Abstract
Necrosis- and ethylene-inducing-like proteins (NLPs) are secreted by fungi, oomycetes and bacteria. Conserved nlp peptides derived from NLPs are recognized as pathogen-associated molecular patterns (PAMPs), leading to PAMP-triggered immune responses. RLP23 is the receptor of the nlp peptides in Arabidopsis thaliana; however, its actual contribution to plant immunity is unclear. Here, we report that RLP23 is required for Arabidopsis immunity against the necrotrophic fungal pathogen Botrytis cinerea. Arabidopsis rlp23 mutants exhibited enhanced susceptibility to B. cinerea compared with the wild-type plants. Notably, microscopic observation of the B. cinerea infection behaviour indicated the involvement of RLP23 in pre-invasive resistance to the pathogen. B. cinerea carried two NLP genes, BcNEP1 and BcNEP2; BcNEP1 was expressed preferentially before/during invasion into Arabidopsis, whereas BcNEP2 was expressed at the late phase of infection. Importantly, the nlp peptides derived from both BcNEP1 and BcNEP2 induced the production of reactive oxygen species in an RLP23-dependent manner. In contrast, another necrotrophic fungus Alternaria brassicicola did not express the NLP gene in the early infection phase and exhibited no enhanced virulence in the rlp23 mutants. Collectively, these results strongly suggest that RLP23 contributes to Arabidopsis pre-invasive resistance to B. cinerea via NLP recognition at the early infection phase.
Similar content being viewed by others
Introduction
Plants activate immunity against pathogenic microorganisms through their perception of pathogen-associated molecular patterns (PAMPs), for protection against pathogen infection1. Many types of PAMPs have been reported, such as flg22 (derived from bacterial flagellin), elf18 (derived from the bacterial elongation factor-Tu), chitin (derived from fungal cell wall), and the nlp peptides derived from secreted proteins termed necrosis- and ethylene-inducing-like proteins (NLPs), conserved in a broad range of fungi, bacteria and oomycetes2,3,4,5,6. PAMPs are recognized by corresponding pattern-recognition receptors (PRRs) localized on the plasma membrane of plant cells1. For instance, the flg22 is recognized by a leucine-rich repeat receptor-like kinase (LRR-RK) termed FLAGELLIN SENSITIVE 2 (FLS2)3. FLS2 interacts with its co-receptor, BRASSINOSTEROID INSENSITIVE 1-ASSOCIATED KINASE 1 (BAK1), and the two factors trans-phosphorylate each other after perception of flg227,8. A series of phosphorylation events lead to the subsequent activation of PAMP-triggered immune responses, such as reactive oxygen species (ROS) burst, mitogen-activated protein kinase (MAPK) activation and callose deposition9. Regarding the limited PRR genes, it was reported that the deletion of a single of these genes reduces the resistance against particular pathogens. For example, Arabidopsis fls2 mutants are more susceptible to the bacterial pathogen Pseudomonas syringae pv. tomato DC3000, as assessed via spray-inoculation of the fls2 mutants with a suspension of the pathogen10. The Arabidopsis CHITIN ELICITOR RECEPTOR KINASE 1 (CERK1) is the lysin motif receptor-like kinase (LysM-RK) for chitin, and cerk1 mutants exhibit enhanced susceptibility to the necrotrophic fungal pathogen Alternaria brassicicola5.
The nlp peptides were identified as a PAMP derived from NLPs2,6. The first purified NLP protein, NEP1, was originally isolated from culture filtrates of Fusarium oxysporum f. sp. erythroxyli, which is the fungus that causes vascular wilt disease in Erythroxylum coca11. NEP1 induces necrosis and the production of the plant hormone ethylene in plants. It was also reported that NLPs, including NEP1, induce necrosis in eudicot, but not monocot, plants11,12,13. Although many NLPs are cytotoxic to plants, some non-cytotoxic NLPs have been identified in fungi and oomycetes in recent years14,15,16. Subsequent studies revealed that NLPs are categorized into three distinct types, i.e., types I, II and III17,18,19. Type I NLPs are the most widely conserved NLPs. They are present in fungi, bacteria and oomycetes. In contrast, type II NLPs are mainly found in bacteria and fungi, and very few are observed in oomycetes. Type III NLPs are mainly observed only in a limited number of ascomycete species19. Subsequent structural analysis of an NLP from the phytopathogenic oomycete Phytium aphanidermatum revealed that NLPs have two opposing antiparallel β-sheets, called β-sandwich, in the central part of the protein, similar to lectins in fungi and actinoporins in sea anemones20. Moreover, it was recently reported that NLPs specifically bind to glycosyl inositol phosphoryl ceramide (GIPC)21, as actinoporins bind to sphingomyelin. Although GIPC is the most abundant class of sphingolipids in plants, GIPCs also occur in fungi and protozoa22. GIPC is composed of inositol phosphoceramide and is anchored in the membrane23. It is speculated that the conformational changes of GIPC–NLP complexes induce pore formation and lead to cell death21,24.
Importantly, two independent articles reported that the amino acids that are conserved in the central part of NLPs (a 20-amino-acid pattern termed nlp20 and a 24-amino-acid pattern termed nlp24) act as a PAMP. The nlp24 peptide contains two conserved regions; conserved region I starts with the AIMY sequence of amino acids, which are highly conserved in type I NLPs, whereas conserved region II starts with the heptapeptide motif GHRHDWE, which is highly conserved in all NLPs6,18. Treatment with the synthetic nlp20/nlp24 peptide induces defence responses, including ethylene production, in A. thaliana2,6. The recognition of nlp20 is also observed in other plants of the Brassicaceae family, i.e., Arabis alpina, Thlaspi arvense and Draba rigida, and the Asteraceae family, i.e., Lactuca sativa2. In contrast, it was reported that cucurbits recognize the C-terminal amino acids of NLPs25. Recently, an LRR-receptor protein (LRR-RP) termed RLP23 was identified as the receptor for nlp20 in A. thaliana26. It was also reported that RLP23 constitutively interacts with the LRR-RK called SUPPRESSOR of BIR1-1 (SOBIR1) and forms a complex with BAK1 after nlp20 perception. The complex triggers the subsequent activation of the nlp peptide-induced immunity26,27,28.
