Catalytic function assays of the 46 PgUGT94s were performed using crude cell extracts of the individual expressed PgUGT94s with UDP-glucose as the putative sugar donor. The results showed that 41 of the 46 PgUGT94s catalyzed the tested substrates, of which 39 PgUGT94s catalyzed Rh2 to produce Rg3 (Fig. 1A and D; Figs. S1A, S7A, S7B, and S8A; Table S1). This result indicates that more than four-fifths of the PgUGT94s exhibit sugar chain elongation activity toward C3-O-Glc of PPD-type saponins, with different efficiencies. We also found that 10 PgUGT94s had catalytic activity toward Rh1 to form Rf (Fig. 1B and D; Figs. S1B, S7C, S7D, S8B, and S8C; Table S1), demonstrating that they can extend the sugar chain at C6-O-Glc of PPT-type saponins. Five PgUGT94s converted CK to gypenoside LXXV (Figs. S1D, S2A, S7I, and S8F-I), Rd to Rb1 (Fig. 1C and D; Figs. S1C, S7E, S7F, and S8D; Table S1), and F1 to notoginsenoside U (Figs. S1H, S2D, S7M, and S8E), suggesting that these five PgUGT94s are able to glycosylate the C20-O-Glc of both PPD- and PPT-type saponins (Table S1). Eight PgUGT94s demonstrated sugar chain elongation for both C3-O-Glc of PPD-type ginsenosides and C6-O-Glc of PPT-type saponins, and five PgUGT94s exhibited sugar chain elongation for both C3-O-Glc and C20-O-Glc of PPD-type ginsenosides (Fig. 1D; Table S1). In addition, PgUGT94Q15 and PgUGT94Q15-V1, the sugar–sugar UGTs for C3-O-Glc of PPD-type ginsenosides, showed sugar chain elongation at C3-O-Glc of 3-O-β-Glc oleanolic acid (3GOA) to form 3-O-[β-D-glucopyranosyl-(1 → 2)-β-D-glucopyranosyl]-oleanolic acid (Fig. S2C, S7L and S8J-O), which has yet to be isolated and detected from Panax plants. PgUGT94Q15-V1 catalyzed calunduloside E to zingibroside R1 by transferring a glucose moiety at C3-O-GlcA (Figs. S1I, S2E, S7J, and S7K). Among the remaining five PgUGT94s, no activity was detected in any of the used substrates (Fig. 1D; Fig. S1; Table S1).
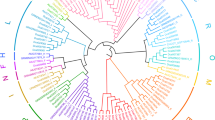
Cloning and functional characterization of Panax ginseng UGT94s with high identity to PgUGT94Q2. (A) High-performance liquid chromatography (HPLC) analysis of in vitro reactions of PgUGT94Q15 toward Rh2 with UDP-glucose as the sugar donor. (B) HPLC analysis of in vitro reactions of PgUGT94Q3 toward Rh1 with UDP-glucose as the sugar donor. (C) HPLC analysis of in vitro reactions of PgUGT94Q6 toward Rd with UDP-glucose as the sugar donor. (D) Phylogenetic analyses of deduced amino acid sequences of PgUGT94s from mixed cDNA of a P. ginseng plant and callus with other characterized plant UGTs, and relative activities of functional PgUGT94s. The relative enzyme activities of PgUGT94Q18, PgUGT94Q3 and PnUGT94Q25-V2 (with the highest enzyme activity toward substrate Rh2, Rh1 and Rd, respectively) were designated as 100%. The relative enzyme activities of the left UGT94s toward substrate Rh2, Rh1 and Rd were standardized by comparing with the three representative ones, respectively. Only bootstrap values above 70% are shown in the tree.
The 46 PgUGT94s were classified into three clades of the phylogenetic tree, consistent with their functions and activities (Fig. 1D). All of the 23 PgUGT94s in clade A exhibited sugar chain elongation activity only at C3-O-Glc of PPD-type saponins, among which PgUGT94Q18 had the highest activities to Rh2, the relative activities of others 22 PgUGT94s ranged from 0.9% to 89% of PgUGT94Q18. Most of the 11 PgUGT94s in clade B exhibited sugar chain elongation activity at both C3-O-Glc of PPD-type and C6-O-Glc of PPT-type saponins, among which PgUGT94Q3 had the highest activities at C6-O-Glc of Rh1 and C3-O-Glc of Rh2. The remaining PgUGT94s in clade B exhibited lower activities to the tested substrates; PgUGT94Q10 had no activity, but PgUGT94Q10-V1 and PgUGT94Q11-V3 had low activity to PPT-type substrates. The five PgUGT94s without activity to the tested substrates and seven functional PgUGT94s clustered in clade C. All of the seven functional PgUGT94s in clade C demonstrated elongation activity at C3-O-Glc of PPD-type saponins, of which five also showed elongation activity at C20-O-Glc of PPD- and PPT-type saponins. Among them, PgUGT94Q6 had the highest activities both towards C3-O-Glc of Rh2 and C20-O-Glc of Rd; the remaining functional PgUGT94s only showed very weak substrate activities.
Several UGTs of the UTG94 family have previously been reported to catalyze sugar chain elongation of natural products including monoterpene, triterpenoid mogrosides, flavonoids, and lignans14,16,18,19,20. For example, CaUGT3 from Catharanthus roseus was found to control sugar chain elongation from the second to fourth positions of the sugar chain14. UGT94P1 from Camellia sinensis, UGT94B1 from Bellis perennis, and PgUGT94Q2 from P. ginseng have been reported as sugar–sugar UGTs, transferring the second sugar moiety to the sugar chain10,19,20. These findings support speculation that the UGT94 family participates extensively in sugar chain elongation in natural products.
