Abstract
DEAD-box helicase 5 (Ddx5) functions as an ATP-dependent RNA helicase and as a transcriptional coactivator for several transcription factors; however, the developmental function of the ddx5 gene in vertebrates is not fully understood. We found that the zebrafish ddx5 gene was expressed in developing gonads. Using the genome editing technology transcription activator-like effector nuclease, we established a ddx5-disrupted zebrafish and examined the morphological phenotypes of the mutant. We found that the majority of ddx5-deficient mutants developed as fertile males with normal testes and a small number of ddx5-deficient mutants developed as infertile females with small ovaries. Apoptotic cell death at 31 days post fertilization was increased in thick immature gonads (presumptive developing ovaries) of the ddx5-deficient mutant compared to those of heterozygous wild-type fish, while the number of apoptotic cells in thin immature gonads (presumptive developing testes) was comparable between the mutant and wild-type animals. Histological analysis revealed that ovaries of adult ddx5-deficient females had fewer vitellogenic oocytes and a larger number of stage I and II oocytes. The amount of cyclic adenosine monophosphate in the ddx5-deficient ovaries was high compared to that of wild-type ovaries, presumably leading to the mitotic arrest of oocyte maturation. Therefore, the ddx5 gene is dispensable for testis development, but it is essential for female sex differentiation and oocyte maturation in zebrafish.
Similar content being viewed by others
Introduction
Vertebrates exhibit various types of sex determination system, leading to the differentiation of gonads into testis or ovary1. Recent findings have suggested that zebrafish possess a ZZ/ZW sex determination system2,3. However, such a system was lost in most laboratory zebrafish stocks4, presumably utilizing a polygenic sex determination system. Furthermore, various environmental factors, including population density and temperature, can influence the sex proportion in zebrafish5,6. Immature gonads during the sex differentiation period start to differentiate as bipotential juvenile ovaries. Immature gonads in approximately half of the zebrafish population develop into ovaries around 30 days post fertilization (dpf), while the immature gonads in the remaining population develop into testes7. Thus, the degree of apoptosis in immature gonads during the sex differentiation period may be essential for testicular and ovarian differentiation in zebrafish. However, it is not fully understood what kind of genes are involved in sex differentiation in zebrafish.
The DEAD-box helicase (Ddx) family is defined by a conserved DEAD (Asp-Glu-Ala-Asp) motif involved in ATP hydrolysis, and family members also possess several conserved motifs that have in ATPase and helicase activity8. DEAD-box helicases play important roles in various cellular processes, such as the regulation of transcription, RNA processing and ribosome biogenesis9. It is well known that vasa/ddx4 is expressed in the germ cells of organisms from fruit flies to mammals10,11. The vasa/ddx4-disrupted zebrafish developed exclusively as infertile males12, indicating a critical role in gametogenesis. The human DDX5 gene is expressed in spermatogonia13. The ablation of the Ddx5 gene using tamoxifen-inducible Ddx5 knockout male mice results in the rapid loss of spermatogonia14. Thus, the physiological function of ddx5 in other vertebrates is not known.
We found that the zebrafish ddx5 gene was expressed in developing gonads. To examine the loss of function of the ddx5 gene, we disrupted the ddx5 gene in zebrafish with TALEN. The majority of ddx5-deficient mutants developed as fertile males, while a small population of ddx5-deficient mutants developed as infertile females with small ovaries. Such phenotypes are different from those of vasa/ddx4-disrupted zebrafish and Ddx5-disrupted male mice12,14. We found that apoptotic cell death was increased in thick immature gonads (presumptive developing ovaries) of ddx5-deficient mutants at 31 dpf. Furthermore, ovaries of adult ddx5-deficient females predominantly possessed stage I and II oocytes and maintained high cyclic adenosine monophosphate (cAMP) concentrations. These results suggest that the ddx5 gene is essential for sex differentiation and oocyte maturation in zebrafish.