However, the actual contribution of RLP23 to plant immunity remains unclear. Here, we report that RLP23 was required for Arabidopsis immunity against the necrotrophic fungal pathogen Botrytis cinerea, which is the causal agent of grey mould. Microscopic observation revealed that the invasion ratio of B. cinerea was higher in Arabidopsis rlp23 mutants compared with wild-type (WT) plants, suggesting the involvement of RLP23 in pre-invasive immunity, which inhibits pathogen invasion. B. cinerea carries two NLP genes termed BcNEP1 and BcNEP2. In the interaction with Arabidopsis, BcNEP1 was preferentially expressed before/during pathogen invasion, whereas BcNEP2 was expressed at the late infection phase. We also discovered that the nlp peptides derived from BcNEP1 and BcNEP2 activated the PAMP-triggered immune response in an RLP23-dependent manner. Together with further studies on A. brassicicola, these results strongly suggest that NLP perception via RLP23 in the early infection phase contributes to Arabidopsis immunity against B. cinerea.
Results
Arabidopsis rlp23 mutants show enhanced susceptibility to the necrotrophic pathogen Botrytis cinerea
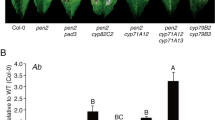
Arabidopsis rlp23 mutants were inoculated with the Botrytis cinerea IuRy-1 strain via dropping of a conidial suspension of the pathogen. We found that the Arabidopsis rlp23-1 plants showed enhanced susceptibility to B. cinerea at 7 days post-inoculation (7 dpi) (Fig. 1A); enhanced susceptibility was also observed in Arabidopsis rlp23-2 plants (Fig. 1A). Lesion size was significantly increased in the rlp23-1 and rlp23-2 mutants compared with the parental WT plant (Col-0) (Fig. 1B). A quantitative analysis of the growth of B. cinerea in planta also supported this finding. The amount of B. cinerea in inoculated Arabidopsis plants was quantified by quantitative PCR (qPCR) of the B. cinerea cutinase A gene (BcCutA) at 6, 12 and 16 h post-inoculation (hpi)29. The expression of BcCutA increased gradually in the inoculated Arabidopsis plants from 6 to 16 hpi (Fig. 1C). Importantly, the amount of the amplified BcCutA at 12 hpi in the rlp23-2 mutant was significantly higher than that detected in the WT plants (Fig. 1C). These results suggest that RLP23 contributes to Arabidopsis immunity against the necrotrophic fungal pathogen B. cinerea.
The Arabidopsis rlp23 mutations enhanced the susceptibility to B. cinerea. 4–5-week-old plants were inoculated with 5 µl of conidial suspensions (1 × 105 conidia/mL) of B. cinerea. (A) Lesion development on each mutant. The photograph was acquired at 7 dpi. (B) Lesion areas were measured at 7 dpi. At least 24 lesions were measured from each line. The statistical significance of differences in lesion size was determined by Tukey’s honestly significant difference (HSD) test (**P < 0.01). The experiment was repeated three times, with similar results. (C) Growth of B. cinerea in planta based on qPCR using B. cinerea- and Arabidopsis-specific primers. BcCutA was quantified by qPCR in the extracted genomic DNA. The Arabidopsis ⍺-shaggy kinase gene (AtASK) was used as a reference. Means and SDs were calculated from three independent samples. The statistical analysis was conducted using two-tailed Student’s t-tests. The level of BcCutA was compared at the same time points between Col-0 and the rlp23-2 mutant plants (*P < 0.05).
The Arabidopsis rlp23 mutant has a defect in pre-invasive resistance against B. cinerea
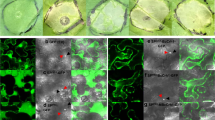
To identify the pathogen infection steps that are affected by the loss of RLP23 function, we performed microscopic observation of the B. cinerea infection behaviour in Arabidopsis plants. At 6 hpi, most conidia had germinated and started to elongate germ tubes, but did not develop invasive hyphae in WT Col-0 or the rlp23-2 mutant. At 12 hpi, some of the germinating conidia exhibited invasive hyphae inside plants (Fig. 2A). Specifically, about 25% of germinating conidia in WT plants displayed invasive hyphae at 12 hpi (Fig. 2B). In contrast, about 45% of germinating conidia in the rlp23-2 mutant showed invasive hyphae at the same time point (Fig. 2B). Thus, the invasion ratio of B. cinerea was clearly higher in the rlp23-2 mutant compared with WT plants at 12 hpi. Considering the higher amount of B. cinerea detected at 12 hpi in the rlp23-2 mutant compared with the wild type (Fig. 1C), these results suggest that the enhanced susceptibility to B. cinerea observed in the Arabidopsis rlp23 mutants is at least partially attributable to the reduction in pre-invasive resistance against the pathogen.
Increased invasion ratio in Arabidopsis rlp23-2 mutants vs. Col-0 plants at 12 hpi. 4–5-week-old plants were drop-inoculated with B. cinerea (1 × 105 conidia/mL) and collected at each time point. The inoculated leaves were stained with trypan blue solution and the B. cinerea hyphae were observed under a light microscope. (A) Arabidopsis Col-0 plants were inoculated with B. cinerea and collected at 12 hpi. The image on the left is focused on conidium and the image on the right is focused on invasive hypha. Bars, 20 µm. c conidium, gt germ tube, ih invasive hypha. (B) Inoculated leaves were collected at each time point and stained with trypan blue solution. At least 100 appressoria were investigated to determine whether they had developed invasive hyphae. The means and SDs were calculated from three independent samples. The statistical analysis was conducted using two-tailed Student’s t-tests. The invasion ratio was compared at the same time points between Col-0 and the rlp23-2 mutant plants (**P < 0.01). The experiment was repeated twice, with similar results.