UGT94 diversity in a single P. ginseng plant
We cloned diverse PgUGT94s with high amino acid identity from the cDNA of a mixed P. ginseng sample (85.87%–99.78%). To explore whether similar diversity exists at the level of a single P. ginseng plant, we cloned UGT94s from the cDNA of the leaves and roots of an individual P. ginseng plant using the same four pairs of primers. A total of 228 and 184 clones were obtained from P. ginseng leaf and root cDNA, respectively (Table S3). Among the 228 clones derived from leaf cDNA, 217 were identical to four detected PgUGT94s from the P. ginseng mixed cDNA (i.e., PgUGT94Q2, Q3, Q4-V1, and Q5-V3). The remaining 11 clones resulted in nine new PgUGT94s. Among the 184 clones derived from root cDNA, 175 were identical to nine detected PgUGT94s from the mixed cDNA (i.e., PgUGT94 Q1-V1, Q2, Q3, Q4-V1, Q4-V2, Q5-V3, Q22, Q23, and Q29). The remaining nine clones resulted in eight new UGT94s that have been not previously obtained (Table S3).
In total, we obtained 26 PgUGT94s, including nine PgUGT94s already obtained from the mixed cDNA sample, from leaf and root cDNA of the individual P. ginseng plant. The deduced amino acids of the 26 PgUGT94s also shared high amino acid identity, ranging from 82.06% to 99.78% (Table S4). Functional assays showed that these PgUGT94s also exhibited sugar chain elongation activity at C3-O-Glc and C20-O-Glc of PPD-type, and at C6-O-Glc and C20-O-Glc of PPT-type saponins. Some of these PgUGT94s showed sugar chain elongation activity at both C3-O-Glc and C20-O-Glc of PPD-type saponins or both C3-O-Glc of PPD-type and C6-O-Glc of PPT-type as well as both C3-O-Glc of PPD-type, and C20-O-Glc of PPD- or PPT-type saponins (Fig. 2A; Fig. S1E–I; Table S3). These results suggest that PgUGT94s from an individual P. ginseng agreed with the sequence and functional diversity results obtained from a mixed cDNA sample.
Diversity analyses of UGT94s from individual Panax plants. (A) Phylogenetic analyses of deduced amino acid sequences of PgUGT94s from an individual P. ginseng plant with other characterized plant UGTs and relative activity of functional UGT94s. The relative enzyme activities of PgUGT94Q18, PgUGT94Q3 and PnUGT94Q25-V2 (with the highest enzyme activity toward substrates Rh2, Rh1 and Rd, respectively) were designated as 100%. The relative enzyme activities of the left UGT94s toward substrates Rh2, Rh1 and Rd were standardized by comparing with the three representative ones, respectively. Only bootstrap values above 70% are shown in the tree. (B) Phylogenetic analyses of deduced amino acid sequences of PnUGT94s from an individual P. notoginseng plant with other characterized plant UGTs and relative activity of functional UGT94s. The relative enzyme activities of PgUGT94Q18, PgUGT94Q3 and PnUGT94Q25-V2 (with the highest enzyme activity toward substrates Rh2, Rh1 and Rd, respectively) were designated as 100%. The relative enzyme activities of the left UGT94s toward substrates Rh2, Rh1 and Rd were standardized by comparing with the three representative ones, respectively. Only bootstrap values above 70% are shown in the tree. (C) Tandem repeat array of PgUGT94 homolog genes in the cloned Pg_scaffold6708 from the P. ginseng genome in our experiment, Pg_scaffold6708 assembled by Nam-Hoon Kim et al. 2018, and Scaffold8624 (partially aligned to Pg_scaffold6708) assembled by Jiang Xu et al. 2017.
The 26 PgUGT94s obtained from the individual P. ginseng plant were also classified into three clades of the phylogenetic tree, consistent with their functions and activities (Fig. 2A). Among these, half were found in clade A; most of these exhibited high substrate conversion ratios to catalyze Rh2 to produce Rg3. 12 PgUGT94s were grouped into clade C; these showed lower substrate conversion ratios to both C3-O-Glc of PPD-type and C20-O-Glc of PPD- or PPT-type saponins, or no activity. Only PgUGT94Q3 was grouped into clade B.
Some UGT94s from Citrullus lanatus, Cucumis melo, Cucumis sativus, and Siraitia grosvenorii; UGT73s from Arabidopsis thaliana and Barbarea vulgaris; and UGT720s from C. lanatus and Siraitia have been reported to preferentially cluster as tandem repeat arrays in their genomes16,25. Published P. ginseng genome data indicates that Pg_scaffold6708 (aligned with Scaffold8624 sequecned in another study6) and Pg_scaffold2289 harbor three and two PgUGT94 homolog sequences in tandem repeat arrays, respectively5. Our cloning and sequencing of a 14–26 kb fragment from Pg_scaffold6708 demonstrated that it contained three PgUGT94 homolog genes, which were identical to three detected PgUGT94 units (PgUGT94Q5-V1, PgUGT94Q11-V1, and PgUGT94Q4-V1) from the P. ginseng mixed cDNA sample, representing clades A, B, and C, respectively (Fig. 2C; Fig. S5A–C). Similarly, our cloning and sequencing of a 417.5–433 kb fragment of Pg_scaffold2289 revealed that it contained two previously detected PgUGT94 units, PgUGT94Q2 and PgUGT94Q3 (Fig. S5D and S5E).
