Abstract
Maternal effects via hormonal transfer from the mother to the offspring provide a tool to translate environmental cues to the offspring. Experimental manipulations of maternally transferred hormones have yielded increasingly contradictory results, which may be explained by differential effects of hormones under different environmental contexts. Yet context-dependent effects have rarely been experimentally tested. We therefore studied whether maternally transferred thyroid hormones (THs) exert context-dependent effects on offspring survival and physiology by manipulating both egg TH levels and post-hatching nest temperature in wild pied flycatchers (Ficedula hypoleuca) using a full factorial design. We found no clear evidence for context-dependent effects of prenatal THs related to postnatal temperature on growth, survival and potential underlying physiological responses (plasma TH levels, oxidative stress and mitochondrial density). We conclude that future studies should test for other key environmental conditions, such as food availability, to understand potential context-dependent effects of maternally transmitted hormones on offspring, and their role in adapting to changing environments.
Similar content being viewed by others
Introduction
Maternal effects may translate environmental cues from the mother to the offspring for example via maternally transferred hormones (hereafter ‘maternal hormones’), potentially increasing offspring survival in the predicted conditions (adaptive maternal effects1,2,3,4). Maternal hormone-mediated effects have been recently highlighted as a potential mechanism and source of phenotypic plasticity to respond to changing climate5,6,7, yet empirical evidence is scarce. Experimental studies on hormone-mediated maternal effects have revealed increasingly contradictory results, for example, elevated maternal androgens both increasing, decreasing or having no effect on offspring growth3. One obvious explanation for such contrasting effects is linked to the well-known pleiotropic effects of (maternal) hormones, due to which prenatal hormone exposure brings both costs (e.g. reduced immune response) and benefits (e.g. faster growth). Therefore, the final fitness outcome is likely to be determined by the potential benefit–cost balance being set by environmental conditions3.
Studies on context-dependent effects of maternal hormones have been repeatedly called for in the literature, but still only few empirical studies are available. In one of the most studied maternal hormone group, glucocorticoids, offspring fitness depend on the matching between maternal and offspring environment (e.g.8,9), such as maternal condition (e.g.10,11), density12 and predictability of food13. In contrast, despite the large body of literature on maternal androgen hormones, to date only one study14 suggests that the effects of maternal androgens on nestling development and immunity depend on the timing (first or second clutch) of breeding and associated food conditions. To our knowledge, the context-dependent effects of other key maternal hormones, such as thyroid hormones, have not been explored. Yet, characterizing context-dependent effects can contribute to our understanding on the cause of the observed large variation in maternal hormone transfer across and within populations4,15 and its potential adaptive function.
We previously reported contrasting effects of maternal thyroid hormones (THs: T3 = triiodothyronine, T4 = thyroxine) on offspring phenotype in two closely related species, the collared flycatcher (Ficedula albicollis) and pied flycatcher (F. hypoleuca)16,17. THs increased early growth, but decreased growth during the second week post-hatching in collared flycatchers16, while they tended to increase growth during the second week post-hatching in pied flycatchers17. The underlying mechanisms and the explanations for these contrasting results remain unknown, but one hypothesis could be that the effects of elevated yolk THs would depend on the post-hatching environmental conditions. For example, if prenatal THs increase resting metabolic rate (RMR), as reported in18, the elevated RMR may lead to increased growth in benign conditions, but decreased growth when resource availability is poor19. Moreover, THs have conserved function in controlling thermoregulation20, which is not fully developed in altricial offspring until late postnatal stage21. If prenatal THs are expected to increase metabolic rates and stimulate thermogenesis, they may benefit nestling survival in low developmental temperatures, while in higher temperatures such effects may not be observable or even turn negative because of higher energy expenditure. Therefore, experimental tests on whether the effects of yolk THs would depend on post-hatching environmental conditions are now duly required.