nlp peptides derived from two types of B. cinerea NLPs induce Arabidopsis immunity in an RLP23-dependent manner
Because RLP23 is required for the perception of nlp peptides derived from NLPs and the subsequent activation of immunity26, next we investigated whether the reduced immunity against B. cinerea observed in the rlp23 mutants is related to the RLP23-dependent recognition of NLP proteins secreted by B. cinerea. It was previously reported that B. cinerea secretes two types of NLPs, i.e., BcNEP1 and BcNEP2, during infection of tomato (Fig. 3)12. To examine whether B. cinerea also secretes these two NLPs during infection of A. thaliana, we sprayed a conidial suspension of B. cinerea on Arabidopsis plants and quantified the expression level of the BcNEP1 and BcNEP2 genes by quantitative reverse transcription PCR (RT–qPCR). BcNEP2 was preferentially expressed in the late phase of B. cinerea infection, which is in accordance with the expression pattern of NLP genes in other fungal pathogens30,31,32 (Fig. 4A). The expression of BcNEP2 was clearly induced at 72 hpi and was highest at 96 hpi. In contrast, the expression level of BcNEP1 was highest at 12 hpi, a time point at which B. cinerea had started to invade, suggesting the preferential expression of BcNEP1 at the early infection phase (Fig. 4A). Specifically, the expression of BcNEP1 reached its peak at 12 hpi, decreased to 48 hpi, and then increased again from 48 to 96 hpi (Fig. 4A). This result seems to be consistent with that reported previously for tomato12. Thus, in Arabidopsis, although B. cinerea expresses BcNEP2 at the late infection phase, the pathogen expresses BcNEP1 at high levels before (6 hpi) and during (12 hpi) invasion, which is distinct from the case of BcNEP2. These findings suggest that the perception of BcNEP1 plays a key role in the pre-invasive resistance of A. thaliana against B. cinerea. We also investigated the expression of RLP23 during B. cinerea infection and found that RLP23 was constitutively expressed, although it started to be induced to some degree at 24 hpi (Fig. 4B).
BcNEP1, BcNEP2, ChNLP1, PpNPP1 and BsNLP1 exhibited a high similarity in amino acid sequence. Sequence data of B. cinerea NEP1 (BcNEP1) and NEP2 (BcNEP2), C. higginsianum NLP1 (ChNLP1), P. parasitica NPP1 (PpNPP1) and B. subtilis NLP1 (BsNLP1) can be found in the GenBank/EMBL data libraries under the accession numbers XP_001555180 for BcNEP1, XP_001551049 for BcNEP2, XP_018154754 for ChNLP1, AAK19753.1 for PpNPP1 and WP_019714591.1 for BsNLP1. Amino acid sequences were aligned using Clustal/Omega33. The putative nlp24 region is indicated by the red open box.
BcNEP1 was preferentially expressed in the early phase of B. cinerea infection. A conidial suspension (3 × 105 conidia/mL) of B. cinerea was spray-inoculated onto Col-0 plants and total RNA was extracted. (A) The transcripts of BcNEP1 and BcNEP2 were quantified by RT–qPCR. BcUBQ was used as internal control. Means and SDs were calculated from four independent samples. The experiment was repeated twice, with similar results. (B) RLP23 was quantified by RT–qPCR. AtUBC was used as internal control. Means and SDs were calculated from three independent samples. The experiment was repeated twice, with similar results.
We then wondered whether BcNEP1 was actually recognized by Arabidopsis RLP23. Focusing on the amino acid sequences of BcNEP1 and BcNEP2, we performed multiple alignment of the amino acid sequences of BcNEP1, BcNEP2, and ChNLP1 of Colletotrichum higginsianum, PpNPP1 of Phytophthora parasitica and BsNLP1 of Bacillus subtilis using Clustal/Omega2,18,33. This analysis showed that these proteins had a high similarity in their amino acid sequences (Fig. 3). However, in the case of the predicted nlp24 region, BcNEP1 exhibited a difference vs. the other four NLPs of microorganisms in three kingdoms. Although the nlp24 region of the five NLPs, including BcNEP1, commonly possessed two conserved regions, i.e., conserved region I and conserved region II, the corresponding sequence of BcNEP1 contained additional three amino acids (NVV) between conserved region I and conserved region II (Fig. 5A).
Bcnlp27 derived from BcNEP1 and Bcnlp24 derived from BcNEP2 triggered ROS accumulation in Arabidopsis in an RLP23-dependent manner. (A) nlp27 from BcNEP1 was aligned with nlp24 from BcNEP2, ChNLP1, PpNPP1 and BsNLP1. The three additional amino acids located between the conserved region I and conserved region II in Bcnlp27 are highlighted by red characters. (B) Leaf discs from true leaves of 4–5-week-old Arabidopsis Col-0, rlp23-1 and rlp23-2 mutant plants were treated with 500 nM Bcnlp27BcNEP1, 500 nM Bcnlp24BcNEP2 or sterile distilled water (DW). Data are reported as relative light units (RLU) and represent the mean ± SE (n = 8). The experiment was repeated three times, with similar results.
The 20 and 24 amino acids derived from the nlp sequence of BcNEP2 were reported to activate Arabidopsis immunity2,6; however, it remains unknown whether the corresponding 27 amino acids derived from BcNEP1 (termed Bcnlp27BcNEP1 here) can activate immunity. Here, we named the 24 amino acids derived from the BcNEP2 nlp sequence as Bcnlp24BcNEP2. Because Bcnlp27BcNEP1 contains three additional amino acids in the nlp24 sequence, we were not able to exclude the possibility that the insertion blocks its recognition by Arabidopsis RLP23. To test whether Bcnlp27BcNEP1 can be recognized by RLP23 and trigger immunity, we measured the amount of ROS after treatment of Arabidopsis leaf discs with each synthetic nlp peptide. We found that Bcnlp24BcNEP2 induced ROS production, as reported previously (Fig. 5B). Notably, Bcnlp27BcNEP1 also induced ROS production, to the same extent as did Bcnlp24BcNEP2 (Fig. 5B). In contrast, ROS production was suppressed in the rlp23 mutants (Fig. 5B). These results demonstrate that not only Bcnlp24BcNEP2 but also Bcnlp27BcNEP1 triggers the PAMP-triggered immune response in A. thaliana in an RLP23-dependent manner, supporting the idea that the recognition of BcNEPs, especially BcNEP1, by RLP23 at the early infection phase contributes to Arabidopsis pre-invasive immunity against B. cinerea.