UGT94 diversity within a single P. notoginseng plant
To confirm whether UGT94 diversity is also present in P. notoginseng, PnUGT94 genes were cloned from leaf and root cDNA of a single P. notoginseng plant using five pairs of primers based on P. notoginseng transcriptome data. We obtained 313 and 210 clones from the leaf and root cDNA, respectively; these were assigned into 34 and 31 individual UGT encoding genes, respectively (Table S5). Owing to low sequence quality, seven PnUGT94s were excluded from further analyses. Nine PnUGT94s, three of which have also been cloned from a P. notoginseng leaf cDNA sample previously (Table S7), could be cloned by two or more pairs of primers or from different tissues (leaves and roots). Multiple clones were detected for 17 PnUGT94s, whereas only one clone was detected for the remaining 34 (Table S5). The deduced amino acid sequence of the left 51 PnUGT94s exhibited high amino acid identity, ranging from 84.08% to 99.78%. Functional assays showed that PnUGT94s also exhibited sugar chain elongation activity at C3-O-Glc of PPD-type saponins and C20-O-Glc of both PPD- and PPT-type saponins, as well as C6-O-Glc of PPT-type saponins, which was consistent with the sequence and functional diversity of PgUGT94s (Fig. 2B; Figs. S3 and S6).
24, 13, and 14 PnUGT94s were classified into clades A, B, and C of the phylogenetic tree, respectively (Fig. 2B). Most of the PnUGT94s in clade A exhibited higher substrate conversion ratios to catalyze sugar elongation at C3-O-Glc of PPD-type saponins, exactly like their corresponding PgUGT94s in clade A. PnUGT94s in clade B were further divided into two sub-clades; four PnUGT94s in one of these sub-clades showed sugar elongation activity at both C3-O-Glc of PPD-type and C6-O-Glc of PPT-type saponins, whereas most members of the other sub-clade showed no activity to any of the tested substrates. All of the 14 PnUGT94s in the clade C exhibited catalyzing activities at both C3-O-Glc and C20-O-Glc of PPD-type saponins, which were higher than those of their corresponding PgUGT94s in clade C. In total, seven PnUGT94s among the three clades were found to have no catalyzing activity to any tested substrates.
Proposed biosynthetic pathways for ginsenosides with two or more sugar chain modifications
Based on the cloning and functional characterization of these PgUGT94s and our previously reported PgUGT71A53, PgUGT71A54, PgUGT71A55, and PgUGT74AE4, the biosynthetic pathways of a series of ginsenosides were elucidated (Fig. 3). CK was glycosylated at C20-O-Glc by PgUGT94Q6 to form gypenoside LXXV (Figs. S2A, S7I, and S8F-I). CK was also glycosylated at C3-OH by PgUGT74AE4 to produce F210, then F2 was transferred another glucose moiety at C3-O-Glc by PgUGT94Q15 to yield Rd (Fig. S2B, S7G, and S7H). Rd was converted to Rb1 by PgUGT94Q6 via transferring a glucose moiety at C20-O-Glc (Fig. 1C, S7E, S7F, and S8D). Rh2 was elongated a glucose moiety at C3-O-Glc by PgUGT94Q15 to yield Rg3 (Fig. 1A, S7A, S7B, and S8A), which could be further converted to Rd by PgUGT71A539. Rh1 was elongated a glucose moiety at C6-O-Glc by PgUGT94Q3 to form Rf (Fig. 1B, S7C, S7D, S8B, and S8C). F1 could be transferred a glucose moiety at C20-O-Glc to yield notoginsenoside U (Fig. S2D, S7M, and S8E). In addition to PPD- and PPT-type saponins, UGT94s were demonstrated to be involved in the biosynthesis of oleanolic acid-type saponins. 3GOA was glycosylated at C3-O-Glc by PgUGT94Q15, Q15-V1, or Q22-V1 to produce 3-O-[β-D-glucopyranosyl-(1 → 2)-β-D-glucopyranosyl]-oleanolic acid (Figure S2C, S7L and S8J-O), and calunduloside E was transferred a glucose moiety at C3-O-GlcA by PgUGT94Q15-V1 to yield zingibroside R1 (Fig. S2E, S7J, and S7K). Combined with functional characterization of UGTs from P. ginseng, the possible Panax biosynthesis pathway net is shown in Fig. 3. Notably, the complete biosynthetic pathway of gypenoside LXXV, notoginsenoside U, Rf, Rb1, 3-O-[β-D-glucopyranosyl-(1 → 2)-β-D-glucopyranosyl]-oleanolic acid, and zingibroside R1 are all elucidated for the first time. These results will provide a foundation for manufacturing these ginsenosides from glucose by building microbial cell factories, and further promote the application of these ginsenosides.
In summary, the results of this study demonstrate the existence of a variety of UGT94 genes with high amino acid identity but diverse functions in an individual P. ginseng or P. notoginseng genome. The phenomenon of several UGTs sharing high amino acid identity but exhibiting diverse functions has been reported in a few plants including A. thaliana and B. vulgaris. Eight BvUGT73Cs (BvUGT73C11, BvUGT73C13, BvUGT73C21, BvUGT73C22, BvUGT73C23, BvUGT73C25, BvUGT73C26, and BvUGT73C27) from B. vulgaris were found to share high amino acid identity, ranging from 70.9% to 93.3%. The functions of these eight BvUGT73Cs were partly overlapping but diverse. BvUGT73C11, BvUGT73C21, BvUGT73C26, and BvUGT73C27 exclusively catalyzed glucosylation at C3-OH of both oleanolic acid and hederagenin. BvUGT73C13 and BvUGT73C22 exhibited glycosylation at both C3-OH and C28-OH of oleanolic acid and hederagenin. BvUGT73C23 and BvUGT73C25 exhibited glycosylation at both C3-OH and C28-OH of oleanolic acid and hederagenin, and at C23-OH of hederagenin25.
