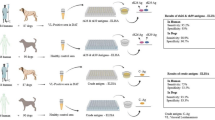
Abstract
Trypanosoma equiperdum is the causative agent of dourine, a parasitic venereal disease of equids. In this work, rabbits were infected with T. equiperdum strain OVI; serological tests (complement fixation test, ELISA and immunoblotting), used for the diagnosis of dourine in horses, were applied to study rabbit humoral immune response and to characterise T. equiperdum antigen pattern recognised by antibodies from infected rabbits. Moreover a protein extract of T. equiperdum strain OVI was produced and tested in skin tests on infected rabbits to detect the cell-mediated response induced by T. equiperdum, in order to evaluate its use in the field diagnosis of dourine. Sera of infected rabbits recognized in immunoblotting Trypanosoma protein bands with molecular weight below 37 kDa, providing a serological response comparable with that already observed in dourine infected horses. Moreover the trypanosome protein extract was capable to produce in vivo delayed-type hypersensitivity (DHT Type IV) in rabbits and proved itself to be non-toxic and non-sensitizing.
Similar content being viewed by others
Introduction
Dourine is a sexually transmitted disease affecting equids and caused by the protozoan Trypanosoma equiperdum (Kinetoplastida, subgenus Trypanozoon). Other members of the subgenus are T. evansi, the causative agent of surra in a wide range of mammals (horses, cattle, goats, buffalos, dogs, camel, deer, wild pigs, capybaras), and T. brucei, the causative agent of sleeping sickness in humans. T. brucei affects also domestic (cattle, pigs, small ruminants) and wild animals1.
In contrast with other trypanosomes, T. equiperdum is not transmitted by an invertebrate vector and it is primarily a tissue parasite that is rarely detected in the blood. Dourine is sexually transmitted; transmission by artificial insemination has not been confirmed, but it could potentially occur since T. equiperdum is present in the seminal fluid and in the genital tissues. The average mortality in horses is about 50%; donkeys and mules are more resistant and may remain unapparent carriers. To date, infected equids are the only known reservoir of the parasite2,3. No vaccines for dourine are available and pharmaceutical therapy is not recommended; the eradication strategy currently applied imposes slaughtering of seropositive animals4. This policy, however, is not economically feasible to be applied in developing countries, where horses are extensively used for transport and agriculture5.
Dourine is endemic in Africa and South America3 and has been also reported in Mongolia and Kazakhstan6. Most countries where dourine and other trypanosomoses, such as surra, are endemic do not regularly notify these diseases, so their spatial distribution remain not well known7. Recent outbreaks of trypanosomosis in Europe, as dourine in Italy8 and surra in Grand Canaria, Spain and France9,10 caused, respectively, by imported infected horses and camels, highlight the risk of importation of equine trypanosomosis into non-endemic countries. In spite of important economic losses caused by equine trypanosomoses, they can still be considered as neglected diseases11. Epidemiological investigations highlighted that knowledge of pathogenesis of dourine and host–pathogen interactions is still limited. Moreover the lack of vaccines, the inability of drugs to cure the neurological stage of the disease, and the limitations of current diagnostics make more difficult the control of the disease7,12. In addition, differential diagnosis is extremely difficult in geographical areas where T. equiperdum coexists with T. evansi, because the two parasites are genetically and antigenically related and the clinical outcome of the two diseases in horses is very similar3.
A better comprehension of the host immune response following infection could allow to identify targets that could improve the diagnosis of the disease. Despite the availability of in silico models or the development of alternative methods, such as cellular cultures that aim to reproduce tissues and organs, animal experimentation is still necessary to understand the pathogenesis of diseases and to develop and evaluate new therapeutic strategies13,14. T. equiperdum is able to infect rabbits, showing that this species could be used in the research on dourine3,15,16 and other trypanosomoses. Small mammals (rodents and lagomorphs), are preferred for laboratory research instead of other mammals as horses, cattle and sheep, due to their lower maintenance costs, shorter reproduction time and availability of transgenic models. Moreover, small mammals could be useful for the study of diseases that affect larger mammals.