The necrotrophic pathogen Alternaria brassicicola does not express the NLP gene at the early infection phase and does not exhibit enhanced virulence in rlp23 plants
A. brassicicola is a necrotrophic fungal pathogen that induces lesions in Arabidopsis leaves, similar to B. cinerea34. Therefore, next we investigated whether the A. brassicicola strain Ryo-1 also expresses its NLP gene at the early infection phase in Arabidopsis. Via the alignment of the A. brassicicola genome sequence with the A. alternata mRNA sequence using Exonerate35, we found that A. brassicicola carries only one NLP homologue in its genome sequence (Supplementary Fig. 1). We designated this gene AbNLP1 and quantified its expression by RT–qPCR during A. brassicicola infection. We observed that AbNLP1 was expressed at very low levels at 24 hpi, increased to 48 hpi and then decreased towards 72 hpi (Fig. 6A). Importantly, AbNLP1 expression was not detected at 4 and 12 hpi. We also found that the A. brassicicola strain Ryo-1 starts to invade Arabidopsis at around 12 hpi36. Collectively, these results indicate that AbNLP1 of A. brassicicola is not expressed before/during invasion, in contrast to BcNEP1 of B. cinerea. Next, to determine whether the lack of RLP23 also reduces the immunity of Arabidopsis against A. brassicicola, a conidial suspension of the A. brassicicola strain was drop-inoculated on the rlp23 mutants. The rlp23-1 and rlp23-2 mutants exhibited immunity against A. brassicicola at 4 dpi, similar to that observed in the WT plants (Fig. 6B) and in contrast to the case of B. cinerea inoculation. The size of the lesions caused by A. brassicicola in the rlp23-1 and rlp23-2 mutants was not significantly different from that of Col-0 (Fig. 6C), whereas the cyp71A12 cyp71A13 mutant exhibited enhanced susceptibility to the pathogen, consistent with our recent finding36. Thus, RLP23 is not essential for the Arabidopsis immunity against A. brassicicola.
The Arabidopsis rlp23 mutation did not enhance the susceptibility to A. brassicicola. (A) A conidial suspension (5 × 105 conidia/mL) of A. brassicicola was spray-inoculated onto 4–5-week-old Col-0 plants. AbNLP1 was quantified by RT–qPCR. AbEF1 was used as internal control. Means and SDs were calculated from three independent samples. AbNLP1 was only detected at 24, 48 and 72 hpi in Arabidopsis Col-0 plants. (B) Leaves from 4–5-week-old plants were drop-inoculated with 5 µl of conidial suspensions (1 × 105 conidia/mL) of A. brassicicola. The susceptibility to A. brassicicola was not affected in Arabidopsis rlp23-1 and rlp23-2 mutants compared with Col-0 plants, whereas the cyp71A12 cyp71A13 mutant exhibited enhanced susceptibility to this pathogen. The photograph was taken at 4 dpi. (C) Lesion areas were measured in the experiments (B). At least 40 lesions from each line were measured at 4 dpi. The statistical significance of differences in lesion size was determined by Tukey’s honestly significant difference (HSD) test (**P < 0.01). The experiment was repeated twice, with similar results.
We also investigated the possible role of RLP23 toward a fungal pathogen taking infection strategies that are distinct from those of B. cinerea. C. higginsianum is a hemi-biotrophic fungal pathogen, i.e., the pathogen initially establishes a biotrophic infection in host cells, which is followed by a necrotrophic phase that leads to cell death and the emergence of pathogenic lesions37. The host plant species of C. higginsianum is Brassica rapa, and it also infects A. thaliana38. We inoculated C. higginsianum (MAFF305635) on the Arabidopsis rlp23 plants by dropping a conidial suspension of the pathogen and measured the lesion size at 5 dpi. The rlp23-1 and rlp23-2 mutants did not exhibit enhanced susceptibility to C. higginsianum, and there was no significant difference compared with Col-0 regarding the size of the lesions caused by C. higginsianum (Supplementary Fig. 2). These results showed that RLP23 is of particular importance to the immunity against B. cinerea, whereas it is dispensable for the immunity against A. brassicicola and C. higginsianum.
Bcnlp peptides enhance the Arabidopsis immunity against A. brassicicola
The results described above strongly suggest that the perception of Bcnlp27BcNEP1 of BcNEP1 at the early infection phase contributes to Arabidopsis immunity against B. cinerea. To address this contention further, we inoculated A. brassicicola on Arabidopsis WT plants by dropping a conidial suspension together with the Bcnlp27BcNEP1 peptide. We found that Arabidopsis plants that were inoculated with the pathogen and Bcnlp27BcNEP1 concomitantly showed enhanced resistance against A. brassicicola compared with plants that were inoculated with the pathogen alone (Fig. 7A). The lesion size caused by A. brassicicola was significantly reduced in the Bcnlp27BcNEP1-treated plants compared with the plants that were inoculated with A. brassicicola alone (Fig. 7B). Similar results were obtained for the co-inoculation of A. brassicicola and the Bcnlp24BcNEP2 peptide (Fig. 7). These results demonstrate that the activation of the nlp-induced immunity in the early infection phase strengthens the Arabidopsis immunity against A. brassicicola.
Inoculation of A. brassicicola together with the Bcnlp peptides reduced its virulence in Arabidopsis Col-0 plants. Leaves from 4–5-week-old plants were drop-inoculated with 5 µl of conidial suspensions (1 × 105 conidia/mL) of A. brassicicola together with 500 nM Bcnlp27BcNEP1, 500 nM Bcnlp24BcNEP2 or sterile distilled water (DW). (A) Lesion development caused by A. brassicicola. The photograph was taken at 5 dpi. (B) Lesion areas were measured in the experiments (A). At least 60 lesions were measured from each line at 5 dpi. The statistical significance of differences in lesion size was determined by Tukey’s honestly significant difference (HSD) test (**P < 0.01). The experiment was repeated twice, with similar results.
