Abstract
Individuals found at bars in slums have several risk factors for HIV and tuberculosis (TB). To determine the prevalence of HIV and TB among individuals found at bars in slums of Kampala, Uganda, we enrolled adults found at bars that provided written informed consent. Individuals with alcohol intoxication were excluded. We performed HIV testing using immunochromatographic antibody tests (Alere Determine HIV-1/2 and Chembio HIV 1/2 STAT-PAK). TB was confirmed using the Xpert MTB/RIF Ultra assay, performed on single spot sputum samples. We enrolled 272 participants from 42 bars in 5 slums. The prevalence of HIV and TB was 11.4% (95% CI 8.1–15.8) and 15 (95% CI 6–39) per 1,000 population respectively. Predictors of HIV were female sex (aOR 5.87, 95% CI 2.05–16.83), current cigarette smoking (aOR 3.23, 95% CI 1.02–10.26), history of TB treatment (aOR 10.19, 95% CI 3.17–32.82) and CAGE scores of 2–3 (aOR 3.90, 95% CI 1.11–13.70) and 4 (aOR 4.77, 95% CI 1.07–21.35). The prevalence of HIV and TB was twice and four times the national averages respectively. These findings highlight the need for concurrent programmatic screening for both HIV and TB among high risk populations in slums.
Similar content being viewed by others
Introduction
HIV and tuberculosis (TB) interact at an epidemiological, clinical, cellular, and molecular level to create a co-epidemic1. In 2018, HIV contributed 251,000 of the 1.2 million TB deaths while TB was the leading cause of death among HIV positive individuals2,3. Notwithstanding, an estimated 3 million TB cases were missed in 2018 and only 79% of HIV-positive individuals knew their HIV status2,3. To increase the detection of TB and HIV, it is important to target high risk and vulnerable populations through active community based screening strategies4,5,6.
Slum dwellers have a higher risk for HIV, TB and HIV/TB co-infection than the national averages7,8. However, slum dwellers are less likely to utilise health services for HIV and TB diagnosis and have a low level of knowledge regarding prevention strategies for either disease9,10. The low utilisation is partly attributed to the perceived poor quality of services at public facilities and thus high risk groups are not covered by facility based screening strategies11,12. Within slum settlements, bars and social drinking places carry the highest risk for TB transmission than other social gathering places such as churches, clinics, hospitals, taxis, community halls, schools, and supermarkets13,14,15,16. As such, bars and social alcohol drinking groups are avenues for TB transmission to bar customers, employees and neighbours, and they propagate outbreaks from an index case17,18,19,20,21. Moreover, alcohol consumption is an established risk factor for tuberculosis in a dose dependent fashion, and exacerbates TB infection by blunting CD4 and CD8 T-lymphocyte cellular responses22,23.
Similarly, there is a high prevalence of HIV in areas that have a high density of bars24. It is likely that the prevalence of HIV among bar customers, employees and neighbours of bars in slums is high due to the high risk sexual behaviour, low risk perception of sex with commercial sex workers and inconsistent condom use observed among individuals at bars in slums21,25,26. Further, individuals who attend bars are less likely to visit health facilities and new TB cases and HIV infections among customers, staff and residents at bars in slums are missed12. The prevalence of HIV and TB among individuals found at bars in slum settings is not widely reported because studies in this setting have focused on each disease in isolation.
The aim of this study was to determine the prevalence of HIV and TB and predictors of HIV infection among individuals found at bars in Kampala slums in Uganda.
Materials and Methods
Study population and setting
Using a cross sectional study design, we enrolled participants from the 5 divisions of Kampala city, the capital city of Uganda, in December of 2019. Kampala city is sub-divided into 5 administrative divisions: Kawempe, Lubaga, Makindye, Nakawa and Central divisions. A map supplied by the Kampala Capital City Authority designates 29 settlements as slums. We randomly selected one slum from each division of Kampala. Bwaise, Kamwokya, Nalukolongo, Kibuli and Naguru Go-down slums were selected from Kawempe, Central, Lubaga, Makindye and Nakawa divisions respectively. At each slum settlement, we identified bars by snowball sampling method with the aid of a village health team leader or a local council chairperson due to lack of organised housing and registration status of bars in slums. The lack of official licensing of the bars and their operation outside the legally recommended hours makes individuals attending these places a “hard-to-reach” and “hidden” population that justifies the use of the snowball sampling method27. A bar was defined as any place from which alcoholic beverages were sold and consumed at the premises. At each bar, study participants were sampled and enrolled consecutively until the desired sample size for the given slum was achieved. The study inclusion criteria were any adult (≥ 18 years) who was found at a bar during the study visit and provided written informed consent. We excluded potential participants with alcohol intoxication as this would impair ability to provide informed consent. Alcohol intoxication was defined as Hack’s impairment index of ≥ 0.628.