Here, we use a full factorial experimental design to, for the first time, study whether maternal THs exert context-dependent effects on early-life phenotype, survival and physiology. We experimentally manipulated both egg TH levels and post-hatching nest-box temperature in a wild population of pied flycatchers. We chose to study interactions between THs and temperature variation, because both factors are involved in thermoregulation7,20, which is crucial for early-life altricial nestlings21. Moreover, we previously found that yolk T4 are higher under relatively lower ambient temperature in passerines22. Testing whether yolk THs have temperature-dependent effects therefore would provide information on whether elevated T4 transfer under lower temperatures could be an adaptive allocation. In addition to measuring postnatal growth and short-term survival (fledging success), we explored changes in potential underlying physiological mechanisms. (1) We measured circulating THs to track any lasting effects of prenatal TH manipulation on the general function of the hormonal axis18 and to assess the direct effects of postnatal temperature on THs. (2) We estimated mitochondrial density (i.e. mitochondrial DNA copy number) as a proxy for the effects of our treatments on cellular bioenergetics23. Mitochondria are the powerhouse of cells, converting nutrients into ATP to sustain cellular functions24. Mitochondrial density and bioenergetics are likely to be influenced by THs across taxa (25,26, reviewed in27) and by ambient temperature28. However, to our knowledge, the effects of prenatal THs on mitochondria have not been characterized beyond mammalian models29. (3) Finally, we measured biomarkers of oxidative stress, the imbalance between oxidizing molecules (e.g. reactive oxygen species, ROS) and antioxidant protection, which ultimately leads to oxidative damage on biomolecules and cellular dysfunction30. Elevated yolk THs may result in increased oxidative stress directly via the stimulating effects of THs on metabolism and mitochondrial ROS production31, or indirectly via increasing developmental speed since fast growth is likely to increase oxidative stress32. Temperature is also likely to influence oxidative stress since both cold and heat stress have been shown to increase oxidative damage levels33, e.g.34.
We summarize our predictions in Table 1. Overall, we predicted that elevated prenatal THs should increase survival in cooler (non-heated) nests, for example due to its stimulating and positive effects on thermogenesis, which might also facilitate growth. On the other side, the increased metabolism in nestlings from heated nests might lead to higher energy expenditure, which albeit not necessarily decreases survival, but might reduce growth (e.g.18). We predict that elevated prenatal THs increase postnatal circulating THs and the effect may be stronger in cooler (non-heated) nests. Mitochondrial density is predicted to be increased by the stimulating effect of (prenatal) THs and low temperature, and thus being highest in the nestlings from TH-injected eggs and non-heated nests. Finally, oxidative damage is also predicted to be higher in prenatal TH elevation group (see above), which could be more pronounced in heated nests due to maladaptive thermogenesis enhancement.
Material and methods
The experiment and all methods we used were in accordance with all relevant guidelines and regulations and have been approved by the Animal Experiment Board of the Administrative Agency of South Finland (ESAVI/2902/2018) and the Environmental Center of Southwestern Finland (license number VARELY549/2018). We conducted our experiment in a nest-box population of pied flycatchers located in Turku, Finland (60° 25′ N, 22° 10′ E). Egg THs were manipulated in unincubated eggs using injections in the yolk, following the procedure in17. The dose was based on the average (± SD) yolk T3 and T4 levels we previously measured from the same population of pied flycatcher eggs (N = 15, T3 = 0.740 ± 0.238 ng/yolk, T4 = 2.307 ± 0.654 ng/yolk). Following35, we aimed to elevate yolk T3 + T4 by 2 × SD (i.e. 0.477 ng/yolk of T3 and 1.308 ng/yolk of T4). All eggs of a nest received the same injection of either thyroid hormones (hereafter, TH clutches, N = 30 clutches) or only vehicle (hereafter, CO clutches, N = 30 clutches). Hatching success was not affected by the hormone treatment (CO: 71.1%, TH: 64.9%, z = − 1.129, p = 0.259).
On the second day after hatching (d2), chicks were individually identified by nail clipping and ca. half of the chicks of each nest were swapped among broods with different hormone treatments whenever possible, to create nests which included both CO and TH treated nestlings. Internal temperature of the nest boxes was then increased between postnatal day 2 and day 8 in ca. half of the boxes (hereafter, ‘heated nests’, N = 24) using heating pads (UniHeat 72 h, USA), installed under the ceiling, and replaced every 2nd day. The period of 2–8 days was selected as pied flycatcher nestlings are not fully thermoregulatory during this period, and thus sensitive to variation in ambient temperature36. The other half served as controls (hereafter ‘non-heated nests’, N = 23 nests) and received non-functional heating pads and similar visits every 2nd day. The sample sizes in the four treatment groups at day 2 are: CO + non-heated 76, CO + heated 50, TH + non-heated 58, and TH + heated 53 nestlings, respectively. The actual temperature within the boxes was recorded with a thermo-logger (iButton thermochron, measuring at 3 min intervals, 0.0625 °C accuracy), placed inside all nest boxes at 5 cm distance above the nest rim, and the daily average temperature from d2 to d8 was calculated for each nest. We averaged the daily temperatures (hereafter “average nest temperature) across the heating period (d2 to d8), which showed that the heating treatment increased the temperature by 2.75 °C (SE = 0.37, marginal means ± SEs controlled for date and iButton position: heated 21.25 ± 0.36, non-heated 18.50 ± 0.35, GLM, t = − 7.40, p < 0.001, Supplementary Fig. S1). From the daily temperature data, we also calculated the minimum and maximum nest temperature per nest (i.e. the lowest and highest daily temperature during d2–d8), which were both highly correlated with the average nest temperature (r > 0.85, Supplementary Fig. S2).
