Abstract
Due to the multistep proton-coupled electron transfer, it remains a huge challenge to accelerate the kinetics of oxygen evolution reaction (OER). Here, we demonstrate that perovskite-type LaCr0.5Fe0.5O3 nanoparticles can be used as highly active and stable OER electrocatalysts, where it shows a low overpotential of 390 mV at 10 mA/cm2, a small Tafel slope of 114.4 mV/dec and excellent stability with slight current decrease after 20 h, superior than that of their individual counterparts (LaFeO3 and LaCrO3). This finding confirms that the present hybrid material would be an effective means to electrocatalyst for catalyzing OER.
Similar content being viewed by others
Introduction
It is very urgent that develop low cost, high stability and efficient energy convert and storage system (eg. metal-air batteries1, fuel cells2 and super capacitors3) for clean energy. Oxygen evolution reaction (OER) is the important step for many energy storage systems4,5,6, where noble metal-based (eg. IrO2/RuO2) catalysts are generally used as the most active catalysts to accelerate the kinetics of OER. However, it is difficult to meet the needs of commercialization, due to its scarcity and expensive of these precious metals7. Therefore, it is inevitable and great interest to explore the efficient and low-cost alternative non-precious electrocatalysts.
Recently, the stable structural, controllable electronic states8 and relatively high ionic/electronic conductivity make oxide perovskite electrocatalysts popular. In particular, as the most important parent oxides in the family of perovskite, LaFeO3 has been widely reported as efficient electrocatalyst. Unfortunately, calculations9,10 and experimental measurements11 results proved that individual LaFeO3 perovskite has poor oxygen reduction reactions (ORR) and oxygen evolution reaction (OER) activity in alkaline electrolyte. As a consequence, many attempts have been used to optimize the electronic structure and further optimize the ORR/OER activity of LaFeO3. For instance, Wang et al. used phosphorus doping strategy to optimize the OER and ORR electrocatalytic activity of LaFeO3-δ perovskite12. Copper and zinc co-doped LaFeO3 perovskite oxides with enhanced electrocatalytic activity were also reported by Mahmoud Omari13. Schon et al. reported the highest catalytic activity for CO oxidation in LaFe0.8Cu0.2O314. Furthermore, the OER activity of LaFeO3 was enhanced through coating an amorphous cobalt-phosphate15. Based on the conclusions reported in the above articles, we can see that the A or B ions in the perovskite oxide (ABO3) are partially replaced by other ions can cause changes in oxygen vacancies, ion valence and structural distortions. These can also lead to the improvement of its catalytic performance. Generally speaking, the stability and electrocatalytic activity of the perovskite oxide are more affected by the B-site cation16. Therefore, the method of replacing the B-site cation by incorporating some heteroatoms is an effective strategy to promote the electrocatalytic activity.
Herein, double perovskite LaCrxFe1−xO3 with enhanced OER activity is reported, where the optimized sample of LaCr0.5Fe0.5O3 displays the lowest overpotential of 390 mV at 10 mA/cm2, Tafel slope of 114.4 mV/dec and long-term durability (20 h). We also through the electrocatalytic test and the first principle calculations results prove that the excellent performance of LaCr0.5Fe0.5O3 are raised from the improved conductivity, tailored electronic structure and strong hybridization between oxygen and imetallic atoms.
Results and discussion
Since the OER electrocatalytic activity of the catalyst is highly correlated with its electronic structure, we therefore first verify this relationship through first principle calculation. Figure 1 shows the calculated the density of states (DOS) results of LaFeO3, LaCr0.5Fe0.5O3 and LaCrO3 models. They all exhibit the full electron occupied states at the Fermi level, revealing the metal-like state of LaFeO3, LaCr0.5Fe0.5O3 and LaCrO3. For LaCr0.5Fe0.5O3 case, there are more electron occupied states at the Fermi level, ascribing the strong hybridization of Fed/Cr-d/O-p orbits. Furthermore, it is well-known that the adsorbate–metal interactions can be predicted by the energy difference between d-band center and Fermi level (∆EO)17,18. Based on the d-band center model, the smaller value of ∆EO, the stronger bond between the metal atom and the adsorbed oxygen-containing substance, which means the higher intrinsic OER activity19,20. Apparently, our calculation results indicate that the LaCr0.5Fe0.5O3 exhibits the smallest ∆EO value [O(− 3.33 eV), Fe(− 1.64 eV) ,Cr(− 1.55 eV)] compared with LaFeO3 [O(− 3.79 eV), Fe(− 2.02 eV)] and LaCrO3 [O(− 4.70 eV), Cr(− 1.49 eV)], revealing the OER activity and electron can be significantly promoted through the strongest binding between surface Cr/Fe atoms and the adsorbed oxygenated species upon electrolysis.