Results
Developmental expression of ddx5 during zebrafish gonad development
Recent accumulating evidence shows that some of Ddx family genes are expressed during gonad development15,16. To examine the developmental expression of the ddx5 gene, we performed whole-mount in situ hybridization (WISH) using several developmental stages of zebrafish embryos and gonads. The ddx5 gene was widely expressed in embryos at 24 h post fertilization (hpf) (Fig. 1a,b). ddx5 expression at 20 dpf was weakly detected in immature gonads (Fig. 1c) and strongly detected in thin and thick immature gonads at 31 dpf, which presumably progress towards initial testicular and ovarian differentiation, respectively (Fig. 1d–f). The ddx5 gene was significantly expressed in stage I and II oocytes of adult ovaries, while ddx5 expression was weakly detected in whole testes at 5 months post fertilization (mpf) (Fig. 1g,h). Thus, these results indicate that the ddx5 gene is predominantly expressed in the immature gonads at 31 dpf and in the early differentiation stages of the ovary at 5 mpf.
Developmental expression of the ddx5 gene in zebrafish gonads. The expression of ddx5 was examined by whole-mount in situ hybridization (WISH) using sense ddx5 RNA probes (b, f) and antisense ddx5 RNA probes (a, c–e, g, h). (a, b) 24 hpf embryos. (c) 20 dpf immature gonad. (d, f) 31 dpf thin immature gonads. (e) 31 dpf thick immature gonad. (g) 5 mpf testis. (h) 5 mpf ovary. ddx5 expression was widely detected in 24 hpf embryos (a) and weakly detected in 20 dpf immature gonads (c). The ddx5 gene was strongly expressed in thin and thick immature gonads at 31 dpf (d, e). The ddx5 expression is strongly detected in stage I and II oocytes (h), while the ddx5 gene is weakly expressed in whole testes at 5 mpf (g). Scale bar 200 μm.
Sex distribution and fertility of ddx5-deficient zebrafish
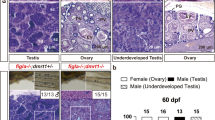
To establish the ddx5-disrupted zebrafish line, ddx5-TALEN constructs were injected into one-cell stage zebrafish embryo, and the F0 embryos were raised to adulthood. The ddx5 mutant allele uy210, which had a total deletion of 5 base pairs (bp), was isolated (***Supplemental Fig. S1–Fig. S3). The Ddx5 mutant protein was functionally disrupted because the mutant did not possess most functional domains, such as the Q motif, helicase ATP-binding domain and transactivation domain (Supplemental Fig. S1). We could not observe any apparent embryonic abnormality in the ddx5-deficient embryos at 5 dpf (Supplemental Fig. S4). Because ddx5 expression is detected in immature gonads at 31 dpf and in adult oocytes and testes at 5 mpf, we examined the sex distribution and fertility in ddx5-deficient adult fish. Fertility was determined by mating individual ddx5-deficient fish with three mature wild-type fish. The ddx5-deficient males were fertile, whereas the ddx5-deficient females were infertile (Table 1). We determined the genotype of adult fish and examined the gonad morphology of the progeny of the ddx5−/− males and ddx5+/− females cross. The homozygous ddx5−/− fish at 5 mpf were predominantly males with testes (77 males and 10 females), while the sex ratio of heterozygous ddx5+/− fish containing the wild-type allele was almost evenly balanced (52 males and 60 females) (Fig. 2). These results suggest that the ddx5 gene is involved in sex differentiation and ovarian development. Therefore, we focused on the loss of function analysis of the ddx5 gene in immature gonads and during oocyte maturation.
Disruption of the ddx5−/− gene causes abnormal sex ratio. Homozygous ddx5−/− male mutants (5 mpf) crossed heterozygous ddx5+/− females (5 mpf), and the sex of growing adult fish was determined by anatomical features (testis or ovary). Total number of progeny: n = 199. Genomic DNA was prepared from individual caudal fins, and the genotype of each fish was determined by genomic PCR using the ddx5 locus-specific primers. The number of heterozygous wild-type animals: n = 112. The number of homozygous mutant animals: n = 87.