Study measurements
We administered a pre-tested questionnaire that sought for socio-demographic data, history of alcohol and cigarette use, history of TB exposure and treatment and past medical history. The socio-economic status of participants was measured by the number of durable household items using the equity tool available at: https://www.equitytool.org/the-equity-tool-2/29. Using possession of common household items as a measure of household wealth is used in national demography surveys in Uganda30. The socioeconomic status was divided into 3 tertiles: rich, middle class and poor after principal component analysis. We used the CAGE questionnaire to assess the level of alcohol consumption. The CAGE questionnaire is a set of 4 simple questions: “Have you ever: (1) felt the need to cut down your drinking; (2) felt annoyed by criticism of your drinking; (3) had guilty feelings about drinking; and (4) taken a morning eye opener?”31. A score of 2–3 is indicative of a high index of suspicion for alcohol abuse while a score of 4 is diagnostic of alcohol dependence32. A study nurse drew 1 ml of blood following standard procedures33. We performed HIV testing on whole blood samples using a rapid immunochromatographic antibody test (Alere Determine HIV-1/2) and sequentially confirmed positive samples with another test (Chembio HIV 1/2 STAT-PAK) according to the Uganda ministry of health HIV testing guidelines34. Participants also provided single spot sputum samples that were tested using the Xpert MTB/RIF Ultra assay for bacteriological confirmation of TB at Mulago Hospital tuberculosis unit laboratory according to World Health Organisation Xpert MTB/RIF user guidelines35. Spot sputum samples are equally sensitive as early morning sputum samples for TB diagnosis36. Twenty one participants were unable to expectorate sputum spontaneously. For these, we induced sputum by nebulising 0.45% hypertonic saline using an ultrasonic nebuliser following normal post bronchodilator spirometry findings according to standard procedures37. To ensure participant privacy and confidentiality, we delivered pre- and post–HIV testing counselling in a designated housing unit in the slum. Newly diagnosed HIV-positive and bacteriologically confirmed TB participants were linked to Makerere University Joint AIDS Program and Mulago Hospital tuberculosis treatment unit for HIV and TB care respectively.
Sample size estimation and statistical analysis
Using the prevalence of sputum positive TB among Kampala slum dwellers with a chronic cough of 18%38, the sample size was estimated to be 283 considering a 95% confidence interval and possible incomplete data rate of 20%. Similarly, a sample size of 178 individuals was deemed adequate for estimating the prevalence of HIV considering a 10.3% prevalence of HIV among youths in slums of Kampala39. The sample size was distributed across the 5 sampled slum settlements proportional to population size of the division using data from the Uganda demographic survey30. Data were entered in EpiData 3.1 and analysed with Stata 15 (StataCorp, College Station, TX, USA). The prevalence of HIV was estimated as the proportion of participants with a positive HIV test to the total number of study participants, expressed as a percentage. Similarly, the prevalence of bacteriologically confirmed TB was estimated as the proportion of participants with a positive Xpert MTB/RIF Ultra assay to the total participants, expressed per 1,000 population. As a secondary objective, we performed logistic regression analysis to determine predictors of HIV infection. At univariable analysis, we examined the associations between categorical and continuous variables and HIV infection using chi-square tests and Rank sum test respectively. Prior to multivariate analysis, we assessed the individual associations between independent variables and HIV infection using individual simple logistic regression analysis. Variables that showed associations with a cut off threshold of p value < 0.2 and didn’t have sparse data in categories were considered at the multivariable analysis. At multivariable analysis, we used a multivariable logistic regression model to obtain factors that independently predicted HIV infection at p < 0.05 and a 95% confidence interval.
Ethical approvals and consent to participate
Study participants provided written informed consent to participate in the study. The study was approved by The AIDS Support Organisation Research and Ethics committee (ref number: TASOREC/044/19-UG-REC-009) and the Uganda National Council of Science and Technology (ref: HS459ES). All methods and experimental protocols were carried out in accordance to the declaration of Helsinki.