Female can be actively brooding until the nestlings are ca. 6–7 days old36. To account for potential behavioral changes of the female linked to the heating treatment that could influence offspring traits (potentially mask any effect of the heating treatment), we collected data on brooding behavior on d4 after hatching. As in passerines, time spent in brooding decreases with the age of the nestlings37,38, d4 provided us a higher chance to detect difference in brooding behavior. We recorded the percentage of time spent brooding during a minimum of 2 h, using miniature video cameras (ca 4 × 4 cm, DashCam, UK) mounted at ca. 2 m distance from the nest-boxes (Nnon-heated = 16, Nheated = 13). Females brooded ca. 33% of the time, but the variation was large (SD 16%) and there was no significant difference in brooding behavior between the treatments (mean ± SD: non-heated nests 34.7 ± 16.3%; heated nests 36.3 ± 15.8%; t = 0.58, p = 0.57), suggesting that any potential effect of the heating treatment would not be influenced by parental behavior. This data also suggests that during daytime, offspring are exposed to ambient temperatures for substantial periods of time (60% of the time at d4).
Nestling survival was checked at every nest visit (d2, d4, d6, d8, d13). Nestling body mass (~ 0.01 g) was recorded at d2, d8 and d13 after hatching, and tarsus length (~ 0.01 mm, proxy for skeletal growth) at d8 and d13 after hatching. A blood sample (ca. 40 µl) from the brachial vein was collected using heparinized capillaries at d13 after hatching and centrifuged (at 10,000g force for 10 min) before being frozen at − 80 °C. Plasma was used for measuring thyroid hormone concentration (see below). Blood cell pellets were used for extracting DNA to molecularly sex the nestlings and assess mitochondrial density (see ESM for details). At both d8 and d13, another small whole blood sample (ca. 20–30 µl) was collected directly in liquid nitrogen and thereafter stored at − 80 °C to analyze oxidative stress biomarkers.
Plasma T3 and T4 levels were analyzed using nano-LC–MS/MS following previously published methods39 and are expressed as ng/ml. Two randomly picked samples per nest were selected due to logistical constraints. Two oxidative stress biomarkers, (1) oxidative damage to lipids (malondialdehyde, MDA, nmol/mg protein, using TBARS assay) and (2) total glutathione (hereafter tGSH; µmol GSH/mg protein), the most abundant endogenous intracellular antioxidant, were measured using established protocols, with all intra-assay CVs < 10%, following17. For oxidative stress biomarkers, we excluded the nests whose nestlings were not involved in cross-fostering and the numbers of nestlings analyzed were 179 and 155 at d8 and d13, respectively. Mitochondrial density, estimated through relative mitochondrial DNA copy number, and molecular sexing were analyzed using qPCR on all nestlings survived to d13 (n = 185), following methods in23,40. See ESM for details.
Statistical analysis
All statistical models were conducted in the environment of R 3.5.1 (R core team 2018). We ran separate generalized linear mixed models (GLMMs, package lme4 and pbkrtest41,42) for each trait of interest (i.e. fledging success, nestling body masses and tarsus lengths, plasma T3 and T4 concentrations, mtDNA density, blood tGSH and MDA concentrations) to assess the interaction between the treatments of yolk TH injection (TH versus control) and nest-box heating (heated versus non-heated). In the meanwhile, we controlled for relevant covariates and random intercepts to account for potential non-independence among nestlings. The estimates of main factors were always reported from a model without any interactions43. Significance level was set at 0.05. For the models with Gaussian error distribution, the significance test was conducted by using Kenward–Roger approximation on the degrees of freedom42. The final sample sizes varied by traits due to both the logistical constraints (see above) and missing values due to random failures in certain assays. The exact sample sizes and model details are reported in ESM. For fledging success, binomial distribution and logit link function were applied, and the model was fit by maximum likelihood using Laplace approximation. We additionally ran Cox proportional hazards models on nestling survival, which allowed us to take into account the exact ages nestlings died. The results from the Cox models were in agreement with the GLMMs and therefore not redundantly presented. For blood MDA, tGSH, and mtDNA density, data were first ln-transformed, and for all models examined, no clear violation on residual normality was visually detected. We found no indications of sex-dependent effects of THs or temperature treatment for any of the response variables (all F < 1.5, p > 0.2), and thus those are not discussed further. We also tested the effect of the actual next-box temperature by replacing the heating treatment in the models with average, minimum, or maximum nest temperature (see above). These models showed generally consistent results with the ones using heating treatment as a predictor, and therefore were only presented in ESM.