Proliferating and dying cells in the immature gonads of ddx5-deficient zebrafish
The maintenance of a sufficient number of germ cells is required for female sex determination in zebrafish. Thus, apoptosis is involved in zebrafish sex differentiation17. We observed thick immature gonads (presumptive developing ovaries) and thin immature gonads (presumptive developing testes) in wild-type fish at 31 dpf (Fig. 3). In the ddx5-deficient gonads, there were thick immature gonads and thin immature gonads at 31 dpf. Thus, the immature gonads in wild-type and the mutant at 31 dpf are similar in appearance.
We examined the number of proliferating and dying cells in immature gonads of ddx5-deficient fish and wild-type fish at 31 dpf. TUNEL analysis revealed that apoptotic cells increased in number in thick immature gonads of ddx5-deficient fish compared to those of wild-type fish (Fig. 4), while the number of apoptotic cells between wild-type and ddx5-deficient thin immature gonads was comparable. The number of proliferating cells marked by anti-phosphorylated histone H3 immunostaining was comparable in the mutant and wild-type immature gonads (Supplemental Fig. S5).
Dying cells in immature gonads of ddx5-deficient fish. (a–f) TUNEL analysis. (a, b) Wild-type. (c, d) ddx5−/− mutant. (a, c, e) Thick immature gonads. (b, d, f) Thin immature gonads. Scale bar 200 μm. (e) The number of apoptotic cells in wild-type (e; n = 12, total 94 positive cells) (f; n = 4, total 113 positive cells) and ddx5−/− mutants (e; n = 7, total 538 positive cells) (f; n = 7, total 160 positive cells) were counted. Error bars indicate standard deviation. Asterisks indicate statistical significance between wild-type and mutant samples (by t test). ***P < 0.001. ns not significant.
ddx5-deficient ovaries exhibited oocyte maturation defects and maintained high cAMP concentrations
Morphologies of ddx5-deficient gonads were examined. We found that the ddx5-deficient female fish had small ovaries at 90 dpf and 5 mpf (Fig. 5, Supplemental Fig. S6). Wild-type ovaries contained various stages18, and most were in intermediate stages II and III, while few were in early stage I and late stage IV–V; however, the ovaries of ddx5-deficient females possessed high amounts of stage I and II oocytes and few stage III–V oocytes (Fig. 6). Distribution of oocyte stages was different between wild-type and ddx5-mutant (P < 0.001 for both wild-type and ddx5-deficient oocytes; Chi square test). In contrast, the testes of ddx5-deficient males had morphology that was similar to that of wild-type males (Fig. 5). Histological analysis confirmed that the ovaries of ddx5-deficient fish predominantly possessed stage I and II oocytes and few stage III-V oocytes, while the testes containing sperm were comparable between the mutant and wild-type fish (Fig. 7).
Homozygous adult ddx5−/− females had small ovaries containing immature oocytes. (a, b, e, f) ddx5+/− wild-type at 5 mpf. (c, d, g, h) The ddx5−/− mutants at 5 mpf. The ovaries of ddx5-deficient females predominantly possessed stage I and II embryos, while various ovarian stages, from I to IV, were observed in wild-type females. The testes of wild-type and ddx5-deficient fish were similar. Genotyping of individual fish was performed by genomic PCR. (a) Scale bar 5 mm. (b) Scale bar 1 mm.
Distribution of the ddx5-deficient oocytes classified by oocyte maturation. Ovarian follicles from wild-type (n = 5) and mutant animals (n = 5) at 5 mpf were classified by diameter (stage I; 7–140 μm, stage II; 140–340 μm, stage III; 340–690 μm, stage IV, V; 690–750 μm). Fifty ovarian follicles from individual ovaries represent the proportion of oocyte stages. Error bars indicate standard deviation.
Hematoxylin and eosin (HE) staining of gonadal sections. (a, b) ddx5+/− wild-type gonads. (c, d) the ddx5−/− mutant gonads. (a, c) Wild-type ovaries possessed stage I–IV oocytes, whereas ddx5-deficient ovaries predominantly contained stage I and II oocytes. (b, d) Both wild-type and ddx5-deficient testes contained differentiating germ cells. Scale bar 200 μm.