Discussion
RLP23 encodes an RLP that is essential for the perception of the nlp24 peptides and subsequent activation of immune responses. However, the actual contribution of RLP23 to the Arabidopsis immunity against pathogens remains poorly understood. In this study, we revealed that RLP23 was involved in Arabidopsis immunity against the necrotrophic fungal pathogen B. cinerea (Fig. 1). In contrast, RLP23 was dispensable for the immunity against the necrotrophic fungus A. brassicicola and the hemi-biotrophic fungus C. higginsianum (Fig. 6B,C and Supplementary Fig. 2). Interestingly, microscopic observation revealed that the invasion ratio of B. cinerea was increased in the rlp23 mutant compared with WT plants (Fig. 2B). Furthermore, BcNEP1 was expressed before/during invasion, in contrast to that observed for BcNEP2, which was expressed at the late infection phase (Fig. 4A). Furthermore, not only the nlp sequence of BcNEP2 (Bcnlp24BcNEP2) but also that of BcNEP1 (Bcnlp27BcNEP1) was recognized by RLP23, although the Bcnlp27BcNEP1 sequence contained three additional amino acids that were absent in BcNEP2 and in the NLPs of C. higginsianum, P. parasitica (oomycete) and B. subtilis (bacterium) (Fig. 5A). Furthermore, we found that A. brassicicola expressed its NLP gene (AbNLP1) preferentially at the late infection phase, rather than before/during pathogen invasion (Fig. 6A). We also showed that the co-inoculation of A. brassicicola with the Bcnlp27BcNEP1 peptide strengthened the immunity against A. brassicicola (Fig. 7). Collectively, these results revealed that RLP23 contributes to the Arabidopsis pre-invasive resistance to B. cinerea via the recognition of the NLP proteins, mainly BcNEP1, which are secreted at the early infection phase.
In hemi-biotrophic pathogens, including Colletotrichum fungi, it was proposed that NLP proteins function in the transition from the biotrophic phase to the necrotrophic phase and in the subsequent maintenance of the necrotrophic phase32. This idea is consistent with the findings that NLP expression is restricted to the late infection phase of hemi-biotrophic pathogens31,39,40. However, our previous work on C. orbiculare–cucurbit interactions suggested another explanation for the restriction of NLP expression to the late phase25. We generated a transgenic strain of C. orbiculare that expressed the NLP gene not only at the late infection phase but also at the early infection phase, and found that the generated C. orbiculare transformants failed to infect cucurbits by activating their pre-invasive resistance25. In this case, cucurbits did not recognize the nlp24 region; rather, they recognized the C-terminal region of the NLP protein25.
The results reported here provide the first example of the activation of pre-invasive resistance by a WT fungal pathogen, rather than an artificial transgenic pathogen25, via plant recognition of the pathogen NLP protein, which further supports the relationship between the restriction of NLP expression to the late phase and the avoidance of pathogen NLP recognition by plants at the early phase. Our study also provides evidence that supports the idea that PRR-dependent PAMP recognition contributes to pre-invasive resistance to fungal pathogens in higher plants. This finding is also consistent with the knowledge that non-host plant resistance against a broad range of fungal pathogens largely relies on pre-invasive immune responses41,42,43,44,45.
Because Arabidopsis recognizes BcNEP1 via RLP23, the expression of BcNEP1 at the early infection phase has a negative impact on B. cinerea infection. Why does B. cinerea express BcNEP1 at the early infection phase? Does B. cinerea benefit from BcNEP1 expression? We consider that B. cinerea expresses BcNEP1 to induce cell death in host plants at the early infection phase, thus facilitating its infection, because B. cinerea is a necrotrophic fungal pathogen. Interestingly, BcNEP1 reportedly induces necrosis more efficiently compared with BcNEP213. However, a single-gene knockout mutant of BcNEP1 was previously generated and the diameters of the lesions caused by the ΔBcnep1 mutant were not significantly different from those caused by the B. cinerea WT strain in detached tomato and Nicotiana benthamiana leaves12. Thus, there is currently no direct evidence that BcNEP1 actually contributes to the B. cinerea virulence. Also, it is noteworthy that the nlp24 peptide is recognized by a limited number of plant species, including several Brassicae species2. Therefore, we speculate that B. cinerea evolved to express BcNEP1 at the early phase to cause cell death in host plants that are not able to recognize BcNEP1, because B. cinerea fungi generally exhibit a broad host range.
The RLP23-triggered antifungal immune pathways that are crucial for pre-invasive resistance against B. cinerea also remain to be elucidated. A recent report revealed that the nlp24 peptide strongly induces ethylene production in an RLP23-dependent manner compared with flg2226. Ethylene is a plant hormone that regulates diverse developmental and physiological processes, including fruit ripening, seed germination, abscission, senescence and immunity to pathogens46. In particular, ethylene contributes to immunity against necrotrophic pathogens, including B. cinerea, compared with other types of pathogens47. For example, ethylene insensitive 2 (EIN2) is an endoplasmic reticulum (ER) membrane-localized positive regulator of ethylene, and the Arabidopsis ein2 mutants are more susceptible to B. cinerea infection48,49. Constitutive expression of ERF1, an early ethylene responsive gene50, also enhances the Arabidopsis resistance against B. cinerea51. Therefore, we speculate that the induction of ethylene production by BcNEP1 might be involved in the activation of antifungal immune responses that restrict the entry of B. cinerea. Further studies are necessary to elucidate at the molecular level the pre-invasive immune responses that are triggered by the RLP23-dependent nlp recognition.
In contrast to that observed for B. cinerea, the rlp23 mutants showed no enhanced susceptibility to A. brassiciacola (Fig. 6B,C). It is likely that RLP23 did not contribute to the immunity against A. brassiciacola because of (i) the late timing of AbNLP1 expression and (ii) the low expression level of AbNLP1 (too low to activate immunity). Interestingly, the ein2 mutants did not exhibit enhanced susceptibility to A. brassicicola, unlike that observed for B. cinerea, even though A. brassicicola is also a necrotrophic pathogen52. Alternatively, the Arabidopsis immune responses that are effective against A. brassicicola might be distinct from those that are effective against B. cinerea. However, we showed that the co-inoculation of A. brassicicola with the Bcnlp peptides enhanced the Arabidopsis immunity to this pathogen (Fig. 7). Therefore, we assume that the immune responses triggered by the nlp peptides are commonly effective against B. cinerea and A. brassicicola.
Materials and methods
Plant growth
Seeds of A. thaliana were sown on soil or rockwool (Grodan), incubated for 2 days at 4 °C in the dark and then grown at 22 °C in 16 h daylight with water for soil or Hoagland medium for rockwool.
Arabidopsis T-DNA insertion lines
All mutant lines used in this study were derived from Col-0. The rlp23-126 and rlp23-226 mutants were provided by the Arabidopsis Biological Resource Center (ABRC), Ohio University, USA, and the cyp71A12 cyp71A13 mutant53 was provided by Dr. Paweł Bednarek, Polish Academy of Sciences, Poland.