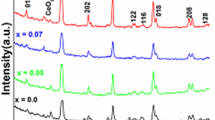
Figure 2a and Figure S1 (Supporting Information) show the X-ray diffraction (XRD) patterns of as-synthesized LaCrxFe1−xO3 (x = 0, 0.2, 0.5, 0.7, 0.9, 1, respectively) perovskite oxides. The diffraction peak of LaFeO3 (x = 0) corresponds to the standard cubic spinel LaFeO3 (PDF# 75-0541). Nevertheless, as the concentration of Cr increased, the characteristic peaks gradually shifts to the body-centered tetragonal spinel structure of LaCrO3 (PDF #75-0441)21. The morphology of the as-prepared LaCr0.5Fe0.5O3 was examined by scanning electron microscopy (SEM) and transmission electron microscopy (TEM) as presented in Figure S2 (Supporting Information) and Fig. 2b, respectively. The morphology of LaCr0.5Fe0.5O3 displays some pore structures formed by aggregated or randomly stacked irregularly shaped particles. Noting that all the samples show the same particles morphology as revealed in Figures S2, S3 (Supporting Information). The high-resolution TEM (HRTEM) was carried out to further characterize the structures of LaCr0.5Fe0.5O3. As shown in Fig. 2c, the obvious lattice fringe of 0.388 nm matched with the (100) plane. Further, as revealed by the high-angle annular dark-field scanning TEM-EDS mapping (Fig. 2d), the element distributions are highly even in LaCr0.5Fe0.5O3.
The oxidation state and composition of the electracatalysts were detected via X-ray photoelectron spectroscopy (XPS). Figure 3a exhibits La 3d spectra of LaFeO3, LaCr0.5Fe0.5O3 and LaCrO3, which are deconvoluted into two different characteristic peaks of La 3d3/2 (850.4 and 854.7 eV) and La 3d5/2 (833.5 and 837.5 eV)22. Noting that the peak positions of LaCr0.5Fe0.5O3 have a slight shift to low energy compared with LaFeO3 and LaCrO3, revealing that there are valence change or electron transfer in LaCr0.5Fe0.5O3. The high resolution spectrums of Cr 2p1/2 and Cr 2p3/2 peaks are consistent with 586.2 and 577.0 eV, respectively23. As can be seen in Fig. 3b, the peak at 577 eV of Cr 2p3/2 can be assigned to Cr3+, and as the literature reports that the catalytic activity will be promoted by Cr3+ of the high-valence Cr species24. As shown in Fig. 3c, the peaks at 709.9 and 723.6 eV were assigned to Fe 2p3/2 and Fe 2p1/2, respectively25. Compared to the LaFeO3, the Fe 2p3/2 peaks of LaCr0.5Fe0.5O3 shift from 709.9 eV to 710.2 eV. The O 1 s spectrum illustrated in Fig. 3d shows three peaks that are matched with lattice oxygen (O1, 529.8 eV), defect oxygen (O2, 531.5 eV) and surface absorbed oxygen (O3, 533.0 eV), respectively26. Furthermore, the result indicates that LaCr0.5Fe0.5O3 possesses higher ratio of oxygen defects (48.4%) than other samples (LaCrO3-31.3%, LaFeO3-40.7%), indicating that Cr doping can introduce more oxygen defects, which will enhance the interaction of hybrids and absorbed oxygen-containing species27.