A high concentration of cyclic AMP (cAMP) in fish oocytes is required for maintaining meiotic arrest19,20. We examined the cAMP concentration in wild-type and ddx5-deficient ovaries. The concentration of cAMP was higher in ddx5-deficient ovaries than it was in wild-type ovaries (Fig. 8). These results suggest that high amounts of stage I and II oocytes are present due to mitotic arrest that was mediated by a maintained high concentration of cAMP.
cAMP concentration relative to the wild-type ovaries. The concentration of cAMP in each ovary (wild-type: n = 4, ddx5−/− mutant: n = 4,) was determined with a cAMP ELISA kit. Error bars indicate standard deviation. Asterisks indicate statistical significance between wild-type and the mutant levels (by t test). *P < 0.05.
Discussion
In this study, we first reported the loss of function of the ddx5 gene in zebrafish. We have demonstrated that the majority of ddx5-deficient mutants develop as fertile males with normal testes, whereas a few ddx5-deficient fish develop into infertile females with aberrant small ovaries (Figs. 2, 5; Table 1). This phenotype was quite different from the failure of Ddx5-disrupted male mice on spermatogenesis14.
The expression of ddx5 was widespread in whole embryos and was not specifically detected in the primordial germ cells at 24 hpf (Fig. 1). ddx5 expression at 24 hpf was different from the germ cell-specific expression of the vasa/ddx4 and nanos1 genes21,22. We found that the ddx5 gene was expressed in developing gonads at 20 dpf. Although ddx5 expression was detected in thin immature gonads (presumptive developing testes) and thick immature gonads (presumptive developing ovaries) at 31 dpf, it was found to have much stronger expression in adult ovaries compared to adult testes. Therefore, ddx5 expression gradually decreased in the developing testis compared to the developing ovary, suggesting important roles of the ddx5 gene in ovarian development.
Recent studies have shown that a threshold number of immature gonads is required for the progression of ovarian fate in zebrafish and mice17,23,24. Induction of apoptosis in the gonad is important for testis development. In fact, Fancl is a member of the Fanconi anemia/BRCA DNA repair pathway, and homozygous zebrafish fancl mutants exclusively develop as fertile males25. The abnormal sex ratio phenotype in the fancl mutant is caused by abnormally increased apoptosis in immature gonads. We observed that most ddx5-deficient mutants developed as fertile males (Fig. 2). We found that thick immature gonads (presumably developing ovaries) in ddx5-deficient fish were accompanied by an abnormal increase in apoptosis compared to wild-type fish during the sex differentiation period (Fig. 4). Therefore, increased apoptosis in the developing gonad provides a cellular mechanism for abnormal sex ratio phenotype of ddx5-deficient zebrafish.
During gonad maturation stages, the ddx5-deficient mutant had morphologically similar testis (Fig. 5) and fertilization activity (Table 1). In clear contrast, the ovaries of ddx5-deficient fish possessed an abundance of stage I and II oocytes, raising the possibility of mitotic arrest during oocyte maturation. It is not clear why a small number of gonads can develop the ovaries. Histological analysis confirmed that the ovaries of adult ddx5-deficient females had fewer vitellogenic oocytes and a high number of stage I and II stage oocytes (Fig. 7). Thus, the ddx5 gene is not required for initial oocyte maturation, but is necessary for vitellogenic oocytes. In most of fish oocytes, elevated intraoocyte cAMP maintains protein kinase A (PKA) in an active state that ascertains cell cycle arrest19. Thus, a decrease in cAMP concentration in oocytes is necessary for the resumption of meiosis. The concentration of cAMP was high in ddx5-deficient ovaries compared to wild-type ovaries (Fig. 8). A high concentration of cAMP contributes to the abundance of stage I and II oocytes. Another possibility is that the loss of the ddx5 gene causes oocyte arrest unrelated to the regulation of cAMP levels and oocyte arrest in the ddx5-deficient mutant results in high cAMP concentration. In summary, the ddx5 gene is dispensable for testis development but indispensable for oocyte maturation in zebrafish.