Results
Participant enrolment
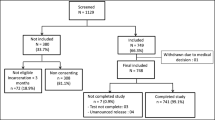
We screened 342 potential participants found at bars in Kampala slums. Of these, 15 were < 18 years of age, 17 had alcohol intoxication and 38 withdrew consent.
Participant characteristics
We enrolled 272 participants from 42 bars, of whom 170 (62.5%) were bar customers, 30 (11.0%) were bar owners, 30 (11.0%) were bar staff while 42 (15.4%) were merely found at the premises. Fourteen (5.2%), 58 (21.3%), 65 (23.9%), 71 (26.1%) and 62 (22.8%) participants were from Kamwokya, Bwaise, Nalukolongo, Kibuli and Naguru Go-down slum settlements respectively. There were 196 (72.1%) males and 251 (92.3%) participants were residents of the slum settlement they were found at with median (interquartile range, IQR) duration of residence of 8 (3–17) years. The median (IQR) age of study participants was 32 (27–38) years. The median (IQR) number of days participants had visited the bar in the preceding week was 7 (3–7) days. Other characteristics of the study participants are shown in Table 1.
Prevalence of HIV infection and bacteriologically confirmed TB among individuals found at bars
The prevalence of HIV was 11.4% (31/272) (95% confidence interval (CI): 8.1–15.8) and 16 (51.6%) individuals were newly diagnosed with HIV. The prevalence of HIV was statistically different across the slum settlements, that is, 25.9% (15/58) in Bwaise, 21.4% (3/14) in Kamwokya, 9.2% (6/65) in Nalukolongo, 7.0% (5/71) in Kibuli and 3.2% (2/62) in Naguru Go-down (p = 0.001). Among the study participants, 235 (82.7%) had ever tested for HIV. Of these, 15 (6.4%) reported to be known HIV positive individuals. Among those that self-reported to be HIV positive, 14 (93.3%) were experienced on antiretroviral therapy (ART) while 6 (40.0%) reported to be taking cotrimoxazole prophylaxis. The proportion of HIV positive participants was higher among those who had never tested for HIV than those who had ever tested (14.9% vs. 10.7%, p < 0.001). The other characteristics of HIV positive individuals are shown in Table 2.
There were 4 participants with newly diagnosed bacteriologically confirmed TB, corresponding to a prevalence of 15 (95%CI: 6–39) per 1,000 population. One individual had HIV/TB co-infection corresponding to a prevalence of 4 per 1,000 population. Thirty (11.0%) participants reported either history of TB treatment, current TB treatment or had newly diagnosed bacteriologically confirmed TB.
Predictors of HIV infection among individuals found at bars in slums in Kampala
In a multivariable logistic regression model, female sex (adjusted odds ratio (aOR) 5.87, 95% confidence interval (CI) 2.05–16.83), current cigarette smoking (aOR 3.23, 95% CI 1.02–10.26), history of TB treatment (aOR 10.19, 95% CI 3.17–32.82) and CAGE scores of 2–3 (aOR 3.90, 95% CI 1.11–13.70) and 4 (aOR 4.77, 95% CI 1.07–21.35) independently predicted HIV infection. The multivariable model is shown in Table 3.
Characteristics of Bacteriologically confirmed TB individuals found at bars in slums of Kampala
Of the 4 participants with bacteriologically confirmed TB, 3 were customers while 1 was a bar owner. Two were from Nalukolongo, 1 from Kibuli and 1 was from Naguru Go-down slum settlements. Also, of the bacteriologically confirmed TB individuals, 3 (75%) were male, 4 (100%) were slum residents, 3 (75%) reported current alcohol use, 3 (75%) reported history of a cough and 3 (75%) had history of contact with a known TB case.
Discussion
The aim of this study was to determine the prevalence of HIV and bacteriologically confirmed TB among individuals found at bars in slums of Kampala. We further analysed for predictors of HIV infection in this population. We found the prevalence of HIV to be twice the national average of 5.7% among adults40. The prevalence of HIV significantly differed by slum settlement and was higher among participants who had never tested for HIV. Female sex, history of tuberculosis treatment, current history of cigarette smoking and CAGE scores of 2–3 and 4 were independent predictors of HIV infection. The prevalence of bacteriologically confirmed TB among individuals found at bars in Kampala slums was 4 times the national estimate of 4 per 1,000 population41. Our study findings show a high prevalence of HIV and TB among individuals found at bars in slums. Moreover, all TB patients and more than half of the HIV infections were newly diagnosed. These data indicate that many new HIV infections and TB cases are missed, and programmatic screening for the two infections is warranted among customers, staff, bar owners and other individuals found at bars in slums. The geospatial variation in the prevalence of HIV within the same city suggests a need for slum specific HIV prevention strategies42.