In this work, rabbits were experimentally infected with T. equiperdum strain OVI and their humoral response was studied in vivo using serological tests (complement fixation test, ELISA and immunoblotting) currently used for the diagnosis of dourine in horses.
Moreover, a protein extract of T. equiperdum strain OVI was produced and used as skin test antigen on rabbits in order to evaluate its performances and its safety. The skin test antigen could be used in the field diagnosis of dourine in horses, in particular in endemic areas, just as brucellin and tuberculin are used in the diagnosis of, respectively, brucellosis and tuberculosis in cattle.
Materials and methods
Animal experimentation
Animal experimentation was carried out in compliance with Italian national law (Legislative Decree 26/2014)17 implementing Directive 2010/63/EU of the Council of the European Communities on the protection of animals used for scientific purposes18. Ethical approval was obtained from the Italian Ministry of Health (Protocol Number 511/2015-PR; DGSAF 11030-A of the 28/04/2015 integrated by the DGSAF 0019112-P of the 08/08/2016, ex Lgs. D. 26/2014, art. 31).
Healthy adult immunocompetent male rabbits, weighting 2.5–3.0 kg, and healthy female guinea pigs, weighting 250 g each, were used. In particular, 18 rabbits were experimentally infected with T. equiperdum, 9 rabbits were used as negative controls, 5 rabbits and 2 guinea pigs were used to assess toxicity and sensitization effects of the skin test antigen.
Animals were kept in an enriched environment, considering the needs of behavioural biology, and were caged individually but ensuring eye contact. Animals were maintained in a conventional facility with 12-h light/dark cycle, at 15 ± 5 °C for rabbits and at 20 ± 4 °C for guinea pigs; free access to regular food and tap water were allowed. Social and food enrichment were guaranteed19.
Rabbit experimental infection
T. equiperdum strain, provided by Onderstepoort Veterinary Institute, Pretoria, South Africa (OVI T. equiperdum), was produced in rats according to the World Organisation for Animal Health (2018)3, and stored at − 80 °C until use. Nine rabbits were infected intrascrotally2,15 with cryopreserved T. equiperdum in order to amplify the parasite and to adapt it to the rabbit. When a marked scrotal edema appeared, rabbits were sacrificed; then the scrotum of each rabbit was removed and the edematous material was taken, homogenized and diluted in 0.01 M phosphate-buffered saline, pH 7.5 (PBS), to get 104 live trypanosomes/ml. Trypanosomes were counted by Bürker counting chamber. This homogenized antigen was inoculated intrascrotally in other 9 rabbits (1 ml for each animal). Blood samples of infected rabbits were collected from the marginal ear vein, after applying local anaesthesia, to verify the presence of antibodies versus T. equiperdum before the infection (T0) and at times 7, 14, 21, 28 and 35 days post infection (T7, T14, T21, T28 and T35). As negative control, nine rabbits were inoculated with PBS only and blood samples were taken at time from T0 to T35. Sera were stored at – 20 °C until use. The second group of 9 inoculated rabbits and negative controls were used also to test the skin test antigen.
Serological tests for the control of rabbit infection
Complement fixation test (CFT) was performed according to the method described for equids in the OIE Manual of Diagnostic Tests and Vaccines for Terrestrial Animals3. Briefly, the antigen was produced in rats using the OVI T. equiperdum, according to the OIE Manual3. Sera were diluted five-times with veronal buffered saline, and those showing more than 50% of fixation level at the dilution 1:5 were considered as positive and tested again to the end point using twofold dilutions.