Methods
Whole-mount in situ hybridization (WISH)
The accession number of zebrafish ddx5 gene is LC565489. The expression of ddx5 was examined by WISH as previously described26. Zebrafish embryos and gonads were hybridized with the digoxygenin (DIG)-labelled RNA probe at room temperature for overnight. After three time washing with PBS containing 0.1% Tween-20 (PBST), the samples were incubated with alkaline phosphatase-conjugated anti-DIG antibody. After three time washing with PBST, the samples were incubated with BM Purple (Roche) as the substrate to visualize the RNA probe recognized by the anti-DIG antibody. After three time washing with PBST, the samples were fixed in 4% paraformaldehyde.
Construction of TALEN plasmids and microinjection of TALEN mRNA
The plasmids for synthesizing TALEN mRNAs were constructed with a two-step assembly system, as described previously27. Initially, six or fewer TAL effector repeat modules were ligated into pFUS vectors. Intermediate array vectors and last TAL effector repeat were then ligated into a pCS2TAL3DDD vector to generate a forward TALEN or a pCS2TAL3RRR vector to generate a reverse TALEN28. The amino acid sequences of the constructed TALENs for ddx5 are shown in Supplementary Table S2.
The plasmids used for synthesizing TALEN mRNAs were linearized by NotI digestion, and mRNAs were transcribed using a mMESSAGE mMACHINE SP6 kit (Life Technologies) and purified using an RNeasy Mini Kit (QIAGEN). Forward and reverse TALEN mRNAs (400 pg each) were simultaneously injected into zebrafish blastomeres at the one-cell stage of embryonic development.
Genotyping for the ddx5 locus and genomic sequencing
To prepare genomic DNA, the embryos and tissues at the indicated stages were incubated in 108 μl of 50 mM NaOH at 98 °C for 10 min. Subsequently, 12 μl of 1 M Tris–HCl (pH 8.0) was added to the solution29. Genomic fragments at the targeted sites were amplified by PCR with PrimeTaq (Primetech), and the locus-specific primers are listed in Supplementary Table S2. PCR conditions were as follows: 40 cycles of 98 °C for 10 s, 55 °C for 30 s and 72 °C for 30 s. To perform the heteroduplex mobility assay (HMA), the resultant PCR amplicons were electrophoresed on a 12.5% polyacrylamide gel29. To confirm individual mutations, genomic fragments for the targeted genomic locus were amplified from 1 μl of the solution using PCR (Supplementary Table S2). The resultant PCR fragments were subcloned into the pGEM-T Easy vector (Promega) and genomic sequences were determined by sequence analysis.
Histological analysis
Embryos were dehydrated in 80% ethanol and embedded using a Technovit kit (Kulzer)30. Embedded embryos were sectioned on a Leica RM2125 microtome at 7 μm and mounted on slides. Embryos were stained with hematoxylin–eosin (HE) after sectioning.
Detection of dying and proliferating cells
To detect proliferating cells, gonads at 31 dpf were incubated with anti-phospho-histone H3 antibody (1/500 dilution) (Upstate, #06-570) in PBST containing sheep serum (10%) at 28 °C overnighty31. After three times washes with PBST, the embryos were incubated with Alexa Fluor 594 goat anti-rabbit IgG (1/500 dilution) (Invitrogen) in PBST containing sheep serum (10%) at 25 °C for 4 h. After three times washes with PBST, the proliferating cells were observed by fluorescence stereomicroscopy. We counted the red, rounded signals as proliferating cells and the other signals as negative cells.
Fixed gonads were dehydrated and treated with proteinase K (10 ng/μl) in PBST for 5 min. After three times washes with PBST, the gonads were incubated with TdT reaction cocktail (400 μl/sample) (Invitrogen) for 60 min at 37 °C. Then they were added to Click-iT reaction cocktail (400 μl/sample), where they were incubated for 30 min at 25 °C, in the dark. Apoptotic cells labelled with Alexa Fluor 594 were observed by fluorescence stereomicroscopy. We counted the red, rounded signals as apoptotic cells and the other signals as negative cells.