Some UGT94s from Panax plants, such as PgUGT94 Q1-V1, Q2, Q3, Q4-V1, Q4-V2, Q5-V3, Q22, Q23, and Q29 for P. ginseng and PnUGT94Q25, Q26-V17, and Q31-V7 for P. notoginseng, were relatively conservative among individuals, but most of these may tend to be polymorphic between individuals. Our results also indicate that most UGT94s tend to be expressed in different organs, since many UGT94s cloned from leaves were different from those from roots within the same Panax plant. However, these UGT94s shared high amino acid identity, even among UGT94s from P. ginseng and P. notoginseng, with amino acid identities ranging from 80.94% to 100% (Table S9), similar to UGT94s from a single P. notoginseng genome (84.08%–99.78%).
Most PgUGT94s, including those in clades B and C, exhibited sugar elongation activity to transfer glucose moieties to C3-O-Glc of PPD-type saponins. The phylogenetic tree implied that PgUGT94s in clade C might first branch off from clades A and B, followed by clades A and B branching off from each other (Fig. S6). Therefore, it is likely that evolution endowed an ancient PgUGT94 belonging to clade C with the ability to glycosylate C20-O-Glc of PPD-type saponins, which further developed into the ability to glycosylate C3-O-Glc of PPD-type saponins. Similarly, PgUGT94s belonging to clade B evolved the ability to glycosylate C6-O-Glc of PPT-type saponins, which further developed into the ability to glycosylate C3-O-Glc of PPD-type saponins. In this manner, more selective and efficient C3-O-Glc glycosylation developed for PPD-type saponins.
Our results confirm that some PgUGT94s, such as PgUGT94Q5-V1, PgUGT94Q11-V1, and PgUGT94Q4-V1, as well as PgUGT94Q2 and PgUGT94Q3, are closely located in tandem repeat arrays within a genome. In their genome sequencing study of P. ginseng6, Xu et al. proposed that the same subfamily of UGTs preferentially clustered as tandem repeat arrays within the P. ginseng genome. Recent whole-genome duplications events have suggested the duplication of subfamily UGT genes to increase their diversity. However, genome sequencing of P. ginseng and P. notoginseng has revealed few subfamily UGTs to support such a proposal; for example, only five and four (identity > 50%; coverage > 50%) of UGT94 homolog sequences were assembled, respectively4,5,6,7. The unprecedented diversity of UGT94s was revealed through cloning of P. ginseng and P. notoginseng cDNA, which avoided bias to complete the establishment of high-identity genes by genome sequencing. The unprecedented diversity revealed in this study may have resulted from whole-genome duplication and tandem duplication, as shown among other subfamily UGT genes of other plants. For example, UGT73C1 to UGT73C6 were discovered to be located in tandem repeat arrays on chromosome 2 of A. thaliana, at least four UGTs of UGT73Cs from B. vulgaris were organized in a tandem array on pseudomolecule 3 of B. vulgaris, and three UGTs from S. grosvenorii, UGT94-289–1, UGT94-289–2, and UGT94-289–3, were localized in tandem repeat arrays on scaffold_127716,25. The organization of tandem repeat arrays likely implies gene duplication events resulting in subfunctionalization and neofunctionalization. In addition to the possible tandem repeats in genome, alleles may also contribute to the high diversity of PgUGT94s or PnUGT94s because P. ginseng is tetraploid and P. notoginseng is diploid. Further evidence is required to determine why there exist so many multi-UGT94 genes with high amino acid identity but diverse functions or activities, even within an individual P. ginseng or P. notoginseng genome.
Experimental procedures
Cloning of UDP-glycosyltransferases
RNA was isolated from callus, leaf, and root of P. ginseng as well as leaf and root of P. notoginseng using RNAprep Pure Plant Kit (TIANGEN, Beijing, China) according to the manufacturer's instructions. RNA was then converted into cDNA using the PrimeScript RT reagent Kit with gDNA eraser (Takara, Dalian, China). PCR amplification of the potential PgUGT94 encoding genes were performed using four pairs of primers (P29-F/R, P75-F/R, P84-F/R, and P85-F/R), and mixed cDNA of a P. ginseng plant and callus, cDNA of P. ginseng leaves, and cDNA of P. ginseng roots as the template, respectively. PCR amplification of the potential PnUGT94 encoding genes were performed using five pairs of primers (PN29-F/R, PN75-F/R, PN84-F/R, PN85-F/R, and PN113586-F/R), and cDNA of P. notoginseng leaves and cDNA of P. notoginseng roots as template, respectively. The PCR amplification was performed with high-fidelity PrimeSTAR HS DNA Polymerase kit. Then PCR products were cloned into the pMD18T vector (Takara, Dalian, China). The sequences of all UGT94 genes, their expression products and related information are deposited in the Registry and Database of Bioparts for Synthetic Biology (https://www.biosino.org/npbiosys/, accession No. OENC 37 to 115, 117, 119 to 128, 130 to 141, 144 to 153, 155 to 163, 165 to 167). The official names of UGTPg1, UGTPg24, UGTPg100, UGTPg101, and all of UGT94s were designated by the UGT nomenclature committee24 (Table S1, S3, S5, and S7).