As displayed in Table S1 and Figures S4, S6 (Supporting Information), the ration of x in LaCrxFe1−xO3 (x = 0, 0.2, 0.5, 0.7, 0.9, 1, respectively) can tune the electrocatalytic activity of the electrocatalysts and the optimized x is about 0.5. Thus, the linear sweep voltammetry (LSV) of LaCr0.5Fe0.5O3 reveals an OER overpotential at 10 mA/cm2 of 390 mV, which is smaller than 510 mV of LaFeO3 and 550 mV of LaCrO3 (Fig. 4a,b). Besides, as shown in Fig. 4c, LaCr0.5Fe0.5O3 owns the lowest Tafel slope (114.4 mV/dec) among LaFeO3 (142.4 mV/dec) and LaCrO3 (192.6 mV/dec), respectively. In addition, the cyclic voltammetry (CV) curve (Figure S5, Supporting Information) illustrates that Cdl (Fig. 4d) of LaCr0.5Fe0.5O3 is about 21.6 mF/cm2, larger than LaFeO3 (11.1 mF/cm2) and LaCrO3 (5.7 mF/cm2). To our knowledge, Cdl is proportional to electrocatalytically active surface areas (ECSA) of electrocatalysts28, and the largest Cdl of LaCr0.5Fe0.5O3 indicates it possesses the largest ESCA. In addition, we also can get the Charge transfer resistance by electrochemial impedance spectra (EIS)29 in Fig. 4e. It can be seen that the charge transfer resistance of LaCr0.5Fe0.5O3 is smaller than that of LaFeO3 and LaCrO3, demonstrating that LaCr0.5Fe0.5O3 owns fastest OER rates and the optimal charge transfer capability. Further, as summarized in Fig. 4f, the LaCr0.5Fe0.5O3 shows better electrocatalytic activity than some recent reported electrocatalysts, such as LFP-512, La0.95FeO3-δ30, SrCo0.9Ti0.1O3-δ31, La0.7 (Ba0.5Sr0.5)0.3Co0.8Fe0.2O332, and SrFe0.9Ti0.1O3-δ31.
At the same time, the excellent stability of the electrocatalyst can also be proved by the following tests. Figure 5a exhibits that the change of the overpotential (η) for LaCr0.5Fe0.5O3 is about 13 mV after 8,000 cycles under 35 mA/cm2. Moreover, the chronoamperometry test also can be carried out to prove the excellent stability of the LaCr0.5Fe0.5O3. It can be acquired from Fig. 5b and Figure S7 (Supporting Information) that the initial activity of LaCr0.5Fe0.5O3 remains almost unchanged after 20 h, demonstrating the outstanding stability of LaCr0.5Fe0.5O3 for OER. In addition, the XRD pattern (Fig. 5c), SEM image (Fig. 5d),TEM image (Figure S6a, Supporting Information) and HRTEM image (Figure S6b, Supporting Information) show that the structure is basically consistent with that before the OER test, confirming the LaCr0.5Fe0.5O3 has good structural stability. In light of all these findings, it is corroborated that LaCr0.5Fe0.5O3 possesses excellent electrocatalytic stability.
Conclusions
In summary, theoretical calculations demonstrate that LaCr0.5Fe0.5O3 possesses the stronger bond between the metal atom and the adsorbed oxygen-containing substance, and greater electrical conductivity for electron transport, thus having better OER electrocatalysis active. Experimentally, electrocatalytic activity of LaCrxFe1−xO3 (x = 0, 0.2, 0.5, 0.7, 0.9, 1, respectively) perovskites are studied, where the sample LaCr0.5Fe0.5O3 shows highly active (lower overpotential and larger ESCA) and excellent stability (structural and test stability). These results prove that doping the B-site perovskite oxide is an effective strategy to conceive more efficient and stable OER electrocatalysts.
Experimental section
Preparation of LaCrxFe1−xO3
The catalysts of LaCrxFe1−xO3 (x represent the amount of Cr incorporated ration in the sample, x = 0, 0.2, 0.5, 0.7, 0.9, 1) nanostructures were prepared by conventional sol–gel method. First of all, (0.005 mol) La(NO3)2.6H2O, (0.005*1−x mol) Fe(NO3)3.9H2O, (0.005*x mol) Cr(NO3)3.9H2O and (0.01 mol) C6H8O7 were added in 30 mL solution (VH2O:VC2H6O = 1:2) with 10 min stirring. Next, the uniform mixture was dried in an oven (90 °C for 24 h). Subsequently, the precursor was annealed under air atmosphere at 600 °C for 5 h with a 2 °C min−1 rate of heating. When the value of x is 0.5, we name it LaCr0.5Fe0.5O3. By analogy we have prepared LaFeO3, LaCr0.2Fe0.8O3, LaCr0.7Fe0.3O3, LaCr0.9Fe0.1O3, LaCrO3, respectively.