Similar to our findings, Lama et al. (2016), found the prevalence of HIV among bar patrons to be 12.7% in the capital of Botswana and 19% of first time testers were HIV positive43. In Tanzania, the prevalence of HIV was 19.0% among female bar and hotel workers which is comparable to our finding of 19.7% among females44. The prevalence of HIV is known to be higher among women than men in sub-Saharan Africa owing to different responses to socio-demographic risk factors, sexual behaviours and HIV/AIDS awareness45,46. However, we suspect that the high HIV risk observed among women found at bars in our study is attributed to commercial sex work engaged in by women at bars in Kampala slums as reported by Mbonye et al. (2013)47. Moreover, the high prevalence of HIV observed in Bwaise slum is consistent with a higher density of female commercial sex workers and men who have sex with men observed in Kawempe division of Kampala48. A further evaluation of the transmission dynamics of HIV in bar setting in slums would better characterise the HIV risk among individuals found at bars. Additionally, an evaluation of the prevalence of other sexually transmitted diseases (STDs) among individuals at bars in slums would be desirable since STDs increase the risk of HIV infection49.
Studies that have evaluated the prevalence of TB in bar settings have been contact investigations of an index case and their findings may not compare to our cross sectional evaluation of the TB prevalence. Nonetheless, using DNA fingerprinting, these studies demonstrate that bars are an avenue for TB transmission and could propagate an outbreak among customers, employees and members of their households15,50,51,52,53. Similar to our finding, Godoy et al.(2017), found that the prevalence of active TB was 16 per 1,000 population upon investigation of contacts of an index case (a bar staff)17. Conversely, Kline, Hedemark and Davies (1996) found it at 144 per 1,000 population among contacts of an index bar patron, including customers and employees19. The prevalence of TB among individuals found at bars in slum settlements is not well reported elsewhere using a cross sectional design other than contact tracing investigations.
Our results underscore the epidemiological and clinical interrelatedness of TB and HIV among individuals found at bars in three ways. First, history of TB treatment was associated with HIV and this could be attributed to HIV-related depletion of CD4 T–lymphocytes which are crucial for immune responses against TB54. Secondly, from our multivariable logistic regression model, the odds ratios of HIV infection increased with a higher CAGE score. This suggests a dose dependent relationship between HIV and problematic alcohol use that leads to high sexual risk behaviour and inconsistent condom use55. The association of a higher risk of HIV with higher CAGE scores is also reported by a meta-analysis of studies across Africa56. This dose dependant relationship has been observed between tuberculosis risk and alcohol use as well22. Lastly, cigarette smoking was associated with HIV infection in our study yet it is an established risk factor for tuberculosis57. Therefore, these results suggest that people living with HIV found at bars also had TB risk factors. This calls for concurrent screening for HIV and TB by national HIV and TB programs among high risk populations in slums58. We did not directly evaluate the association between HIV and TB in the multivariable model population due to the small number of TB outcomes. However, current TB treatment was commoner among HIV-positive individuals than HIV-negative persons (p = 0.035). We have not found reports that have concurrently evaluated the prevalence HIV and tuberculosis among individuals in bars in slum settings and thus our findings fill an important knowledge gap.
Our study had some limitations. We did not perform sputum cultures which could have increased our detection of bacteriologically confirmed tuberculosis. Further, by excluding participants with alcohol intoxication, we might have inadvertently excluded individuals with a higher risk for TB and HIV. However, this was necessary to ensure that consent to participate was obtained wilfully. Lastly, the small sample size affected the precision of the effect sizes of predictors of HIV infection. This analysis did not account for other confounders such as sexual behaviour, knowledge and practices regarding HIV and TB prevention and ease of access to HIV and TB diagnostic services, all of which could influence the risk of TB and HIV in this population. Subsequent studies could evaluate these factors among individuals found at bars with a larger sample size.
Conclusion
There was a high prevalence of HIV and TB among individuals found at bars in the slums of Kampala, Uganda. Female sex, current cigarette smoking, history of TB treatment, and CAGE scores of 2–3 and 4 predicted HIV infection. These study findings highlight the need for concurrent programmatic screening for HIV and TB among high risk populations in slums to identify the missed infections that drive the two epidemics. We recommend slum specific programs for HIV and TB prevention that focus on women and reduction of cigarette smoking and alcohol use among individuals at bars in slum settings.