Phylogenetic tree construction
The deduced amino acid sequences of UGT94s and three other UGT94 family members were aligned using clustal W in MEGA7 program (Version 7.0.26) with default parameters. The MEGA7 program was used to construct the Neighbor–Joining phylogenetic tree with set parameters (bootstrap method with 1,000 bootstrap replicates, poisson model, uniform rates and complete deletion). The scale bar means 0.1 amino acid substitution per site. The percentage of bootstrap values were marked near the nodes as numbers.
Heterologous expression of the UGT94 genes
Heterologous expression of the UGT94 genes in E. coli BL21 (DE3) was performed as described previously with slight modifications11. UGT94 genes with a C-terminal 6 × His-tag were constructed into the pET28a vector using the ClonExpress Multis one Step Cloning Kit (Vazyme Biotech) and transformed into E. coli BL21 (DE3). After induction with 0.2 mM isopropyl β-D-1-thiogalactopyranoside, the cells were collected by centrifugation at 8,000 g for 5 min, and suspended with 50 mM Tris–HCl buffer (pH 8.0). The cells were disrupted with a French Press at 25 kpsi. The homogenate was centrifuged at 12, 000 g for 20 min to remove cell debris and the left supernatant was used for in vitro enzymatic assays and western blotting. The E. coli BL21 (DE3) cells harboring the empty vector pET28a were carried out in parallel as the control.
Enzymatic assays for UGT94s
Enzymatic assays for UGT94s were carried out as described by Yan et al.9 The in vitro enzymatic reaction was conducted in a 50 μl volume containing 50 mM Tris–HCl buffer (pH 8.0), 1% Tween-20, 5 mM UDP-glucose, 0.5 mM acceptor substrate, and 40 ul crude enzyme (the recombinant E. coli extract) in a 35 °C water bath for 12 h. The reaction was terminated by adding 50 μl of n-butanol. The n-butanol phase was evaporated and then analyzed by thin layer chromatography (TLC) and high-performance liquid chromatography (HPLC). The concentration of each His-tagged UGT94 in crude enzyme was quantified by dot blot analysis as previous described26. A series of diluted (8, 12, 14, 16, 18, and 32 ng/μL) of purified C-terminal 6× His-tagged UGT OleD (NCBI accession number, ABA42119.2) were used to draw a standard curve for protein quantitative. To compare the enzymatic activities of UGT94s, the enzymatic reaction condition was the same as describe above except the incubation time was 1 h. The enzyme activity of each UGT94 was calculated as the amount of substrate (nM) converted by per unit protein (mg) in 1 min, and all data are means of three independent experiments (Table S11). The relative enzyme activities of three representative UGT94s with the highest enzyme activity toward substrate Rh2 (C3), Rh1 (C6) and Rd (C20) respectively, were designated as 100%. The relative enzyme activities of each of the left UGT94s toward Rh2, Rh1 and Rd were standardized by comparing with the three representative ones, PgUGT94Q18, PgUGT94Q3 and PnUGT94Q25-V2, respectively.
Cloning of Pg_scaffold6708 and Pg_scaffold2289
The 14–26 kb of Pg_scaffold6708 was divided in five parts. The each parts were cloned using the genome DNA of P. ginseng callus as the template and linked into pEASY-Blunt Simple cloning Vector (Transgen, Beijing, China) to sequence. The 417.5–419.9 kb and 430.2–433 kb of Pg_scaffold2289 were PCR amplified using the genome DNA of P. ginseng callus as template and also linked into the pEASY-Blunt vector to sequence.
TLC and HPLC analysis
TLC analysis was performed using silica gel 60 F254 plates (Merck KGaA, Darmstadt, Germany) as described previously11. Ginsenoside standards were purchased from Nantong FeiYu biological technology Co., Ltd. (Nantong, China). The concentration of ginsenoside standards is 1 mg/ml each. HPLC analysis was performed on a Shimadzu LC 20A system (Shimadzu, Kyoto, Japan). Chromatographic separations were performed with a Shim-pack XR-ODS column (100 mm × 2.0 mm, 2.2 μm, Shimadzu, Kyoto, Japan). Water (A) and acetonitrile (B) were used in the gradient elution system. The HPLC analysis program for reactants was carried out as follows: 0–2 min (15% B), 16 min (70% B), and 16.01–20 min (95% B), and 20.01–22 min (15% B). The flow rate was kept at 0.45 ml/min and the temperature of column compartment was set as 35℃. The products were detected at 203 nm.
HPLC/ESIMS analysis
The chromatographic analysis was carried out on a Waters LC system (Waters, Massachusetts, USA) using a Shim-pack XR-ODS column (100 mm × 2.0 mm, 2.2 μm, Shimadzu, Kyoto, Japan). The HR-ESIMS data were acquired using a Thermo Scientific Q Exactive mass spectrometer (Thermo Fisher Scientific, Massachusetts, USA) in the positive or negative ionization mode.
NMR analysis
1H NMR, 13C NMR, 2D-COSY NMR, 2D-NOE NMR, 2D-HSQC NMR and 2D-HMBC NMR were recorded on Bruker AM–500 (500 MHz) spectrometers in the C5D5N or d6-DMSO.