References
Wang, H.-F. & Xu, Q. Materials design for rechargeable metal-air batteries. Matter1, 565 (2019).
Wei, C. et al. Recommended practices and benchmark activity for hydrogen and oxygen electrocatalysis in water splitting and fuel cells. Adv. Mater.31, 180629631 (2019).
Yan, J., Li, S., Lan, B., Wu, Y. & Lee, P. S. Rational design of nanostructured electrode materials toward multifunctional supercapacitors. Adv. Funct. Mater.30, 1902564 (2019).
Wan, W. et al. 3D carbon framework-supported CoNi nanoparticles as bifunctional oxygen electrocatalyst for rechargeable Zn-air batteries. Appl. Catal. B240, 193–200 (2019).
Zhang, C. et al. Boosting hydrogen evolution activity of vanadyl pyrophosphate nanosheets for electrocatalytic overall water splitting. Chem. Commun.55, 10511 (2019).
Liu, X. et al. Nanoporous Zn-doped Co3O4 sheets with single-unit-cell-wide lateral surfaces for efficient oxygen evolution and water splitting. Nano Energy44, 371–377 (2018).
Xu, J. et al. Amorphous MoOX-stabilized single platinum atoms with ultrahigh mass activity for acidic hydrogen evolution. Nano Energy70, 104529 (2020).
Wang, H., Zhou, M., Choudhury, P. & Luo, H. Perovskite oxides as bifunctional oxygen electrocatalysts for oxygenevolution/reduction reactions—A mini review. Appl. Mater. Today16, 56 (2019).
Lee, Y., Gadre, M. J., Shaohorn, Y. & Morgan, D. Ab initio GGA+U study of oxygen evolution and oxygen reduction electrocatalysis on the (001) surfaces of lanthanum transition metal perovskites LaBO3 (B=Cr, Mn, Fe, Co and Ni). Phys. Chem. Chem. Phys.17, 21643–21663 (2015).
Wang, Y. & Cheng, H. Oxygen reduction activity on perovskite oxide surfaces: A comparative first-principles study of LaMnO3, LaFeO3, and LaCrO3. J. Phys. Chem. C117, 2106–2112 (2013).
Sunarso, J., Torriero, A. A., Zhou, W., Howlett, P. C. & Forsyth, M. Oxygen reduction reaction activity of la-based perovskite oxides in alkaline medium: A thin-film rotating ring-disk electrode study. J. Phys. Chem. C116, 5827–5834 (2012).
Li, Z. et al. Engineering phosphorus-doped LaFeO3-δ perovskite oxide as robust bifunctional oxygen electrocatalysts in alkaline solutions. Nano Energy47, 199–209 (2018).
Omari, E., Omari, M. & Barkat, D. Oxygen evolution reaction over copper and zinc co-doped LaFeO3 perovskite oxides. Polyhedron156, 116–122 (2018).
Schon, A., Dacquin, J.-P., Dujardin, C. & Granger, P. Catalytic activity and thermal stability of LaFe12xCuxO3 and La2CuO4 perovskite solids in three-way-catalysis. Catal. Today320, 30–39 (2019).
Peng, Q., Wen, Y., Shan, B. & Chen, R. Enhanced oxygen evolution reaction activity of LaFeO3 coated with Co-Pi. ECS Trans.64, 27–35 (2015).
Banerjee, S., Debata, S., Madhuri, R. & Sharma, P. K. Electrocatalytic behavior of transition metal (Ni, Fe, Cr) doped metal oxide nanocomposites for oxygen evolution reaction. Appl. Surf. Sci.449, 660–668 (2018).
Zhou, Y. et al. Enlarged Co–O covalency in octahedral sites leading to highly efficient spinel oxides for oxygen evolution reaction. Adv. Mater.30, 1802912 (2018).
Wang, H. et al. Double perovskite LaFexNi1@xO3 nanorods enable efficient oxygen evolution electrocatalysis. Angew. Chem.131, 2338–2342 (2019).