Results and discussion
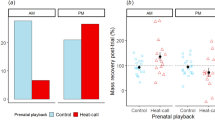
To our knowledge, we present the first experimental study on context-dependent effects of prenatal yolk THs. We found no clear evidence for temperature-dependent effects of elevated yolk THs, mimicking higher maternal transfer, on offspring survival (fledging success, CO-non-heated 69.7% CO-heated 78.0% TH-non-heated 75.9% TH-heated 76.9%, z = 0.156, p = 0.876, Supplementary Table S1). These results suggest that contrary to our predictions: while the minimum nest-box temperature positively predicted nestling fledging success, elevated prenatal THs do not clearly benefit nestlings’ survival in cooler environment (Fig. 1). Egg TH concentrations were previously observed to increase with decreasing ambient temperature in passerines22. If that was an adaptive allocation, we would expect elevated yolk THs to benefit offspring survival under cooler condition, but our results do not provide clear support for such an adaptive explanation.
Effects of minimum nest-box temperature during heating treatment (day 2–8 post-hatching) on nestling fledging success. The fitted logistic curves of TH-nestlings (blue line and dots) and of CO-nestlings (red line and dots) were highly similar and very close to the grand mean (the gold line). Shaded areas represent the 95% confidence interval range.
The growth of offspring (tarsus or body mass) from TH elevated and control eggs was not differentially affected by the postnatal temperature treatments at any of the measured age-points (All F < 1.30, p > 0.26, Fig. 2a, Supplementary Tables S2, S3). Our results therefore suggest that the previously reported discrepancies in the effects of prenatal THs on offspring growth across sister species (collared and pied flycatchers16,17), and other altricial birds18,44 are unlikely driven by context-dependent effects of THs related to early postnatal temperature differences. Yet, there are several possible alternative explanations: (1) the study took place in a relatively warm year, with the average temperature during the nestling phase (June-July) being ca. 2 °C higher than the averages in the past 15 years (2018: + 18.4 °C vs 2003–2018 + 16.5 °C). We may expect that the differential effects of THs on growth to be evident only under relatively cold conditions. (2) Our experimental temperature manipulation was rather small, which could have either been compensated by maternal brooding (though brooding did not seem to differ among the groups) or simply too small to induce measurable changes in growth. In previous studies, ca 5 °C elevation led to a decrease in body mass in blue tits (Cyanistes caeruleus34) and great tits (Parus major45,46) and an increase in body mass in tree swallows (Tachycineta bicolor47); (3) if maternal hormone transfer varies according to environmental context, our egg injection treatment might have resulted in different doses depending on the initial yolk hormone levels. Quantifying egg hormonal content in unmanipulated eggs across experiments, years and contexts is now needed to test this possibility. The logical next steps to test other context-dependent effects of maternal THs would be either to reduce ambient temperature during postnatal development or to manipulate some other environmental factors, such as food availability following similar experimental set-ups.
Effects of prenatal hormone manipulation (TH = experimentally elevated yolk thyroid hormone treatment, CO = control) and postnatal temperature manipulation (non-heated vs. heated nests) on offspring phenotype and physiology. (a) Nestling body mass growth pattern (g, average ± SE); (b) plasma triiodothyronine (T3) concentration (pg/ml, marginal means ± SE), (c) plasma thyroxine (T4) concentration (pg/ml, marginal means ± SE), (d) mitochondrial density in blood cells (ln-transformed, marginal means ± SE), (e) blood total glutathione concentration (tGSH, nmol/mg protein, ln-transformed means ± SE) and (f) lipid peroxidation (MDA concentration, µmol/mg protein, ln-transformed means ± SE). Heated nests were on average 2.75 °C warmer than non-heated ones. See text and ESM for details on statistics and sample sizes.