Indirect-ELISA (i-ELISA) was performed according to Bonfini et al.20. Briefly, rabbit sera were diluted 1:100 in PBS containing 0.05% Tween 20 and 0.1% BSA (dilution buffer); as secondary antibody, a peroxidase-conjugated goat Anti-Rabbit IgG (H + L) (Bio-Rad, Hercules, CA, USA) diluted 1:30,000 in dilution buffer was used. Results were expressed as percentage of positivity (PP), calculated using the following formula:
As positive and negative controls, a lyophilized serum obtained from a rabbit experimentally infected with OVI T. equiperdum15 and the serum of a healthy rabbit were used. The cut-off value of the ELISA was calculated by adding 4 times the standard deviation to the mean PP value of sera collected from the 9 rabbits in the control group at times from T0 to T3521. Sera showing a PP value greater than the cut-off value were considered positive.
The immunoblotting assay was performed according to Luciani et al.22, using rabbit sera diluted 1:5,000 and the peroxidase-conjugated goat Anti-Rabbit IgG (H + L) (Bio-Rad) diluted 1:3,000. Image acquisition was carried out with Chemidoc MP (Bio-Rad) using Image Lab Software version 4.0.1 (Bio-Rad). Sera reacting with bands with molecular weight (MW) equal or less than 37 kDa were considered as positive for Trypanosoma antibodies22,23.
Skin test
The antigen was produced from blood collected from rats that had been experimentally infected with OVI T. equiperdum3 and containing approximately 90% live trypanosomes, as assessed by a Bürker counting chamber. After washing with PBS at 2000 rpm for 20 min at 4 °C, the supernatant was discarded and the buffy coat containing trypanosomes was collected and, after further washings to remove red cells, the pellet was collected, resuspended in PBS and lysed by freeze/thawing cycles in liquid nitrogen. The lysed cells were filtered through 0.8 µm, 0.45 µm and 0.22 µm filters (Millipore, Carrigtwohill, Co. Cork, Ireland). The protein concentration of the antigen was determined using the BCA Protein Assay Kit (Thermo Scientific, Rockford, IL, USA) and adjusted to 1.4–2.0 mg/ml with PBS.
Six weeks after infection with OVI T. equiperdum, the 9 infected rabbits and the negative controls were shaved at two rectangular areas of about 3.0 × 9.0 cm on both flanks. Each of the two areas was divided into 3 equal squares. T. equiperdum skin test antigen (0.2 ml) was inoculated intradermally into 3 squares according to Latin square design and physiological solution (0.2 ml) into the remaining 3 squares as control. Skin reactions (diameters of erythema) were measured 24 h after antigen injection. The reaction was considered positive if an erythema of 8–25 mm of diameter was present.
Evaluation of toxicity and sensitization effects of the skin test antigen
Toxicity and sensitization effects of the skin test antigen were evaluated using, respectively, guinea pigs and rabbits.
Two guinea pigs were inoculated subcutaneously with 0.5 ml of T. equiperdum skin test antigen and observed for 7 days to evaluate toxicity effects (body weight loss, systemic effects and behavioural variations).
Six rabbits divided into 2 groups (test group and control group) were used to evaluate skin sensitization effects of the antigen. Three rabbits (test group) were inoculated intradermally 3 times at 5-day intervals with 0.1 ml of T. equiperdum skin test antigen. Twenty days after the third inoculation, another dose was injected into the same rabbits and into other 3 rabbits, previously not-inoculated with T. equiperdum skin test antigen (control group). Animals (test group and control group) were observed from the first inoculation until the end of the experiment (48 h after the last inoculation) to check for signs of sensitization (erythema and/or edema) at the point of injection.
Statistical analyses
Statistical analysis was performed with the calculation of the percentage of reactor animals (observed or presumed) and the relative uncertainty, according to a Bayesian approach and using a Beta distribution, at 95% confidence interval, considering the percentage of rabbits that gave a skin test positive reaction, and taking into consideration two types of reactions: rabbits that showed positive reactions in all the skin treated areas and rabbits that showed positive reaction in at least one skin treated area.
Results
Serological tests for the control of rabbit infection
All the nine rabbits infected with OVI T. equiperdum became positive after 7 days from inoculation (T7) (CFT titres ≥ 1:40) and maintained high antibody titers (CFT titres ≥ 1:320) for the entire duration of the experiment (Table 1). The control animals remained negative throughout the experiment.