Pan, Y. et al. Electronic structure and d-band center control engineering over M-doped CoP (M=Ni, Mn, Fe) hollow polyhedron frames for boosting hydrogen production. Nano Energy56, 411–419 (2019).
He, D. et al. Active electron density modulation of Co3O4-based catalysts enhances their oxygen evolution performance. Angew. Chem.132, 6996–7002 (2020).
Paul Blessington Selvadurai, A. et al. Influence of Cr substitution on structural, magnetic and electrical conductivity spectra of LaFeO3. J. Alloys Compd.646, 924–931 (2015).
Ran, J. et al. Modulation of electronics of oxide perovskites by sulfur doping for electrocatalysis in rechargeable Zn−Air batteries. Chem. Mater.32, 3439–3446 (2020).
Wu, Y. et al. Cr-doped FeNi–P nanoparticles encapsulated into N-doped carbon nanotube as a robust bifunctional catalyst for efficient overall water splitting. Adv. Mater.31, 1900178 (2019).
Yang, Y. et al. Highly active trimetallic NiFeCr layered double hydroxide electrocatalysts for oxygen evolution reaction. Adv. Energy Mater.8, 1703189 (2018).
Li, J.-S. et al. Nitrogen-doped Fe/Fe3C@graphitic layer/carbon nanotube hybrids derived from MOFs: Efficient bifunctional electrocatalysts for ORR and OER. Chem. Commun.51, 2710–2713 (2015).
Ji, D. et al. The Kirkendall effect for engineering oxygen vacancy of hollow Co3O4 nanoparticles toward high performance portable zinc-air batteries. Angew. Chem. Int. Ed.58, 13840–13844 (2019).
Li, X. et al. MOF derived Co3O4 nanoparticles embedded in Ndoped mesoporous carbon layer/MWCNT hybrids: extraordinary bifunctional electrocatalysts for OER and ORR. J. Mater. Chem. A3, 17392 (2015).
Diao, J. et al. Interfacial engineering of W2N/WC heterostructures derived from solid-state synthesis: A highly efficient trifunctional electrocatalyst for ORR, OER, and HER. Adv. Mater.32, 1905679 (2020).
Garcıa-Osorio, D. A., Jaimes, R., Vazquez-Arenas, J., Lara, R. H. & Alvarez-Ramirezc, J. The kinetic parameters of the oxygen evolution reaction (OER) calculated on inactive anodes via EIS transfer functions: OH formation. J. Electrochem. Soc.164, E3321–E3328 (2017).
Zhu, Y. et al. Enhancing electrocatalytic activity of perovskite oxides by tuning cation deficiency for oxygen reduction and evolution reactions. Chem. Mater.28, 1691–1697 (2016).
Liu, P. et al. Bifunctional oxygen electrocatalyst of mesoporous Ni/NiO nanosheets for flexible rechargeable Zn–Air batteries. Nano-Micro Lett.12, 68 (2020).
Jung, J. et al. Optimizing nanoparticle perovskite for bifunctional oxygen electrocatalysis. Energy Environ. Sci.9, 176 (2016).
Acknowledgements
This work was financially supported by the Application Research and Development Plan of Gansu Academy of Sciences (2018JK-16) and Key Research and Development Plan of Gansu Province (No. 18YF1GA088) and Innovative Team Construction Project of Gansu Academy of Science (2020CX005-01).
Author information
Authors and Affiliations
Contributions
X.G., J.R. and D.G. wrote the main manuscript text. J.L. prepared Fig. 1, J.Z. prepared transmission test and data processing. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gao, X., Sun, Z., Ran, J. et al. High efficiency electrocatalyst of LaCr0.5Fe0.5O3 nanoparticles on oxygen-evolution reaction. Sci Rep 10, 13395 (2020). https://doi.org/10.1038/s41598-020-70283-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-70283-9
This article is cited by
-
Influence of iso-valent Al3+ doping on the electrocatalytic activity of La0.5Sr0.5Co0.8Fe0.2−xAlxO3−δ (x = 0–0.2) perovskite oxides
Journal of Solid State Electrochemistry (2023)
-
Magnetic Properties and Mössbauer Investigation of LaFe0.75Cr0.25O3 Perovskite
Journal of Superconductivity and Novel Magnetism (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.