We aimed to characterize the nestling physiological changes underlying potential context-dependent effects of prenatal THs in response to variation in postnatal ambient temperature. Physiological biomarkers are commonly used as early proxies of responses to environmental variation, and are often sensitive even in cases where effects on growth and (early-life) survival are not visible (e.g.48,49). In contrast to our predictions, we observed no apparent differences in the circulating T3 or T4 concentration in nestlings exposed to higher levels of THs prenatally (T3: F1,28.3 = 1.553 , p = 0.223; T4: F1,34.5 = 1.032, p = 0.317), or in interaction with postnatal temperature treatments (hormone × heating, T3: F1,37.3 = 1.896 , p = 0.177; T4: F1,32.1 = 0.028, p = 0.867, Fig. 2b,c, Supplementary Table S4). Yet both T3 and T4 levels correlated positively with nestling body mass (estimate ± SE, T3: 0.196 ± 0.035, F1.36.5 = 26.9, p < 0.001; T4: 0.563 ± 0.219, F1,33.2 = 5.65, p = 0.023, Supplementary Table S4). Maternal hormones are suggested to cause long-lasting effects on offspring via changes in the function and sensitivity of the corresponding hormonal axis3, so-called organizational effects. Changes in hypothalamus–pituitary–thyroid (HPT)-axis in response to prenatal THs during embryonic development has been characterized in chicken50, pigeons18 as well as mammalian models (reviewed in51). Our results suggest an absence of such long-term programming effects of the HPT-axis in our study system—however it must be noted that circulating TH levels are highly variable in response to internal and external (food, temperature, circadian) variations4,49, which could mask potential organizing effects. Effects of maternal THs might also appear in the interactions with other endocrine axis, such as glucocorticoids52,53 or gonads54,55,56. Experimental challenges with thyrotropin-releasing hormones (TRH) or thyriod-stimulating hormone (TSH) are now needed to test for the effects of prenatal THs on the sensitivity of the HPT-axis in birds.
For the first time, we characterized variation in mitochondrial density in relation to prenatal THs and temperature in birds, and in a wild population. In contrast to our predictions, we did not observe clear context-dependent effects of THs on mtDNA copy number (hormone × heating, F1,155.8 = 0.55, p = 0.458, Fig. 2d, Supplementary Table S5), and also no apparent effect of prenatal TH elevation (F1,31.0 = 0.739, p = 0.397, Supplementary Table S5) or heating treatment (F1,41.5 = 0.003, p = 0.958, Supplementary Table S5) per se. While THs are known to affect mitochondrial biogenesis and function postnatally26,27, only one previous study to our knowledge has investigated the effects of prenatal THs on mitochondrial parameters in a hypothyroid rat model. This study found no clear effects of prenatal THs on mitochondrial traits29. Furthermore, mitochondria are generally responsive to varying (both high and low) ambient temperature (reviewed in28). The lack of effects of our heating experiment may potentially be explained by the timing of the measurements: mitochondrial density was measured 5 days after the heating treatment had ceased, and given that mitochondrial traits are very plastic (as already shown in this species23), the effect of the temperature treatment may have vanished by the time of measurement. Finally, although mitochondria in avian blood cells have been shown moderately correlated with muscle mitochondria57, we cannot rule out that both TH and temperature effects on mitochondrial density could be tissue-specific, and not visible in blood cells.
We predicted that any effects of the treatments on growth, circulating TH levels or mitochondria could lead to oxidative stress, due to the altered production of free radicals, and/or antioxidant defenses. In line with the results above, we found no evidence for context-dependent effects of prenatal THs and postnatal temperature on the endogenous antioxidant glutathione or oxidative damage to lipids at the end of the heating period (d8) or at 13 days of age (all F < 1.61, p > 0.21, Fig. 2e,f, Supplementary Tables S6, S7). These results support our previous findings where elevated prenatal THs did not appear to influence postnatal oxidative stress biomarkers in birds16,17. Yet, effects of prenatal THs on oxidative stress could be tissue-dependent and/or only visible during embryo development, which needs to be further tested.
In conclusion, we found no clear evidence for context-dependent effects of prenatal THs depending on the ambient early postnatal temperature, nor support for the hypothesis that higher TH transfer to eggs in cold conditions benefits offspring in cooler rearing conditions. This seems to suggest that the previously found discrepancy in the effects of elevated yolk THs in collared and pied flycatchers16,17 is more likely due to species difference instead of environment. Nevertheless, more studies examining other environmental factors are still needed before we can disregard the potential context-dependent effects of maternal THs. To that end, our study suggests multiple avenues for further research on the potential context-dependence of maternal effects on offspring phenotype and the potential underlying physiological mechanisms. As maternal effect has been suggested to enable rapid adaptation to climate change by providing an additional source of phenotypic plasticity5,6,7, it is important to understand their context-dependent effects.