Indirect-ELISA PP values of infected rabbits ranged from 6.3 to 18.2% at 7 days post inoculation (T7) and gradually increased till 83.7–116.7% at time T35. Calculated cut-off was 9.2%; according to this value, at time T7 rabbits #1, #5, #6, #7 and #8 tested positive and rabbits #2, #3, #4 and 9 tested negative, while at time T14 all the rabbits tested positive. PP values for the rabbits of the control group were less than 6.9% throughout the experiment (Fig. 1).
Immunoblotting results showed that rabbit serum antibodies from the control group at times from T0 to T35 identified bands with MW ranging from 40 to 180 kDa (Fig. 2). On the contrary, starting from time T7, antibodies in the sera of infected rabbits showed an increasing reaction with Trypanosoma proteins, and in particular with low MW proteins (6–37 kDa) (Fig. 3). More in detail, rabbits #3, #4, #5 and #7 tested negative at T7 but positive at T14; the other 5 rabbits (#1, #2, #6, #8, #9) tested positive at both T7 and T14. At time T7 rabbits #1 and #2 reacted with a weak band of 37 kDa, rabbit #6 reacted with a weak band of 26 kDa, rabbit #8 with bands of 37, 24 and 20 kDa and rabbit #9 with bands of 37, 25, 20 and 15 kDa (images not shown).
The gradual increase in the number and intensity of protein bands recognized by rabbit positive sera during the time, has been already described for horse positive sera in Luciani et al.22,23.
Results of the three serological tests of infected rabbits at times T0, T7 and T14 were summarized in Table 2.
Skin test
Skin reactions to T. equiperdum skin test antigen were observed in 8 out of 9 infected rabbits (Fig. 4). In particular, a positive reaction (diameters of erythema of 8–25 mm) was observed in five rabbits in all the three treated skin areas; in two rabbits, in two out of three treated areas and in one rabbit, in one out of three treated areas. Negative reaction in the three treated skin areas was observed in one rabbit. Moreover, negative reaction was observed in the skin negative control areas.
Statistically significant differences were observed between negative controls and rabbits that showed positive reactions in all the three treated skin areas or in at least one treated skin area (Table 3).
Evaluation of toxicity and sensitization effects of the skin test antigen
No clinical signs, changes in body weight or behavioural variations were observed in the two guinea pigs inoculated subcutaneously with T. equiperdum skin test antigen.
No dermal responses as erythema and/or oedema were found in rabbits of both test and control group from the first inoculation until the end of the experiment.
Discussion
Diagnosis of dourine depends on the identification of clinical signs and lesions, the isolation of the parasite and the use of serological tests. The first ones can be similar to those caused by other diseases, such as surra, and differential diagnosis and epidemiological investigations are needed. T. equiperdum is present in the blood and tissue fluids of infected horses in small numbers, so its identification is difficult. Moreover, T. equiperdum is morphologically very similar to T. evansi3,11.
Genetic markers for the differential diagnosis between dourine and surra are under evaluation; to date no T. equiperdum-specific PCR method is available3.
Phylogenetic analysis has suggested an evolution of T. equiperdum and T. evansi from T. brucei brucei; for this reason, and considering the high degree of similarity among the three trypanosomes, some authors have proposed to consider T. equiperdum and T. evansi as subspecies of T. brucei (T. brucei equiperdum and T. b. evansi)24,25.
OIE-prescribed serological tests for dourine are CFT and indirect fluorescent antibody test (IFAT); the latter is currently used to confirm infection or resolve inconclusive CFT results. Other serological tests used in the diagnosis of dourine are ELISA, radioimmunoassay, counter immunoelectrophoresis, agar gel immunodiffusion (AGID), immunoblotting and a card agglutination test3. These tests are not specific for T. equiperdum, due to antigenic similarities among T. equiperdum, T. evansi and other Trypanosoma species3,26.