Ginsenoside Rg3
1H NMR (C6H5N, 500 MHz) δ ppm: 5.38 (d, J = 7.6 Hz, 1H), 5.31 (t, J = 7.0 Hz, 1H), 4.56 (d, J = 11.6 Hz, 1H), 4.52–4.44 (m, 2H), 4.39–4.29 (m, 3H), 4.28–4.21 (m, 2H), 4.19–4.11 (m, 2H), 3.95–3.84 (m, 3H), 3.29 (dd, J = 11.8, 4.4 Hz, 1H), 2.67–2.55 (m, 1H), 2.41–2.24 (m, 2H), 2.22–2.15 (m, 1H), 2.09–1.98 (m, 3H), 1.96–1.76 (m, 2H), 1.75–1.67 (m, 1H), 1.65 (s, 3H), 1.62 (s, 3H), 1.60–1.44 (m, 5H), 1.43 (s, 3H), 1.42–1.32 (m, 3H), 1.29 (s, 3H), 1.24–1.18 (m, 1H), 1.11 (s, 3H), 1.07–1.00 (m, 1H), 0.97 (s, 3H), 0.80 (s, 3H), 0.78–0.70 (m, 1H), 0.68 (d, J = 11.2 Hz, 1H).
Ginsenoside Rf
1H NMR (C6H5N, 500 MHz) δ ppm: 5.93 (d, J = 7.6 Hz, 1H), 5.38–5.30 (m, 1H), 4.95–4.90 (m, 1H), 4.53–4.43 (m, 3H), 4.40–4.06(m, 8H), 3.98–3.82 (m, 3H), 3.50–3.44 (m, 1H), 2.65–2.55 (m, 1H), 2.45–2.40 (m, 1H), 2.35–2.25 (m, 2H), 2.14–1.99 (m, 6H), 1.98–1.76 (m, 5H), 1.72–1.60 (m, 8H), 1.55–1.50 (m, 2H), 1.47 (s, 3H), 1.43–1.29 (m, 5H), 1.20–1.12 (m, 4H), 1.01–0.91 (m, 4H), 0.80 (s, 3H);
13C NMR (C6H5N, 125 MHz) δ ppm: 130.70, 126.27, 103.85, 103.78, 79.82, 79.66, 78.56, 78.37, 78.02, 77.79, 75.98, 72.90, 71.67, 70.95, 63.29, 61.36, 54.71, 51.61, 50.80, 48.22, 44.97, 41.09, 40.14, 39.58, 39.37, 35.75, 32.01, 31.18, 27.70, 26.96, 26.76, 25.74, 22.93, 17.61, 17.53, 17.35, 16.74, 16.68.
Ginsenoside Rb1
1H NMR (C6H5N, 500 MHz) δ ppm: 5.36 (d, J = 7.6 Hz, 1H), 5.32–5.27 (m, 1H), 5.13–5.06 (m, 2H), 4.90 (d, J = 7.6 Hz, 1H), 4.71 (d, J = 11.2 Hz, 1H), 4.58–4.43 (m, 4H), 4.36–4.00 (m, 14H), 3.25 (dd, J = 11.6, 4.0 Hz, 1H), 2.64–2.28 (m, 4H), 2.21–2.06 (m, 1H), 2.01–1.90 (m, 2H), 1.88–1.73 (m, 3H), 1.64 (s, 6H), 1.59 (m, 3H), 1.57–1.40 (m, 4H), 1.38–1.14 (m, 11H), 1.09 (s, 3H), 1.02–0.96 (m, 1H), 0.95 (s, 6H), 0.80 (s, 3H), 0.75–0.60 (m, 2H).
Notoginsenoside U
1H NMR (d6-DMSO, 500 MHz) δ ppm: 5.07 (t, J = 6.5 Hz, 1H), 4.44 (d, J = 7.4 Hz, 1H), 4.24 (d, J = 7.6 Hz, 1H), 3.94–3.84 (m, 2H), 3.68–3.62 (m, 1H), 3.59–3.53 (m, 1H), 3.43–3.28 (m, 2H), 3.21–3.08 (m, 2H), 3.08–3.00 (m, 3H), 2.22–2.13 (m, 1H), 2.06–1.86 (m, 3H), 1.80–1.66 (m, 2H), 1.63 (s, 3H), 1.65–1.58 (m, 1H), 1.56 (s, 3H), 1.54–1.34 (m, 6H), 1.26–1.19 (m, 8H), 1.08–1.00 (m, 1H), 0.94 (s, 3H), 0.95–0.87 (m, 1H), 0.83 (s, 9H), 0.74 (d, J = 10.2 Hz, 1H).
Gypenoside LXXV
1H NMR (C6H5N, 500 MHz) δ ppm: 5.35–5.29 (m, 1H), 5.12 (d, J = 7.8 Hz, 1H), 5.09 (d, J = 7.8 Hz, 1H), 4.73 (d, J = 11.4 Hz, 1H), 4,52 (d, J = 11.8 Hz, 1H), 4.39–4.28 (m, 2H), 4.26–4.12 (m, 3H), 4.10–3.99 (m, 3H), 3.98–3.87 (m, 2H), 3.76–3.62 (m, 1H), 3.44–3.37 (m, 1H), 2.66–2.23 (m, 2H), 2.47–2.32 (m, 2H), 2.08–1.97 (m, 2H), 1.90–1.75 (m, 4H), 1.66 (s, 6H), 1.71–1.65 (m, 1H), 1.60 (s, 3H), 1.59–1.30 (m, 7H), 1.26–1.23 (m, 1H), 1.22 (s, 3H), 1.03 (s, 3H), 1.02–0.97 (m, 1H), 0.99 (s, 3H), 0.94 (s, 3H), 0.91–0.85 (m, 1H), 0.88 (s, 3H), 0.80 (d, J = 11.4 Hz, 1H);
13C NMR (C6H5N, 125 MHz) δ ppm: 130.99, 125.90, 105.31, 98.01, 83.39, 79.21, 78.33, 78.27, 77.98, 76.99, 75.18, 74.78, 71.62, 71.49, 70.16, 70.08, 62.73, 56.28, 51.54, 51.32, 50.24, 49.39, 39.99, 39.48, 39.32, 37.28, 36.14, 35.09, 30.78, 30.66, 28.60, 28.18, 26.55, 25.73, 23.12, 22.29, 18.68, 17.89, 17.35, 16.27, 16.25, 15.98.