Fungal materials
The B. cinerea strain IuRy-1 was provided by Dr. Katsumi Akutsu, Ibaraki University, Japan. The A. brassicicola strain Ryo-1 was provided by Dr. Akira Tohyama. The C. higginsianum isolate MAFF305635 was obtained from the Ministry of Agriculture, Forestry and Fisheries (MAFF) Genebank, Japan. B. cinerea was cultured on 3.9% (w/v) potato dextrose agar (PDA, Difco) medium at 24 °C in the dark. For sporulation of B. cinerea, the fungus was cultured under a cycle of 16 h black light and 8 h dark for 4–5 days. A. brassicicola and C. higginsianum were cultured on 3.9% PDA medium (Nissui) at 24 °C in the dark.
Pathogen inoculation, lesion development analysis and trypan blue staining assay
4–5-week-old plants grown on rockwool were used for the inoculation assay. Conidial suspensions (5 µl) of B. cinerea (1 × 105 conidia/mL), A. brassicicola (1 × 105 conidia/mL) or C. higginsianum (1 × 105 conidia/mL) were placed onto each leaf without wounds. In the case of B. cinerea inoculation, conidia were dissolved in Sabouraud maltose broth buffer [1% (w/v) peptone (Difco) and 4% maltose were dissolved in distilled water and the pH was adjusted to 5.6 with HCl]54. The inoculated plants were kept at high humidity and transferred to a growth chamber with 21 °C light and 18 °C dark temperatures with a 12 h light/12 h dark cycle. For A. brassicicola and C. higginsianum, conidia were dissolved in sterile distilled water and the inoculated plants were kept at high humidity and at 22 °C in 16 h daylight.
For the analysis of lesion development after the inoculation assay, four drops of 5 µl of a conidial suspension of each pathogen were placed onto each leaf without wounds, and at least 24 lesions were evaluated in each experiment. The developed lesions were quantified using the ImageJ image analysis software (https://imagej.net). For pathogen invasion assay, trypan blue staining of inoculated leaves was conducted according to Koch and Slusarenko (1990)55. For the trypan blue assay, at least 200 conidia were investigated in each treatment area.
Quantitative RT–PCR analysis and quantitative PCR analysis
For the quantification of the amount of B. cinerea, 4–5-week-old plants grown on rockwool were drop-inoculated with B. cinerea (1 × 105 conidia/mL) and three leaves were collected. Total DNA was extracted using a DNeasy Plant Mini Kit (Qiagen). For the quantification of gene expression levels during pathogen infection, 4–5-week-old plants grown on rockwool were spray-inoculated with B. cinerea (3 × 105 conidia/mL) or A. brassicicola (5 × 105 conidia/mL) and three leaves were collected. Total RNA was extracted using an RNeasy Plant Mini Kit (Qiagen). The Takara Prime Script RT Master Mix (Takara Bio Inc.) was used for cDNA synthesis.
Takara TB Green Premix Ex Taq I and a Thermal Cycler Dice Real Time System TP800 (Takara) were used for both quantitative RT–PCR and quantitative PCR using the primers listed in Supplementary Table 1. For quantitative RT–PCR, the Botrytis ubiquitin (BcUBQ, XM_001556819.1), Arabidopsis ubiquitin-conjugating enzyme 2 (AtUBC, At5g25760) and A. brassicicola elongation factor 1 (AbEF1, GEMY01015044) genes were used as internal controls to normalize the levels of cDNA. For quantitative PCR, Arabidopsis α-shaggy kinase (AtASK, At5g26751) was used as the control. The primers used for the amplification of BcCutA29, AtASK29, AtUBC56, BcUBQ57 and AbEF158 were as described previously. Relative gene expression was calculated with ∆Ct method, subtracting Ct values of internal control gene from that of target gene, and represented as 2^(−∆Ct). Relative fold change was calculated with ∆∆Ct method, normalizing ∆Ct values with ΔCt value of Col-0 at 0 h, and represented as 2^(−∆∆Ct)59.
Synthetic peptides
The synthetic peptides used in this study were as follows: Bcnlp27BcNEP1, GIMYAWYFPKDQPAAGNVVGGHRHDWE; and Bcnlp24BcNEP2, AIMYSWYMPKDEPSTGIGHRHDWE. Each peptide was dissolved in sterile distilled water.
ROS measurements
The ROS assay was performed as described previously, with some modifications60. Leaf discs from true leaves of 4–5-week-old plants grown on soil were kept in the dark overnight with 50 µl of sterile distilled water in a 96-well plate. A reaction solution (50 μl) including 400 µM luminol, 20 µg/ml of horseradish peroxidase and 1 µM synthetic peptides (Bcnlp27BcNEP1 and Bcnlp24BcNEP2) was added into each well just before measurement. Luminescence was measured for about 60 min every 2 min using Luminoskan Ascent (Thermo Fisher Scientific). At least eight leaf discs were measured per experimental plot.
References
Couto, D. & Zipfel, C. Regulation of pattern recognition receptor signalling in plants. Nat. Rev. Immunol. 16, 537–552 (2016).
Böhm, H. et al. A conserved peptide pattern from a widespread microbial virulence factor triggers pattern-induced immunity in Arabidopsis. PLOS Pathog. 10, e1004491. https://doi.org/10.1371/journal.ppat.1004491 (2014).
Gómez-Gómez, L. & Boller, T. FLS2: An LRR receptor–like kinase involved in the perception of the bacterial elicitor flagellin in Arabidopsis. Mol. Cell 5, 1003–1011 (2000).
Kunze, G. et al. The N terminus of bacterial elongation factor Tu elicits innate immunity in Arabidopsis plants. Plant Cell 16, 3496–3507 (2004).
Miya, A. et al. CERK1, a LysM receptor kinase, is essential for chitin elicitor signaling in Arabidopsis. Proc. Natl. Acad. Sci. USA 104, 19613–19618 (2007).
Oome, S. et al. Nep1-like proteins from three kingdoms of life act as a microbe-associated molecular pattern in Arabidopsis. Proc. Natl. Acad. Sci. 111, 16955–16960 (2014).
Chinchilla, D. et al. A flagellin-induced complex of the receptor FLS2 and BAK1 initiates plant defence. Nature 448, 497–500 (2007).