Data availability
All data are available in https://figshare.com/articles/dataset/TH_temperature-dependent_effects_data_and_code_zip/12833711.
Change history
17 March 2021
A Correction to this paper has been published: https://doi.org/10.1038/s41598-021-85657-w
References
Moore, M. P., Whiteman, H. H. & Martin, R. A. A mother’s legacy: The strength of maternal effects in animal populations. Ecol. Lett. 22, 1620–1628 (2019).
Yin, J. J., Zhou, M., Lin, Z. R., Li, Q. S. Q. & Zhang, Y. Y. Transgenerational effects benefit offspring across diverse environments: A meta-analysis in plants and animals. Ecol. Lett. 22, 1976–1986 (2019).
Groothuis, T. G. G., Hsu, B.-Y., Kumar, N. & Tschirren, B. Revisiting mechanisms and functions of prenatal hormone-mediated maternal effects using avian species as a model. Philos. Trans. R. Soc. B 374, 20180115 (2019).
Ruuskanen, S. & Hsu, B.-Y. Maternal thyroid hormones: An unexplored mechanism underlying maternal effects in an ecological framework. Physiol. Biochem. Zool. 91, 904–916 (2018).
Meylan, S., Miles, D. B. & Clobert, J. Hormonally mediated maternal effects, individual strategy and global change. Philos. Trans. R. Soc. B 367, 1647–1664 (2012).
Donelson, J. M., Salinas, S., Munday, P. L. & Shama, L. N. S. Transgenerational plasticity and climate change experiments: Where do we go from here?. Glob. Change Biol. 24, 13–34 (2018).
Ruuskanen, S., Hsu, B.-Y. & Nord, A. Endocrinology of thermoregulation of birds in a changing climate. https://doi.org/10.32942/osf.io/jzam3 (2020).
Sheriff, M. J. et al. Integrating ecological and evolutionary context in the study of maternal stress. Integr. Comp. Biol. 57, 437–449 (2017).
Schoech, S. J., Rensel, M. A. & Heiss, R. S. Short- and long-term effects of developmental corticosterone exposure on avian physiology, behavioral phenotype, cognition, and fitness: A review. Curr. Zool. 57, 514–530 (2011).
Love, O. P. & Williams, T. D. The adaptive value of stress-induced phenotypes: Effects of maternally derived corticosterone on sex-biased investment, cost of reproduction, and maternal fitness. Am. Nat. 172, E135–E149 (2008).
Weber, B. M. et al. Pre- and postnatal effects of experimentally manipulated maternal corticosterone on growth, stress reactivity and survival of nestling house wrens. Funct. Ecol. 32, 1995–2007 (2018).
Dantzer, B. et al. Density triggers maternal hormones that increase adaptive offspring growth in a wild mammal. Science 340, 1215–1217 (2013).
Zimmer, C., Boogert, N. J. & Spencer, K. A. Developmental programming: Cumulative effects of increased pre-hatching corticosterone levels and post-hatching unpredictable food availability on physiology and behaviour in adulthood. Horm. Behav. 64, 494–500 (2013).
Muriel, J. et al. Context-dependent effects of yolk androgens on nestling growth and immune function in a multibrooded passerine. J. Evol. Biol. 28, 1476–1488 (2015).
Gil, D. Hormones in avian eggs: Physiology, ecology and behavior. Adv. Study Behav. 38, 337–398 (2008).
Hsu, B.-Y., Doligez, B., Gustafsson, L. & Ruuskanen, S. Transient growth-enhancing effects of elevated maternal thyroid hormones at no apparent oxidative cost during early postnatal period. J. Avian Biol. 50, jav-01919 (2019).
Sarraude, T., Hsu, B.-Y., Groothuis, T. G. G. & Ruuskanen, S. Manipulation of prenatal thyroid hormones does not influence growth or physiology in nestling pied flycatchers. Physiol. Biochem. Zool. 93, 255–266 (2020).
Hsu, B.-Y., Dijkstra, C., Darras, V. M., de Vries, B. & Groothuis, T. G. G. Maternal thyroid hormones enhance hatching success but decrease nestling body mass in the rock pigeon (Columba livia). Gen. Comp. Endocrinol. 240, 174–181 (2017).