3-O-[β-D-glucopyranosyl-(1 → 2)-β-D-glucopyranosyl]-oleanolic acid:
1H NMR (C6H5N, 500 MHz) δ ppm: 5.46 (brs, 1H), 5.37–5.33 (m, 1H), 4.90 (dd, J = 7.3, 2.2 Hz, 1H), 4.53 (d, J = 12.0 Hz, 1H), 4.49–4.41 (m, 2H), 4.39–4.27 (m, 3H), 4.25–4.19 (m, 2H), 4.18–4.07 (m, 2H), 3.99–3.87 (m, 2H), 3.31–3.24(m, 2H), 2.25–1.69 (m, 1H), 1.50–1.36 (m, 4H), 1.28 (s, 6H), 1.25–1.15 (m, 4H), 1.08 (s, 3H), 1.00 (s, 3H), 0.97 (s, 3H), 0.94 (s, 3H), 0.88–0.83 (m, 1H), 0.80 (s, 3H), 0.71 (d, J = 11.6 Hz, 1H);
13C NMR (C6H5N, 125 MHz) δ ppm: 179.97, 144.65, 122.28, 105.79, 104.81, 88.74, 83.17, 77.99, 77.78, 77.68, 76.88, 71.40, 71.35, 62.57, 62.44, 55.59, 47.75, 46.43, 46.26, 41.92, 41.76, 39.49, 39.27, 38.47, 36.71, 34.00, 33.66, 32.97, 30.73, 28.07, 27.98, 26.34, 25.95, 23.46, 18.23, 17.15, 16.59, 15.23.
References
Shao, H. et al. Crystal structures of a multifunctional triterpene/flavonoid glycosyltransferase from Medicago truncatula. Plant Cell. 17, 3141–3154. https://doi.org/10.1105/tpc.105.035055 (2005).
Bowles, D., Lim, E. K., Poppenberger, B. & Vaistij, F. E. Glycosyltransferases of lipophilic small molecules. Annu. Rev. Plant Biol. 57, 567–597. https://doi.org/10.1146/annurev.arplant.57.032905.105429 (2006).
Vogt, T. & Jones, P. Glycosyltransferases in plant natural product synthesis: characterization of a supergene family. Trends Plant Sci. 5, 380–386 (2000).
Chen, W. et al. Whole-genome sequencing and analysis of the Chinese herbal plant Panax notoginseng. Mol. Plant 10, 899–902. https://doi.org/10.1016/j.molp.2017.02.010 (2017).
Kim, N. H. et al. Genome and evolution of the shade-requiring medicinal herb Panax ginseng. Plant Biotechnol. J. 16, 1904–1917. https://doi.org/10.1111/pbi.12926 (2018).
Xu, J. et al. Panax ginseng genome examination for ginsenoside biosynthesis. Gigascience 6, 1–15. https://doi.org/10.1093/gigascience/gix093 (2017).
Zhang, D. et al. The medicinal herb Panax notoginseng genome provides insights into ginsenoside biosynthesis and genome evolution. Mol. Plant 10, 903–907. https://doi.org/10.1016/j.molp.2017.02.011 (2017).
Yang, W. Z., Hu, Y., Wu, W. Y., Ye, M. & Guo, D. A. Saponins in the genus Panax L. (Araliaceae): a systematic review of their chemical diversity. Phytochemistry 106, 7–24. https://doi.org/10.1016/j.phytochem.2014.07.012 (2014).
Yan, X. et al. Production of bioactive ginsenoside compound K in metabolically engineered yeast. Cell Res. 24, 770–773. https://doi.org/10.1038/cr.2014.28 (2014).
Wang, P. P. et al. Production of bioactive ginsenosides Rh2 and Rg3 by metabolically engineered yeasts. Metab. Eng. 29, 97–105. https://doi.org/10.1016/j.ymben.2015.03.003 (2015).
Wei, W. et al. Characterization of Panax ginseng UDP-glycosyltransferases catalyzing protopanaxatriol and biosyntheses of bioactive ginsenosides F1 and Rh1 in metabolically engineered yeasts. Mol. Plant 8, 1412–1424. https://doi.org/10.1016/j.molp.2015.05.010 (2015).
Yue, C. J. & Zhong, J. J. Purification and characterization of UDPG: ginsenoside Rd glucosyltransferase from suspended cells of Panax notoginseng. Process. Biochem. 40, 3742–3748. https://doi.org/10.1016/j.procbio.2005.05.001 (2005).
Jung, S. C. et al. Two ginseng UDP-glycosyltransferases synthesize ginsenoside Rg3 and Rd. Plant Cell Physiol. 55, 2177–2188. https://doi.org/10.1093/pcp/pcu147 (2014).
Masada, S. et al. Functional and structural characterization of a flavonoid glucoside 1,6-glucosyltransferase from Catharanthus roseus. Plant Cell Physiol. 50, 1401–1415. https://doi.org/10.1093/pcp/pcp088 (2009).
Frydman, A. et al. Citrus fruit bitter flavors: isolation and functional characterization of the gene Cm1,2RhaT encoding a 1,2 rhamnosyltransferase, a key enzyme in the biosynthesis of the bitter flavonoids of citrus. Plant J. 40, 88–100. https://doi.org/10.1111/j.1365-313X.2004.02193.x (2004).