Schulze, B. et al. Rapid heteromerization and phosphorylation of ligand-activated plant transmembrane receptors and their associated kinase BAK1. J. Biol. Chem. 285, 9444–9451 (2010).
Yu, X., Feng, B., He, P. & Shan, L. From chaos to harmony: Responses and signaling upon microbial pattern recognition. Annu. Rev. Phytopathol. 55, 109–137 (2017).
Zipfel, C. et al. Bacterial disease resistance in Arabidopsis through flagellin perception. Nature 428, 764–767 (2004).
Bailey, B. A. Purification of a protein from culture filtrates of Fusarium oxysporum that induces ethylene and necrosis in leaves of Erythroxylum coca. Phytopathology 85, 1250–1255 (1995).
Cuesta Arenas, Y. et al. Functional analysis and mode of action of phytotoxic Nep1-like proteins of Botrytis cinerea. Physiol. Mol. Plant Pathol. 74, 376–386 (2010).
Schouten, A., Baarlen, P. V. & Kan, J. A. L. V. Phytotoxic Nep1-like proteins from the necrotrophic fungus Botrytis cinerea associate with membranes and the nucleus of plant cells. New Phytol. 177, 493–505 (2008).
Cabral, A. et al. Nontoxic Nep1-like proteins of the downy mildew pathogen Hyaloperonospora arabidopsidis: Repression of necrosis-inducing activity by a surface-exposed region. Mol. Plant Microbe Interact. 25, 697–708 (2012).
Zhou, B.-J., Jia, P.-S., Gao, F. & Guo, H.-S. Molecular characterization and functional analysis of a necrosis- and ethylene-inducing, protein-encoding gene family from Verticillium dahliae. Mol. Plant Microbe Interact. 25, 964–975 (2012).
Dong, S. et al. The NLP toxin family in Phytophthora sojae includes rapidly evolving groups that lack necrosis-inducing activity. Mol. Plant Microbe Interact. 25, 896–909 (2012).
Gijzen, M. & Nürnberger, T. Nep1-like proteins from plant pathogens: Recruitment and diversification of the NPP1 domain across taxa. Phytochemistry 67, 1800–1807 (2006).
Oome, S. & Van den Ackerveken, G. Comparative and functional analysis of the widely occurring family of Nep1-Like proteins. Mol. Plant Microbe Interact. 27, 1081–1094 (2014).
Seidl, M. F. & Van den Ackerveken, G. Activity and phylogenetics of the broadly occurring family of microbial Nep1-like proteins. Annu. Rev. Phytopathol. 57, 367–386 (2019).
Ottmann, C. et al. A common toxin fold mediates microbial attack and plant defense. Proc. Natl. Acad. Sci. USA 106, 10359–10364 (2009).
Lenarčič, T. et al. Eudicot plant-specific sphingolipids determine host selectivity of microbial NLP cytolysins. Science 358, 1431–1434 (2017).
Cacas, J.-L. et al. Biochemical survey of the polar head of plant glycosylinositolphosphoceramides unravels broad diversity. Phytochemistry 96, 191–200 (2013).
Gronnier, J., Germain, V., Gouguet, P., Cacas, J.-L. & Mongrand, S. GIPC: Glycosyl inositol phospho ceramides, the major sphingolipids on earth. Plant Signal. Behav. 11, e1152438. https://doi.org/10.1080/15592324.2016.1152438 (2016).
Van den Ackerveken, G. How plants differ in toxin-sensitivity. Science 358, 1383–1384 (2017).
Azmi, N. S. A. et al. Inappropriate expression of an NLP effector in Colletotrichum orbiculare impairs infection on Cucurbitaceae cultivars via plant recognition of the C-terminal region. Mol. Plant Microbe Interact. 31, 101–111 (2018).
Albert, I. et al. An RLP23–SOBIR1–BAK1 complex mediates NLP-triggered immunity. Nat. Plants 1, 15140. https://doi.org/10.1038/nplants.2015.140 (2015).
Albert, I., Zhang, L., Bemm, H. & Nürnberger, T. Structure-function analysis of immune receptor, AtRLP23 with its ligand nlp20 and coreceptors AtSOBIR1 and AtBAK1. Mol. Plant Microbe Interact. 32, 1038–1046 (2019).
Bi, G. et al. Arabidopsis thaliana receptor-like protein AtRLP23 associates with the receptor-like kinase AtSOBIR1. Plant Signal. Behav. 9, e27937. https://doi.org/10.4161/psb.27937 (2014).
Gachon, C. & Saindrenan, P. Real-time PCR monitoring of fungal development in Arabidopsis thaliana infected by Alternaria brassicicola and Botrytis cinerea. Plant Physiol. Biochem. 42, 367–371 (2004).
Gan, P. et al. Comparative genomic and transcriptomic analyses reveal the hemibiotrophic stage shift of Colletotrichum fungi. New Phytol. 197, 1236–1249 (2013).
Irieda, H. et al. Colletotrichum orbiculare secretes virulence effectors to a biotrophic interface at the primary hyphal neck via exocytosis coupled with SEC22-mediated traffic. Plant Cell 26, 2265–2281 (2014).
Kleemann, J. et al. Sequential delivery of host-induced virulence effectors by appressoria and intracellular hyphae of the phytopathogen Colletotrichum higginsianum. PLoS Pathog. 8, e1002643. https://doi.org/10.1371/journal.ppat.1002643 (2012).
Sievers, F. et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 7, 539. https://doi.org/10.1038/msb.2011.75 (2011).
Lawrence, C. B. et al. At Death’s door: Alternaria pathogenicity mechanisms. Plant Pathol. J. 24, 101–111 (2008).
Gs, S. Automated generation of heuristics for biological sequence comparison. BMC Bioinform. 6, 31. https://doi.org/10.1186/1471-2105-6-31 (2005).
Kosaka, A. et al. bak1–5 mutation uncouples tryptophan-dependent and independent postinvasive immune pathways triggered in Arabidopsis by multiple fungal pathogens. bioRxiv https://doi.org/10.1101/2020.04.26.052480 (2020).
Cannon, P. F., Damm, U., Johnston, P. R. & Weir, B. S. Colletotrichum—current status and future directions. Stud. Mycol. 73, 181–213 (2012).
O’Connell, R. J. et al. Lifestyle transitions in plant pathogenic Colletotrichum fungi deciphered by genome and transcriptome analyses. Nat. Genet. 44, 1060–1065 (2012).