Measurement of cAMP concentration
Cell extract was prepared from 5 mpf ovaries of wild-type and ddx5-deficient fish. The concentration of cAMP in each sample was analyzed with a cAMP ELISA kit (Caymen Chemical Company) following the manufacturer’s instructions.
Ethics statement
All animal experiments were performed in accordance with the animal protocol approved by the Institutional Animal Care and Use Committee (IACUC) and the ethics committee of the University of Yamanashi. The IACUC and the ethics committee of the University of Yamanashi approved this study (Approval Identification Number: A30-25).
References
Santos, D., Luzio, A. & Coimbra, A. M. Zebrafish sex differentiation and gonad development: A review on the impact of environmental factors. Aquat. Toxicol. 191, 141–163 (2017).
Wilson, C. A. et al. Wild sex in zebrafish: Loss of the natural sex determinant in domesticated strains. Genetics 198, 1291–1308 (2014).
Kossack, M. E. & Draper, B. W. Genetic regulation of sex determination and maintenance in zebrafish (Danio rerio). Curr. Top Dev. Biol. 134, 119–149 (2019).
Liew, W. C. et al. Polygenic sex determination system in zebrafish. PLoS One 7, e34397 (2012).
Uchida, D., Yamashita, M., Kitano, T. & Iguchi, T. An aromatase inhibitor or high water temperature induce oocyte apoptosis and depletion of P450 aromatase activity in the gonads of genetic female zebrafish during sex-reversal. Comp. Biochem. Physiol. A 137, 11–20 (2004).
Ribas, L., Valdivieso, A., Dıáz, N. & Piferrer, F. Appropriate rearing density in domesticated zebrafish to avoid masculinization: Links with the stress response. J. Exp. Biol. 220, 1056–1064 (2017).
Sun, D. et al. Sox9-related signaling controls zebrafish juvenile ovary-testis transformation. Cell Death Dis. 4, e930 (2013).
Linder, P. Dead-box proteins: A family affair-active and passive players in RNP-remodeling. Nucleic Acids Res. 34, 4168–4180 (2006).
Cordin, O., Banroques, J., Tanner, N. K. & Linder, P. The DEAD-box protein family of RNA helicases. Gene 367, 17–37 (2006).
Hay, B., Jan, L. Y. & Jan, Y. N. A protein component of Drosophila polar granules is encoded by vasa and has extensive sequence similarity to ATP-dependent helicases. Cell 18, 577–587 (1988).
Castrillon, D. H., Quade, B. J., Wang, T. Y., Quigley, C. & Crum, C. P. The human VASA gene is specifically expressed in the germ cell lineage. Proc. Natl. Acad. Sci. USA 97, 9585–9590 (2000).
Hartung, O., Forbes, M. M. & Marlow, F. L. Zebrafish vasa is required for germ-cell differentiation and maintenance. Mol. Reprod. Dev. 81, 946–961 (2014).
Neuhaus, N. et al. Single-cell gene expression analysis reveals diversity among human spermatogonia. Mol. Hum. Reprod. 23, 79–90 (2017).
Legrand, J. M. D. et al. DDX5 plays essential transcriptional and post-transcriptional roles in the maintenance and function of spermatogonia. Nat. Commun. 10, 2278 (2019).
Tsai-Morris, C. H., Sheng, Y., Lee, E., Lei, K. J. & Dufau, M. L. Gonadotropin-regulated testicular RNA helicase (GRTH/Ddx25) is essential for spermatid development and completion of spermatogenesis. Proc. Natl. Acad. Sci. USA 101, 6373–6378 (2004).
Tian, C. et al. DExD/H-box RNA helicase genes are differentially expressed between males and females during the critical period of male sex differentiation in channel catfish. Comp. Biochem. Physiol. D 22, 109–119 (2017).