Data availability
Datasets generated and/or analysed during this study are available from the corresponding author upon reasonable request.
References
Kwan, C. K. & Ernst, J. D. HIV and tuberculosis: a deadly human syndemic. Clin. Microbiol. Rev.24, 351–376 (2011).
World Health Organization. Global tuberculosis report 2019. in Global tuberculosis report 2019 (2019).
Joint United Nations Programme on HIV/AIDS (UNAIDS) & UNAIDS, D. Geneva, Switzerland; 2018. North Am. West. Cent. Eur. AIDS Epidemic Update Reg. Summ. 1–16 (2019).
Figueroa-Munoz, J. I. & Ramon-Pardo, P. Tuberculosis control in vulnerable groups. Bull. World Health Organ.86, 733–735 (2008).
Sharma, M., Ying, R., Tarr, G. & Barnabas, R. A systematic review and meta-analysis of community and facility-based approaches to address gaps in HIV testing and linkage in sub-Saharan Africa. Nature528, S77–S85 (2015).
Jensen, S. G. et al. Impact of contact investigation and tuberculosis screening among high-risk groups in Denmark. Int. J. Tuberc. Lung Dis Off. J. Int. Union Tuberc. Lung Dis.20, 1580–1587 (2016).
Noykhovich, E., Mookherji, S. & Roess, A. The Risk of Tuberculosis among Populations Living in Slum Settings: a Systematic Review and Meta-analysis. J. Urban Health Bull N. Y. Acad. Med.96, 262–275 (2019).
Madise, N. J. et al. Are slum dwellers at heightened risk of HIV infection than other urban residents? Evidence from population-based HIV prevalence surveys in Kenya. Health Place18, 1144–1152 (2012).
Obuku, E. A. et al. Socio-demographic determinants and prevalence of Tuberculosis knowledge in three slum populations of Uganda. BMC Public Health12, 536 (2012).
Mberu, B. U., Haregu, T. N., Kyobutungi, C. & Ezeh, A. C. Health and health-related indicators in slum, rural, and urban communities: a comparative analysis. Glob. Health Action9, 58–66 (2016).
Banerjee, A., Bhawalkar, J. S., Jadhav, S. L., Rathod, H. & Khedkar, D. T. Access to health services among slum dwellers in an industrial township and surrounding rural areas: a rapid epidemiological assessment. J. Fam. Med. Prim. Care1, 20–26 (2012).
McCreesh, N. et al. Coverage of clinic-based TB screening in South Africa may be low in key risk groups. Public Health Action6, 19–21 (2016).
Murray, E. J. et al. A multidisciplinary method to map potential tuberculosis transmission ‘hot spots’ in high-burden communities. Int. J. Tuberc. Lung Dis Off. J. Int. Union Tuberc. Lung Dis.13, 767–774 (2009).
Jackson, A. D. et al. Characterising transmission of a tuberculosis genotype in Scotland: a qualitative approach to social network enquiry. Int. J. Tuberc. Lung Dis Off. J. Int. Union Tuberc. Lung Dis.13, 486–493 (2009).
Klovdahl, A. S. et al. Networks and tuberculosis: an undetected community outbreak involving public places. Soc. Sci. Med.1982(52), 681–694 (2001).
Chamie, G. et al. Identifying locations of recent TB transmission in rural Uganda: a multidisciplinary approach. Trop. Med. Int. Health TM IH20, 537–545 (2015).
Godoy, P. et al. A highly transmissible tuberculosis outbreak: the importance of bars. Epidemiol. Infect.145, 3497–3504 (2017).
Classen, C. N. et al. Impact of social interactions in the community on the transmission of tuberculosis in a high incidence area. Thorax54, 136–140 (1999).
Kline, S. E., Hedemark, L. L. & Davies, S. F. Outbreak of tuberculosis among regular patrons of a neighborhood bar. N. Engl. J. Med.333, 222–227 (1995).
Weis, S. E. et al. Transmission dynamics of tuberculosis in Tarrant county, Texas. Am. J. Respir. Crit. Care Med.166, 36–42 (2002).
White, A. et al. Transmission of tuberculosis in bars in Stroud: A cluster of 19 cases linked by MIRU-VNTR and/or epidemiology over a 30 year period. Eur. Respir. J.44, 1699 (2014).