Three serological tests, developed for the diagnosis of dourine in horses, were applied in the present study to evaluate the humoral response of OVI T. equiperdum infected rabbits and to compare the results with the horse humoral response. Infected animals tested positive for anti-Trypanosoma antibodies at CFT after 7 days post infection (p.i.) and at i-ELISA and immunoblotting at times between 7 and 14 days p.i.. Moulton et al.16 and Hiepe et al.27 observed an increase in antibody titres after, respectively, 7 and 9 days p.i. in rabbits infected with T. equiperdum. In another work of Bishop et al.28, antibodies versus T. equiperdum were detected after 14 days p.i. in experimentally infected rabbits and horses. According to Luciani et al.22 a mare, experimentally infected by tranfusion of blood collected from dourine naturally infected horses (2011 Italian outbreak), became positive at CFT and immunoblotting after 14 days post infection (p.i.), and tested positive also at IFAT 3 days later (17 days p.i.). Hébert et al.29 carried out experimentally infections on horses to validate a model for assessing drug efficacy against T. equiperdum infection: 9 horses, intravenously inoculated with OVI T. equiperdum (dose 5 × 104 trypanosomes/animal), became seropositive at CFT after 7–9 days p.i., while among 3 horses intravaginally infected with different doses of the parasite, only one became positive at CFT at day 23 p.i.. In a work of Hagos et al.5 6 stallions were intravenously infected with T. equiperdum Dodola 834/940 strain (dose 50,000 parasites/ml); seroconversion was observed after 32 days p.i. by CATT/T. evansi.
So, just as for other pathogens, the time of seroconversion in T. equiperdum infections varies depending on the dose and route of inoculation, and the strain of the parasite.
Moreover, immunoblotting results obtained in the present work demonstrated that antibodies from infected rabbits, in contrast to antibodies from healthy rabbits, specifically recognise a T. equiperdum antigenic profile with low molecular weight bands (< 37 kDa), just as observed in dourine positive horses22,23. These results seem to confirm the hypothesis that T. equiperdum low molecular proteins are important in the humoral immunitary response against this pathogen both in horse and rabbit and could represent possible candidate diagnostic antigens for the development of more specific tests for dourine.
Some authors evaluated the cellular immune response of laboratory animals (mice, rabbits) infected with T. cruzi or other Trypanosoma species using a delayed type hypersensitivity test30,31,32,33,34. Mansfield and Kreier33 described positive skin test reactions of the Arthus type (type III hypersensitivity reactions) in rabbits infected with T. congolense. Delayed hypersensitivity reactions were observed in rabbits and mice infected with T. brucei and T. rhodesiense27. Thé et al.34 described a DTH response in a skin test performed on mice infected with T. cruzi. Regarding the cell-mediated response induced by T. equiperdum in horse, to date no scientific data are available in the literature.
Delayed hypersensitivity reactions are usually used to detect infections caused by Mycobacterium bovis in cattle; serological tests are used in parallel or serial testing to improve the detection of infected animals and confirm the results of intra-dermal skin tests. The use of tuberculin testing and stamping-out of reactors has eliminated M. bovis infection from farmed cattle in some countries35,36. Brucellin skin test, performed using a standardised and s-LPS-free antigen preparation, is used for the screening of unvaccinated cattle in the diagnosis of brucellosis. The brucellin skin test has high sensitivity and specificity and is also used to detect Brucella infections in unvaccinated sheep, goats and pigs. Moreover, the test may aid when false positive serological reactions due to cross-reacting bacteria are suspected, as false positive reacting animals always give negative results in the skin test37, and being independent of circulating antibodies, it might improve the diagnosis of brucellosis in latent carriers of Brucella38,39.