Kanneganti, T. D., Huitema, E., Cakir, C. & Kamoun, S. Synergistic interactions of the plant cell death pathways induced by phytophthora infestans Nep1-Like protein PiNPP1.1 and INF1 elicitin. Mol. Plant Microbe Interact. 19, 854–863 (2006).
Qutob, D., Kamoun, S. & Gijzen, M. Expression of a Phytophthora sojae necrosis-inducing protein occurs during transition from biotrophy to necrotrophy. Plant J. 32, 361–373 (2002).
Shimada, C. et al. Nonhost resistance in Arabidopsis-Colletotrichum interactions acts at the cell periphery and requires actin filament function. Mol. Plant Microbe Interact. 19, 270–279 (2006).
Hiruma, K. et al. Entry mode–dependent function of an indole glucosinolate pathway in Arabidopsis for nonhost resistance against anthracnose pathogens. Plant Cell 22, 2429–2443 (2010).
Hiruma, K. et al. Arabidopsis ENHANCED DISEASE RESISTANCE 1 is required for pathogen-induced expression of plant defensins in nonhost resistance, and acts through interference of MYC2-mediated repressor function. Plant J. 67, 980–992 (2011).
Lipka, V. et al. Pre- and postinvasion defenses both contribute to nonhost resistance in Arabidopsis. Science 310, 1180–1183 (2005).
Maeda, K. et al. AGB1 and PMR5 contribute to PEN2-mediated preinvasion resistance to Magnaporthe oryzae in Arabidopsis thaliana. Mol. Plant Microbe Interact. 22, 1331–1340 (2009).
Guo, H. & Ecker, J. R. The ethylene signaling pathway: new insights. Curr. Opin. Plant Biol. 7, 40–49 (2004).
Bari, R. & Jones, J. D. G. Role of plant hormones in plant defence responses. Plant Mol. Biol. 69, 473–488 (2009).
Li, W. et al. EIN2-directed translational regulation of ethylene signaling in Arabidopsis. Cell 163, 670–683 (2015).
Thomma, B. P. H. J., Eggermont, K., Tierens, K.F.M.-J. & Broekaert, W. F. Requirement of functional ethylene-insensitive 2 gene for efficient resistance of Arabidopsis to infection by Botrytis cinerea. Plant Physiol. 121, 1093–1101 (1999).
Solano, R., Stepanova, A., Chao, Q. & Ecker, J. R. Nuclear events in ethylene signaling: A transcriptional cascade mediated by ethylene-insensitive3 and ethylene-response-factor1. Genes Dev. 12, 3703–3714 (1998).
Berrocal-Lobo, M., Molina, A. & Solano, R. Constitutive expression of ethylene-response-factor1 in Arabidopsis confers resistance to several necrotrophic fungi. Plant J. 29, 23–32 (2002).
van Wees, S., Chang, H.-S., Zhu, T. & Glazebrook, J. Characterization of the early response of Arabidopsis to Alternaria brassicicola infection using expression profiling. Plant Physiol. 132, 606–617 (2003).
Müller, T. M. et al. Transcription activator-like effector nuclease-mediated generation and metabolic analysis of camalexin-deficient cyp71a12 cyp71a13 double knockout lines. Plant Physiol. 168, 849–858 (2015).
Jiang, Y. & Yu, D. The WRKY57 transcription factor affects the expression of jasmonate ZIM-domain genes transcriptionally to compromise Botrytis cinerea resistance. Plant Physiol. 171, 2771–2782 (2016).
Koch, E. & Slusarenko, A. Arabidopsis is susceptible to infection by a downy mildew fungus. Plant Cell 2, 437–445 (1990).
Czechowski, T., Stitt, M., Altmann, T., Udvardi, M. K. & Scheible, W.-R. Genome-wide identification and testing of superior reference genes for transcript normalization in Arabidopsis. Plant Physiol. 139, 5–17 (2005).
Ren, H. et al. Selection of reliable reference genes for gene expression studies in Botrytis cinerea. J. Microbiol. Methods 142, 71–75 (2017).
Cho, Y. et al. Transcriptional responses of the Bdtf1-deletion mutant to the phytoalexin brassinin in the necrotrophic fungus Alternaria brassicicola. Mol. Basel Switz. 19, 10717–10732 (2014).
Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−∆∆CT method. Methods 25, 402–408 (2001).
Gómez-Gómez, L., Felix, G. & Boller, T. A single locus determines sensitivity to bacterial flagellin in Arabidopsis thaliana. Plant J. 18, 277–284 (1999).
Acknowledgements
We thank Dr. Paweł Bednarek (Polish Academy of Sciences, Poland) for cyp71A12 cyp71A13, Arabidopsis Biological Resource Center (Ohio University, USA) for rlp23-1, and rlp23-2, Dr. Katsumi Akutsu (Ibaraki University, Japan) for B. cinerea IuRy-1, Dr. Akira Tohyama for A. brassicicola Ryo-1 and Ministry of Agriculture, Forestry and Fisheries Genebank (Japan) for C. higginsianum MAFF305635. This work was supported by Grants-in-Aid for Scientific Research (18H02204, 18H04780, 18K19212) (KAKENHI), by grants from the Project of the NARO Bio-oriented Technology Research Advancement Institution (Research program on development of innovative technology), and by the Asahi Glass Foundation.
Author information
Authors and Affiliations
Contributions
Y.T. and E.O. designed this research. E.O performed the experiments and analyzed the data. E.O., Y.T. and K.M. wrote the manuscript and prepared the figures.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ono, E., Mise, K. & Takano, Y. RLP23 is required for Arabidopsis immunity against the grey mould pathogen Botrytis cinerea. Sci Rep 10, 13798 (2020). https://doi.org/10.1038/s41598-020-70485-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-70485-1
This article is cited by
-
Amplification of cell signaling and disease resistance by an immunity receptor Ve1Ve2 heterocomplex in plants
Communications Biology (2022)
-
Identification of genetic loci in lettuce mediating quantitative resistance to fungal pathogens
Theoretical and Applied Genetics (2022)
-
The necrosis- and ethylene-inducing peptide 1-like protein (NLP) gene family of the plant pathogen Corynespora cassiicola
Current Genetics (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.