Auer, S. K., Salin, K., Rudolf, A. M., Anderson, G. J. & Metcalfe, N. B. The optimal combination of standard metabolic rate and aerobic scope for somatic growth depends on food availability. Funct. Ecol. 29, 479–486 (2015).
McNabb, F. M. A. The hypothalamic–pituitary–thyroid (HPT) axis in birds and its role in bird development and reproduction. Crit. Rev. Toxicol. 37, 163–193 (2007).
Price, E. R. & Dzialowski, E. M. Development of endothermy in birds: Patterns and mechanisms. J. Comp. Physiol. B 188, 373–391 (2018).
Ruuskanen, S. et al. Temperature-induced variation in yolk androgen and thyroid hormone levels in avian eggs. Gen. Comp. Endocrinol. 235, 29–37 (2016).
Stier, A., Bize, P., Hsu, B.-Y. & Ruuskanen, S. Plastic but repeatable: Rapid adjustments of mitochondrial function and density during reproduction in a wild bird species. Biol. Lett. 15, 20190536 (2019).
Salin, K., Auer, S. K., Rey, B., Selman, C. & Metcalfe, N. B. Variation in the link between oxygen consumption and ATP production, and its relevance for animal performance. Proc. R. Soc. B 282, 20151028 (2015).
Lassiter, K., Dridi, S., Greene, E., Kong, B. & Bottje, W. G. Identification of mitochondrial hormone receptors in avian muscle cells. Poult. Sci. 97, 2926–2933 (2018).
Lanni, A., Moreno, M. & Goglia, F. Mitochondrial actions of thyroid hormone. Compr. Physiol. 6, 1591–1607 (2016).
Weitzel, J. M. & Iwen, K. A. Coordination of mitochondrial biogenesis by thyroid hormone. Mol. Cell. Endocrinol. 342, 1–7 (2011).
Clarke, A. & Portner, H. O. Temperature, metabolic power and the evolution of endothermy. Biol. Rev. 85, 703–727 (2010).
Xia, T., Zhang, X., Wang, Y. & Deng, D. Effect of maternal hypothyroidism during pregnancy on insulin resistance, lipid accumulation, and mitochondrial dysfunction in skeletal muscle of fetal rats. Biosci. Rep. 38, BSR20171731 (2018).
Halliwell, B. & Gutteridge, J. M. C. Free Radicals in Biology and Medicine (Oxford University Press, New York, 2015).
Villanueva, I., Alva-Sanchez, C. & Pacheco-Rosado, J. The role of thyroid hormones as inductors of oxidative stress and neurodegeneration. Oxid. Med. Cell. Longev. 2013, 218145 (2013).
Stier, A. et al. Elevation impacts the balance between growth and oxidative stress in coal tits. Oecologia 175, 791–800 (2014).
Stier, A., Massemin, S. & Criscuolo, F. Chronic mitochondrial uncoupling treatment prevents acute cold-induced oxidative stress in birds. J. Comp. Physiol. B 184, 1021–1029 (2014).
Andreasson, F., Nord, A. & Nilsson, J. -Å. Experimentally increased nest temperature affects body temperature, growth and apparent survival in blue tit nestlings. J. Avian Biol. 49, jav-01620 (2018).
Podmokła, E., Drobniak, S. M. & Rutkowska, J. Chicken or egg? Outcomes of experimental manipulations of maternally transmitted hormones depend on administration method—a meta-analysis. Biol. Rev. 93, 1499–1517 (2018).
Lundberg, A. & Alatalo, R. The Pied Flycatcher (Poyser, London, 1992).
Haggerty, T. M. Effects of nestling age and brood size on nestling care in the Bachman’s sparrow (Aimophila aestivalis). Am. Midl. Nat. 128, 115–125 (1992).
Chastel, O. & Kersten, M. Brood size and body condition in the house sparrow Passer domesticus: The influence of brooding behaviour. Ibis 144, 284–292 (2002).
Ruuskanen, S. et al. A new method for measuring thyroid hormones using nano-LC-MS/MS. J. Chromatogr. B 1093–1094, 24–30 (2018).
Chang, H.-W. et al. High-throughput avian molecular sexing by SYBR green-based real-time PCR combined with melting curve analysis. BMC Biotechnol. 8, 12 (2008).
Bates, D., Maechler, M., Bolker, B. & Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48 (2015).
Halekoh, U. & Højsgaard, S. Kenward–Roger approximation and parametric bootstrap methods for tests in linear mixed models—the R package pbkrtest. J. Stat. Softw. 59, 1–32 (2014).