In this work a protein extract of OVI T. equiperdum was also produced and used as skin test antigen on T. equiperdum infected rabbits in order to evaluate its ability to produce an in vivo delayed type hypersensitivity, to obtain a diagnostic tool for the use in the field for dourine and other diseases caused by trypanosomes of the subgenus Trypanozoon, just as it is commonly done for brucellin and tuberculin in the detection of Brucella spp. and M. bovis infections in ruminants. Skin test antigen produced a delayed-type hypersensitivity (DHT, Type IV) response and the diameter of skin reactions obtained was in a range 8–25 mm, similarly to what can be observed in animals treated with other kind of allergens, namely tubercolin or brucellin. Moreover skin test antigen proved to be non-toxic and non-sensitizing, when tested, respectively, on guinea pigs and rabbits. These encouraging results support further studies to standardize the manufacturing process and chemical characteristics of the new skin test antigen described in this paper, in order to guarantee reproducibility of its diagnostic performances. It is also necessary to evaluate the cell-mediated response induced by T. equiperdum in healthy and infected horses. Since the dourine cases reported in Italy in 2011, no dourine outbreaks have been detected in Italy and in other European countries, so to date it has not been possible to test skin test antigen on naturally infected horses. However, since skin test antigens do not interfere with serological diagnosis, being independent from humoral immunitary response, they could be employed in parallel with serology to improve the evaluation of the health status of equids when dourine infection is suspected. Moreover, the lack of vaccines for dourine and surra reduces false positive results that could be obtained using skin test on vaccinated animals. Further studies should be done to evaluate diagnostic performances of skin test antigens on T. equiperdum infected horses in areas where dourine is endemic and to compare the cell-mediated response induced by T. equiperdum in both rabbits and horses. OVI T. equiperdum skin test antigen should also be tested on T. evansi infected horses to evaluate possible cross-reactions.
According to the results obtained from this study, the rabbit model could be used to increase the pathogenic knowledge of dourine, and it could help to develop more effective diagnostic tools and possibly new strategies for dourine control and prophylaxis.
References
Radwanska, M., Vereecke, N., Deleeuw, V., Pinto, J. & Magez, S. Salivarian trypanosomosis: a review of parasites involved, their global distribution and their interaction with the innate and adaptive mammalian host immune system. Front. Immunol. 9, 1–20. https://doi.org/10.3389/fimmu.2018.02253 (2018).
Ahmed, Y. et al. Trypanosoma equiperdum in the horse—a neglected threat?. Vlaams Diergeneeskd. Tijdschr. 87, 66–87 (2018).
World Organisation for Animal Health (Office International des Épizooties: OIE). Chapter 3.5.3. Dourine. In Manual of Diagnostic Tests and Vaccines for Terrestrial Animals 1–10 (OIE, Paris, 2018).
World Organisation for Animal Health (Office International des Épizooties: OIE). Technical disease cards: Dourine 1–4 (OIE, Paris, 2013).
Hagos, A. et al. Efficacy of Cymelarsan and Diminasan against Trypanosoma equiperdum infections in mice and horses. Vet. Parasitol. 171, 200–206 (2010).
Suganuma, K. et al. Isolation, cultivation and molecular characterization of a new Trypanosoma equiperdum strain in Mongolia. Parasites Vectors 9, 481. https://doi.org/10.1186/s13071-016-1755-3 (2016).
Büscher, P. et al. Equine trypanosomosis: enigmas and diagnostic challenges. Parasites Vectors 12(1), 234. https://doi.org/10.1186/s13071-019-3484-x (2019).
Calistri, P. et al. Dourine re-emergence in Italy. J. Equine Vet. Sci. 33, 83–89 (2013).
Gutierrez, C., Desquesnes, M., Touratier, L. & Büscher, P. Trypanosoma evansi: recent outbreaks in Europe. Vet. Parasitol. 174, 26–29 (2010).
Tamarit, A. et al. Trypanosoma evansi infection in mainland Spain. Vet. Parasitol. 167(1), 74–76. https://doi.org/10.1016/j.vetpar.2009.09.050 (2010).