Itkin, M. et al. The biosynthetic pathway of the nonsugar, high-intensity sweetener mogroside V from Siraitia grosvenorii. Proc. Natl. Acad. Sci. USA 113, E7619–E7628. https://doi.org/10.1073/pnas.1604828113 (2016).
Nagatoshi, M. et al. UGT75L6 and UGT94E5 mediate sequential glucosylation of crocetin to crocin in Gardenia jasminoides. Febs. Lett. 586, 1055–1061. https://doi.org/10.1016/j.febslet.2012.03.003 (2012).
Noguchi, A. et al. Sequential glucosylation of a furofuran lignan, (+)-sesaminol, by Sesamum indicum UGT71A9 and UGT94D1 glucosyltransferases. Plant J. 54, 415–427. https://doi.org/10.1111/j.1365-313X.2008.03428.x (2008).
Ohgami, S. et al. Volatile glycosylation in tea plants: sequential glycosylations for the biosynthesis of aroma beta-primeverosides are catalyzed by two Camellia sinensis glycosyltransferases. Plant Physiol. 168, 464–477. https://doi.org/10.1104/pp.15.00403 (2015).
Osmani, S. A., Bak, S., Imberty, A., Olsen, C. E. & Moller, B. L. Catalytic key amino acids and UDP-sugar donor specificity of a plant glucuronosyltransferase, UGT94B1: molecular modeling substantiated by site-specific mutagenesis and biochemical analyses. Plant Physiol. 148, 1295–1308. https://doi.org/10.1104/pp.108.128256 (2008).
Sayama, T. et al. The Sg-1 glycosyltransferase locus regulates structural diversity of triterpenoid saponins of soybean. Plant Cell 24, 2123–2138. https://doi.org/10.1105/tpc.111.095174 (2012).
Shibuya, M., Nishimura, K., Yasuyama, N. & Ebizuka, Y. Identification and characterization of glycosyltransferases involved in the biosynthesis of soyasaponin I in Glycine max. Febs. Lett. 584, 2258–2264. https://doi.org/10.1016/j.febslet.2010.03.037 (2010).
Yano, R. et al. Isolation and characterization of the soybean Sg-3 gene that is involved in genetic variation in sugar chain composition at the C-3 position in soyasaponins. Plant Cell Physiol. 59, 797–810. https://doi.org/10.1093/pcp/pcy019 (2018).
Mackenzie, P. I. et al. The UDP glycosyltransferase gene superfamily: recommended nomenclature update based on evolutionary divergence. Pharmacogenetics 7, 255–269 (1997).
Erthmann, P. O., Agerbirk, N. & Bak, S. A tandem array of UDP-glycosyltransferases from the UGT73C subfamily glycosylate sapogenins, forming a spectrum of mono- and bisdesmosidic saponins. Plant Mol Biol 97, 37–55. https://doi.org/10.1007/s11103-018-0723-z (2018).
Stanta, G. Guidelines for molecular analysis in archive tissues (Springer, New York, 2011).
Acknowledgements
This work was financially supported by the National Key Research and Development Program of China (Grant No. 2018YFA0900700), the Strategic Priority Research Program of the Chinese Academy of Sciences (Grant No. XDB27020206), the Key Deployment Projects of the Chinese Academy of Sciences (Grant No. KFZD-SW-215), the Strategic Biological Resources Service Network Plan of the Chinese Academy of Sciences (Grant No. KFJ-BRP-009), the International Great Science Program of the Chinese Academy of Sciences (Grant No. 153D31KYSB20170121), and Science and Technology Commission of Shanghai Municipality (Grant No. 17YF1422500). The authors thank Drs Yan Wang, Wenjuan Yuan and Yining Liu for NMR spectroscopic and mass spectrometry assistance in Shanghai Institute of Plant Physiology and Ecology, CAS. The authors also thank UGT nomenclature committee for offering the official names of all UGTs cloned characterized in this study.
Author information
Authors and Affiliations
Contributions
C.Y., C.L., and W.W. performed the biological experiments; C.Y., Q.L., X.Y., and J.Y. carried out the chemical analyses; C.Y. and Y.W. performed the bioinformatics analyses; P.W., Z.Z., X.Y., J.Y., and G.Z. supervised and coordinated the experiments; C.Y., P.W., and Z.Z. wrote the manuscript. C.Y., C.L., and W.W. contributed equally to this work.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yang, C., Li, C., Wei, W. et al. The unprecedented diversity of UGT94-family UDP-glycosyltransferases in Panax plants and their contribution to ginsenoside biosynthesis. Sci Rep 10, 15394 (2020). https://doi.org/10.1038/s41598-020-72278-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-72278-y
This article is cited by
-
Effect of far-red light on the production and diversity of ginsenosides in leaves of Panax ginseng Meyer
Applied Biological Chemistry (2023)
-
Identification of two key UDP-glycosyltransferases responsible for the ocotillol-type ginsenoside majonside-R2 biosynthesis in Panax vietnamensis var. fuscidiscus
Planta (2023)
-
Advances in the biosynthesis and metabolic engineering of rare ginsenosides
Applied Microbiology and Biotechnology (2023)
-
Production of ginsenoside compound K by microbial cell factory using synthetic biology-based strategy: a review
Biotechnology Letters (2023)
-
Pathway elucidation of bioactive rhamnosylated ginsenosides in Panax ginseng and their de novo high-level production by engineered Saccharomyces cerevisiae
Communications Biology (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.