Schielzeth, H. Simple means to improve the interpretability of regression coefficients. Methods Ecol. Evol. 1, 103–113 (2010).
Ruuskanen, S., Darras, V. M., Visser, M. E. & Groothuis, T. G. G. Effects of experimentally manipulated yolk thyroid hormone levels on offspring development in a wild bird species. Horm. Behav. 81, 38–44 (2016).
Rodríguez, S., Diez-Méndez, D. & Barba, E. Negative effects of high temperatures during development on immediate post-fledging survival in great tits Parus major. Acta Ornithol. 51, 235–244 (2016).
Rodríguez, S. & Barba, E. Nestling growth is impaired by heat stress: An experimental study in a Mediterranean great tit population. Zool. Stud. 55, 13 (2016).
Dawson, R. D., Lawrie, C. C. & O’Brien, E. L. The importance of microclimate variation in determining size, growth and survival of avian offspring: Experimental evidence from a cavity nesting passerine. Oecologia 144, 499–507 (2005).
Stier, A., Massemin, S., Zahn, S., Tissier, M. L. & Criscuolo, F. Starting with a handicap: Effects of asynchronous hatching on growth rate, oxidative stress and telomere dynamics in free-living great tits. Oecologia 179, 999–1010 (2015).
Wikelski, M. & Cooke, S. J. Conservation physiology. Trends Ecol. Evol. 21, 38–46 (2006).
Darras, V. M. The role of maternal thyroid hormones in avian embryonic development. Front. Endocrinol. 10, 66 (2019).
Huget-Penner, S. & Feig, D. S. Maternal thyroid disease and its effects on the fetus and perinatal outcomes. Prenat. Diagn. https://doi.org/10.1002/pd.5684 (2020).
Kulkami, S. S. & Buchholz, K. R. Beyond synergy: Corticosterone and thyroid hormone have numerous interaction effects on gene regulation in Xenopus tropicalis tadpoles. Endocrinology 153, 5309–5324 (2012).
Watanabe, Y., Grommern, S. V. H. & de Groef, B. Corticotropin-releasing hormone: Mediator of vertebrate life stage trasitions?. Gen. Comp. Endocrinol. 228, 60–68 (2016).
Sechman, A. The role of thyroid hormones in regulation of chicken ovarian steroidogenesis. Gen. Comp. Endocrinol. 190, 68–75 (2013).
Flood, D. E. K., Fernandino, J. I. & Langlois, V. S. Thyroid hormones in male reproductive develoment: Evidence for direct crosstalk between the androgen and thyroid hormones axes. Gen. Comp. Endocrinol. 192, 2–14 (2013).
Duarte-Guterman, P., Navarro-Martín, L. & Trudeau, V. L. Mechanisms of crosstalk between endocrine systems: Regulation of sex steroid hormone synthesis and action by thyroid hormones. Gen. Comp. Endocrinol. 203, 69–85 (2014).
Stier, A. et al. How to measure mitochondrial function in birds using red blood cells: A case study in the king penguin and perspectives in ecology and evolution. Methods Ecol. Evol. 8, 1172–1182 (2017).
Acknowledgements
We thank all field assistants, especially Lucas Bousseau and Thomas Rosille and Päivi Kotitalo for their great effort, Janina Stauffer for help with laboratory analysis and Tuija Koivisto for analyzing the brooding videos. Turku Biocenter Finland supported the facilities for thyroid hormone measurements.
Funding
The project was funded by an Academy of Finland Grant (# 286278) to SR. BYH was supported by a Grant from The Ella and Georg Ehrnrooth Foundation, TS by a Grant from the University of Groningen, NCS by Erasmus+, Boussole Grand Est and EDUFI (TM-19-11246) Grants, MC by Erasmus+ and Boussole Grand Est Grants, and AS by a ‘Turku Collegium for Science and Medicine’ Fellowship.
Author information
Authors and Affiliations
Contributions
B.Y.H., A.S. and S.R. designed the study and conducted the fieldwork. All authors contributed to data analysis. B.Y.H. conducted the final statistical analysis. A.S., N.C.S., M.C. and S.R. conducted laboratory work. B.Y.H., A.S. and S.R. wrote the manuscript, with input from T.S., M.C. and N.C.S.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hsu, BY., Sarraude, T., Cossin-Sevrin, N. et al. Testing for context-dependent effects of prenatal thyroid hormones on offspring survival and physiology: an experimental temperature manipulation. Sci Rep 10, 14563 (2020). https://doi.org/10.1038/s41598-020-71511-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-71511-y
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.