Gizaw, Y., Megersa, M. & Fayera, T. Dourine: a neglected disease of equids. Trop. Anim. Health Prod. 49(5), 887–897. https://doi.org/10.1007/s11250-017-1280-1 (2017).
Hébert, L. et al. Melarsomine hydrochloride (Cymelarsan) fails to cure horses with Trypanosoma equiperdum OVI parasites in their cerebrospinal fluid. Vet. Parasitol. 264, 47–51 (2018).
Committee on the Use of Laboratory Animals in Biomedical and Behavioral Research, Commission on Life Sciences, National Research Council (US), Institute of Medicine (US). Use of laboratory animals in biomedical and behavioral research. National Academy Press (US), Washington (DC), 1–112. ISBN: 0-309-56422-0 (1988).
Freires, I. A., Sardi, J. C., de Castro, R. D. & Rosalen, P. L. Alternative animal and non-animal models for drug discovery and development: bonus or burden?. Pharm. Res. 34(4), 681–686. https://doi.org/10.1007/s11095-016-2069-z (2017).
Pascucci, I. et al. Diagnosis of dourine in outbreaks in Italy. Vet. Parasitol. 193, 30–38. https://doi.org/10.1016/j.vetpar.2012.12.006 (2013).
Moulton, J. E., Coleman, J. L. & Gee, M. K. Pathogenesis of Trypanosoma equiperdum in rabbits. Am. J. Vet. Res. 36(4), 357–366 (1975).
Legislative Decree 4 March 2014 n. 26. Transposition of directive 2010/63/UE on the protection of animals used for scientific purposes. Off. J. of the Italian Republic n. 61 of 14/03/2014 (2014).
Commission, E. Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes. Off. J. Eur. Union 276, 33–79 (2010).
Brønstad, A. et al. Current concepts of harm-benefit analysis of animal experiments—report from the AALAS-FELASA working group on harm-benefit analysis—part 1. Lab Anim. 50(1 Suppl), 1–20. https://doi.org/10.1177/0023677216642398 (2016).
Bonfini, B. et al. Development of an indirect ELISA for the serological diagnosis of dourine. Vet. Parasitol. 261, 86–90 (2018).
Desquesnes, M. et al. Development and application of an antibody-ELISA to follow up a Trypanosoma evansi outbreak in a dromedary camel herd in France. Vet. Parasitol. 162, 214–220. https://doi.org/10.1016/j.vetpar.2009.03.033 (2009).
Luciani, M. et al. IgG antibodies from dourine infected horses identify a distinctive Trypanosoma equiperdum antigenic pattern of low molecular weight molecules. Vet. Immunol. Immunopathol. 151, 140–146 (2013).
Luciani, M. et al. Trypanosoma equiperdum low molecular weight proteins as candidates for specific serological diagnosis of dourine. Front. Vet. Sci. 5(40), 1–6 (2018).
Desquesnes, M. et al. Trypanosoma evansi and Surra: a review and perspectives on origin, history, distribution, taxonomy, morphology, hosts, and pathogenic effects. Biomed. Res. Int. https://doi.org/10.1155/2013/194176 (2013).
Carnes, J. et al. Genome and phylogenetic analyses of Trypanosoma evansi reveal extensive similarity to T. brucei and multiple independent origins for dyskinetoplasty. PLOS Negl. Trop. Dis. 9(1), 1–21 (2015).
World Organisation for Animal Health (Office International des Épizooties: OIE). Chapter 3.1.21. Trypanosoma evansi infection (Surra). In: Manual of Diagnostic Tests and Vaccines for Terrestrial Animals 660–674 (OIE, Paris, 2018).
Hiepe, F., Jungnitz, S. & Hiepe, T. Production of autoantibodies against DNA and collagen after inoculation of rabbits with Trypanosoma equiperdum. Appl. Parasitol. 37(4), 266–274 (1996).