Imtiaz, S. et al. Alcohol consumption as a risk factor for tuberculosis: meta-analyses and burden of disease. Eur. Respir. J.50, 1700216 (2017).
Mason, C. M., Dobard, E., Zhang, P. & Nelson, S. Alcohol exacerbates murine pulmonary tuberculosis. Infect. Immun.72, 2556–2563 (2004).
Nichols, B. E., Nkalamo, D. & Whitcomb, B. W. Density of drinking establishments and HIV prevalence in a migrant town in Namibia. AIDS Behav.16, 829–834 (2012).
Pitpitan, E. V. & Kalichman, S. C. Reducing HIV risks in the places where people drink: prevention interventions in alcohol venues. AIDS Behav.20, 119–133 (2016).
Morojele, N. K. et al. Alcohol use and sexual behaviour among risky drinkers and bar and shebeen patrons in Gauteng province, South Africa. Soc. Sci. Med.1982(62), 217–227 (2006).
Magnani, R., Sabin, K., Saidel, T. & Heckathorn, D. Review of sampling hard-to-reach and hidden populations for HIV surveillance. AIDS Lond. Engl.19(Suppl 2), S67-72 (2005).
Hack, J. B., Goldlust, E. J., Gibbs, F. & Zink, B. The H-Impairment Index (HII): a standardized assessment of alcohol-induced impairment in the Emergency Department. Am. J. Drug Alcohol Abuse40, 111–117 (2014).
The EquityTool - Equity Tool. https://www.equitytool.org/the-equity-tool-2/.
UDHS, I. Uganda demographic and health survey. Uganda Bur. Stat. Kampala Uganda (2011).
Ewing, J. A. Detecting alcoholism: The CAGE Questionnaire. JAMA252, 1905–1907 (1984).
O’Brien, C. P. The CAGE Questionnaire for detection of alcoholism. JAMA300, 2054–2056 (2008).
World Health Organization. WHO guidelines on drawing blood: best practices in phlebotomy. (World Health Organization, 2010).
Ministry of Health. National HIV Testing Services Policy and Implementation Guidelines. (2016).
World Health Organization. Xpert MTB/RIF implementation manual: technical and operational ‘how-to’; practical considerations. (2014).
Datta, S., Shah, L., Gilman, R. H. & Evans, C. A. Comparison of sputum collection methods for tuberculosis diagnosis: a systematic review and pairwise and network meta-analysis. Lancet Glob. Health5, e760–e771 (2017).
Weiszhar, Z. & Horvath, I. Induced sputum analysis: step by step. Breathe9, 300–306 (2013).
Sekandi, J., Neuhauser, D., Smyth, K. & Whalen, C. Active case finding of undetected tuberculosis among chronic coughers in a slum setting in Kampala, Uganda. Int. J. Tuberc. Lung Dis.13, 508–513 (2009).
Swahn, M. H., Culbreth, R., Salazar, L. F., Tumwesigye, N. M. & Kasirye, R. Psychosocial correlates of self-reported HIV among youth in the slums of Kampala. BMC Public Health19, 1176 (2019).
UNAIDS, U. Factsheet: global AIDS update. 2019. (2019).
Ministry of Health. The Uganda National Tuberculosis Prevalence Survey, 2014–2015 Survey Report. (2017).
Dwyer-Lindgren, L. et al. Mapping HIV prevalence in sub-Saharan Africa between 2000 and 2017. Nature570, 189–193 (2019).
Lama, T. P., Kumoji, E., Ketlogetswe, D., Anderson, M. & Brahmbhatt, H. Alcohol consumption and risky sexual behavior among persons attending alcohol consumption venues in Gaborone, Botswana. Prev. Sci.17, 227–236 (2016).
Ao, T. T. H., Sam, N. E., Masenga, E. J., Seage, G. R. & Kapiga, S. H. Human immunodeficiency virus type 1 among bar and hotel workers in northern Tanzania: the role of alcohol, sexual behavior, and herpes simplex virus type 2. Sex. Transm. Dis.33, 163–169 (2006).
Sia, D. et al. What explains gender inequalities in HIV/AIDS prevalence in sub-Saharan Africa? Evidence from the demographic and health surveys. BMC Public Health16, 1136 (2016).