Uchida, D., Yamashita, M., Kitano, T. & Iguchi, T. Oocyte apoptosis during the transition from ovary-like tissue to testes during sex differentiation of juvenile zebrafish. J. Exp. Biol. 205, 711–718 (2002).
Selman, K., Wallace, R. A., Sarka, A. & Qi, X. Stages of oocyte development in the zebrafish, brachydanio rerio. J. Morphol. 218, 203–224 (1993).
Conti, M. et al. Role of cyclic nucleotide signaling in oocyte maturation. J. Mol. Endocrinol. 187, 153–159 (2002).
Maitra, S. et al. High cAMP attenuation of insulin-stimulated meiotic G2–M1 transition in zebrafish oocytes: Interaction between the cAMP-dependent protein kinase (PKA) and the MAPK3/1 pathways. J. Mol. Endocriol. 393, 109–119 (2014).
Yoon, C., Kawakami, K. & Hopkins, N. Zebrafish vasa homologue RNA is localized to the cleavage planes of 2- and 4-cell-stage embryos and is expressed in the primordial germ cells. Development. 124, 3157–3166 (1997).
Köprunner, M., Thisse, C., Thisse, B. & Raz, E. A zebrafish nanos-related gene is essential for the development of primordial germ cells. Genes Dev. 15, 2877–2885 (2001).
Pepling, M. E. & Spradling, A. C. Mouse ovarian germ cell cysts undergo programmed breakdown to form primordial follicles. Dev. Biol. 234, 339–351 (2001).
Tzung, et al. Early depletion of primordial germ cells in zebrafish promotes testis formation. Stem Cell Rep. 4, 61–73 (2015).
Rodríguez-Marí, A. et al. Sex reversal in zebrafish fancl mutants is caused by tp53-mediated germ cell apoptosis. PLoS Genet. 6, e1001034 (2010).
Kawahara, A., Chien, C. B. & Dawid, I. B. The homeobox gene mbx is involved in eye and tectum development. Dev. Biol. 248, 107–117 (2002).
Hisano, Y. et al. Maternal and zygotic sphingosine kinase 2 are indispensable for cardiac development in zebrafish. J. Biol. Chem. 290, 14841–14851 (2015).
Dahlem, T. J. et al. Simple methods for generating and detecting locus-specific mutations induced with TALENs in the zebrafish genome. PLoS Genet. 8, e1002861 (2012).
Kotani, H., Taimatsu, K., Ohga, R., Ota, S. & Kawahara, A. Efficient multiple genome modifications induced by the crRNAs, tracrRNA and Cas9 protein complex in zebrafish. PLoS One 10, e0128319 (2015).
Suzuki, H. et al. Characterization of biklf/klf17-deficient zebrafish in posterior lateral line neuromast and hatching gland development. Sci. Rep. 9, 13680 (2019).
Yanagi, K., Sone, R., Ohga, R. & Kawahara, A. Involvement of the centrosomal protein 55 (cep55) gene in zebrafish head formation. Genes Cells. 24, 642–649 (2019).
Acknowledgements
We would like to thank A. Nagase for zebrafish maintenance and T. Saigoh for helpful discussion. This work was supported by the Japan Society for the Promotion of Science (A.K.) and the Takeda Science Foundation (A.K.).
Author information
Authors and Affiliations
Contributions
A.K. conceived and designed the work and wrote the manuscript. R.S., K.T., R.O. and A.K. performed the experiments. R.S., T.N., M.T. and A.K. conducted the methodology. All authors performed the data analysis and reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sone, R., Taimatsu, K., Ohga, R. et al. Critical roles of the ddx5 gene in zebrafish sex differentiation and oocyte maturation. Sci Rep 10, 14157 (2020). https://doi.org/10.1038/s41598-020-71143-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-71143-2
This article is cited by
-
Role of the DEAD-box RNA helicase DDX5 (p68) in cancer DNA repair, immune suppression, cancer metabolic control, virus infection promotion, and human microbiome (microbiota) negative influence
Journal of Experimental & Clinical Cancer Research (2023)
-
Sexual determination in zebrafish
Cellular and Molecular Life Sciences (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.