Bishop, S., Rae, P. F., Phipps, L. P., Boid, R. & Luckins, A. G. Trypanosoma equiperdum: detection of trypanosomal antibodies and antigen by enzyme-linked-immunosorbent assay. Br. Vet. J. 151(6), 715–720 (1995).
Hébert, L. et al. Validation of a new experimental model for assessing drug efficacy against infection with Trypanosoma equiperdum in horses. Vet. Parasitol. 263, 27–33 (2018).
Cazorla, S. I. et al. Redirection of the immune response to the functional catalytic domain of the cystein proteinase cruzipain improves protective immunity against Trypanosoma cruzi infection. J. Infect. Dis. 202(1), 136–144 (2010).
da Silva Guerreiro, M. L., Brito Morais, I. R. & Gumes Andrade, S. Immunological response to re-infections with clones of the Colombian strain of Trypanosoma cruzi with different degrees of virulence: influence on pathological features during chronic infection in mice. Mem. Inst. Oswaldo Cruz 110(4), 500–506 (2015).
Finerty, J. F., Krehl, E. P. & McKelvin, R. L. Delayed-type hypersensitivity in mice immunized with Trypanosoma rhodesiense antigens. Infect. Immun. 20(2), 464–467 (1978).
Mansfield, J. M. & Kreier, J. P. Tests for antibody- and cell-mediated hypersensitivity to Trypanosome antigens in rabbits infected with Trypanosoma congolense. Infect. Immun. 6(1), 62–67 (1972).
Thé, T. S., Portella, R. S., Guerreiro, M. L. & Andrade, S. G. Effect of treatment with cyclophosphamide in low doses upon the onset of delayed type hypersensitivity in mice chronically infected with Trypanosoma cruzi: involvement of heart interstitial dendritic cells. Mem. Inst. Oswaldo Cruz 108(6), 691–698 (2013).
World Organisation for Animal Health (Office International des Épizooties: OIE). Chapter 3.4.6. Bovine tuberculosis. In: Manual of Diagnostic Tests and Vaccines for Terrestrial Animals 1058–1074 (OIE, Paris, 2018).
Duignan, A., Kenny, K., Bakker, D. & Good, M. Tuberculin PPD potency assays in naturally infected tuberculous cattle as a quality control measure in the Irish Bovine Tuberculosis eradication programme. Front. Vet. Sci. 6, 328. https://doi.org/10.3389/fvets.2019.00328 (2019).
World Organisation for Animal Health (Office International des Épizooties: OIE). Chapter 3.1.4. Brucellosis (B, abortus, B. melitensis and B. suis). In: Manual of Diagnostic Tests and Vaccines for Terrestrial Animals 355–398 (OIE, Paris, 2018).
Bercovich, Z. The use of skin delayed-type hypersensitivity as an adjunct test to diagnose brucellosis in cattle: a review. Vet. Q. 22(3), 123–130 (2000).
Nyanhongo, N., Hansen, M., Nymo, I. H., Godfroid, J. & Michel, A. L. Evaluating the brucellin skin test as an additional test to control bovine brucellosis. SOJ Microbiol. Infect. Dis. 5(4), 1–6 (2017).
Funding
The present study was funded by the Italian Ministry of Health (Grant IZS AM 07/11 RC).
Author information
Authors and Affiliations
Contributions
M.T., O.M.: designed the study. E.R., M.P.V., O.M. carried out animal experimentation. B.B., D.R., G.O., I.K., M.L., T.D.F.: carried out the laboratory investigations and acquired the data. B.B., G.O., I.K., M.L., M.T., O.M., T.D.F.: analysed and interpreted the data. B.B., M.L., T.D.F.: drafted the manuscript. E.R., L.I., M.P.V., M.T., O.M.: critically revised the article for important intellectual content. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Di Febo, T., Krasteva, I., Bonfini, B. et al. Humoral immune response and delayed-type hypersensitivity in rabbits infected with Trypanosoma equiperdum. Sci Rep 10, 14914 (2020). https://doi.org/10.1038/s41598-020-71992-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-71992-x
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.