Hegdahl, H. K., Fylkesnes, K. M. & Sandøy, I. F. Sex differences in HIV prevalence persist over time: evidence from 18 countries in Sub-Saharan Africa. PLoS ONE11, e0148502 (2016).
Mbonye, M. et al. ‘It is like a tomato stall where someone can pick what he likes’: structure and practices of female sex work in Kampala, Uganda. BMC Public Health13, 741 (2013).
Ministry of Health. Synthesis, Consolidation and Building Consensus on Key and Priority Population Size Estimation Numbers in Uganda. (2019).
Mayer, K. H. & de Vries, H. HIV and sexually transmitted infections: responding to the “newest normal”. J. Int. AIDS Soc.21, (2018).
Centers for Disease Control and Prevention (CDC). Tuberculosis outbreak associated with a homeless shelter - Kane County, Illinois, 2007–2011. MMWR Morb. Mortal. Wkly. Rep.61, 186–189 (2012).
Ijaz, K., Yang, Z., Matthews, H. S., Bates, J. H. & Cave, M. D. Mycobacterium tuberculosis transmission between cluster members with similar fingerprint patterns. Emerg. Infect. Dis.8, 1257–1259 (2002).
Ishibatake, H. & Onizuka, R. A report of outbreaks of pulmonary tuberculosis in two bars. Kekkaku72, 623–628 (1997).
Castilla, J. et al. Population-based contact investigation of a cluster of tuberculosis cases in a small village. Epidemiol. Infect.137, 1426–1435 (2009).
Ellis, P. K., Martin, W. J. & Dodd, P. J. CD4 count and tuberculosis risk in HIV-positive adults not on ART: a systematic review and meta-analysis. PeerJ5, e4165 (2017).
Schwitters, A. et al. HIV and alcohol knowledge, self-perceived risk for HIV, and risky sexual behavior among young HIV-negative men identified as harmful or hazardous drinkers in Katutura, Namibia. BMC Public Health15, 1182 (2015).
Fisher, J. C., Bang, H. & Kapiga, S. H. The association between HIV infection and alcohol use: a systematic review and meta-analysis of African studies. Sex. Transm. Dis.34, 856–863 (2007).
Bates, M. N. et al. Risk of tuberculosis from exposure to tobacco smoke: a systematic review and meta-analysis. Arch. Intern. Med.167, 335–342 (2007).
Long, E. F., Vaidya, N. K. & Brandeau, M. L. Controlling co-epidemics: analysis of HIV and tuberculosis infection dynamics. Oper. Res.56, 1366–1381 (2008).
Acknowledgements
Research reported in this publication was supported by the Fogarty International Centre of the National Institutes of Health, U.S. Department of State’s Office of the U.S. Global AIDS Coordinator and Health Diplomacy (S/GAC), and President’s Emergency Plan for AIDS Relief (PEPFAR) under award number 1R25TW011213. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and Affiliations
Contributions
J.B.B.: conceptualisation, data accrual, formal data analysis, interpretation of results, drafting manuscript and approved submitted version; G.A.: formal data analysis, interpretation of results, revised manuscript and approved submitted version; S.N.: data accrual, interpretation of results, revised manuscript and approved submitted version; F.B.: data accrual, interpretation of results, revised manuscript and approved submitted version; S.N.: data accrual, interpretation of results, revised manuscript and approved submitted version; R.B.: data accrual, interpretation of results, revised manuscript and approved submitted version; M.S.: data accrual, interpretation of results, revised manuscript and approved submitted version; A.W.: data accrual, interpretation of results, revised manuscript and approved submitted version; R.M.: data accrual, interpretation of results, revised manuscript and approved submitted version; S.N.: data accrual, interpretation of results, revised manuscript and approved submitted version; W.W.: conceptualisation, interpretation of results, revised manuscript and approved submitted version; I.A.B.: conceptualisation, interpretation of results, revised manuscript and approved submitted version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Baluku, J.B., Anguzu, G., Nassozi, S. et al. Prevalence of HIV infection and bacteriologically confirmed tuberculosis among individuals found at bars in Kampala slums, Uganda. Sci Rep 10, 13438 (2020). https://doi.org/10.1038/s41598-020-70472-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-70472-6
This article is cited by
-
Prevalence of HIV in slums area: a systematic review and meta-analysis
BMC Infectious Diseases (2024)
-
Relational Factors and HIV Testing Practices: Qualitative Insights from Urban Refugee Youth in Kampala, Uganda
AIDS and Behavior (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.