Abstract
We conducted a series of experiments to test insect embryo capability to survive and increase reproductive investment during early development after short exposure to essential oils. We used Callosobruchus maculatus as a model insect and eucalyptus leaf and flower essential oils. Both essential oils exhibited toxicity against C. maculatus embryos and adults. However, flower essential oil was more toxic. A fetus exposed to essential oils tried to make the best of a bad situation and compensate essential oils harmful effects in the later life stages. Insect progeny production guarantee resulted in a trade-off between reproduction and female longevity. The insect also could alter fitness and reproductive behavior including, mating latency reduction, copulation duration increase, and copulation success rate raise in adulthood. Flower essential oil-exposed embryos were more successful in increasing copulation duration, and leaf essential oil-exposed embryos achieved more copulation success and less mating latency. These consequences persisted until F1 generation that was not directly exposed to essential oil. However, the F2 generation could concur with the harmful effects of essential oils. C. maculatus embryo might use epigenetic mechanisms to guarantee progeny production. Reproductive behavior changes and the trade-off can be evolutionary mechanisms to save species from possible extinction in deleterious situations.
Similar content being viewed by others
Introduction
Embryo exposure to some substances can have profound impacts on the life history strategies of many vertebrates lead to differences in adult competitive abilities and alternative reproductive tactics that possess evolutionary importance1.
Insects, with 450 million years history of living on earth, are the most successful life and useful models for the research of invertebrate animal features. Terrestriality, fight, complete metamorphosis, and eusociality have mentioned as four major adaptive features of insects2. However, insects possess other evolutionary features worth studying, such as insect behavioral immunity against different environmental stressors3. Environmental stressors such as nutrient availability, toxin or pathogen exposure, can severely restrict the reproduction ability of an organism and cause parental attempts to fight against it4.
Plant essential oils have been used for arthropod pest control as promising attractive alternatives to many synthetic pesticides because of their fast degradability properties, safety to humans and environment, and especially in case of pesticide resistance developing. Essential oils as fumigants or contact insecticides influence insect physiology by disruption of primary metabolic pathways result in rapid death, longevity reduction, and alteration of oviposition. Plant volatiles could also cause behavioral responses in insects and synergize or increase insect responses to sex pheromones5,6,7,8,9,10.
Due to the potential use of essential oils as natural biocides, lethality or effects on development have been well studied. Non-lethal consequences, however, remain under-documented11.
Scientists have demonstrated environmental stimulus (such as a toxin or toxicant exposure) could lead to organism gene expression changes to overcome a new situation. Epigenetic is changes in gene expression by heritable modifications to the DNA molecule but not gene sequence base changes because of different environmental stressors. It can lead to heritable adaptation in natural populations12.
Epigenetic mechanisms can be exploited to alter gene expression. Immediate changes in gene expression are involved in not only the toxin metabolism but also critical biological processes. Some of the changes in gene expression are transient, and some epigenetic changes could be persistent and last after termination of exposure. Some even inherited in later generations that did not experience exposure directly. Epigenetic changes take place in hours, but the results could proceed for a lifetime13,14.
To the best of our knowledge, insect embryo ability to survive against exposure to plant essential oil and compensate the damages to product progeny has not well-discussed. In this study, we used the cowpea weevil, Callosobruchus maculatus (Coleoptera: Chrysomelidae), as a model insect and Eucalyptus camaldulensis (Myrtales: Myrtaceae) flower and leaf essential oils to study probable attempt and success of embryo to avoid reproduction failure in adulthood by improving behavioral fitness.
Results and discussion
Fumigant toxicity
The results demonstrated that eucalyptus, both leaf and flower essential oils were toxic to C. maculatus and exhibited intense insecticidal activity. However, flower essential oil was more toxic. Besides, fumigant toxicity varied with the type of plant part, oil concentration, and exposure time (Supplementary Tables 1 and 2).
Mortality of C. maculatus adults increased with increasing oil concentration and time of exposure. The flower essential oil showed a more robust fumigant activity. For example, LC50 at 24 h was 132.7 µl/lair for the flower essential oil compared to 174.2 µl/lair for the leaves (Supplementary Table 1).
Mortality caused by two essential oils compared 24 h post-treatment. Two concentrations of 189.25 and 227.1 µl/lair flower essential oil induced significantly more mortality (86.6 and 100%, respectively) than leaf essential oil (50 and 70%, respectively) in the same concentration (Fig. 1).
The effectiveness of essential oils depends on many factors, such as the chemical composition of essential oils, the mode of application and the post-application temperature of the environment, etc. For example, the toxicity of Thymus vulgaris essential oil and its major constituents against the larvae of Culex quinquefasciatus and Spodoptera littoralis were increased with rising temperature15.
Some studies have documented that sublethal exposure to plant essential oils, or environmental changes (e.g., host changes) could impact the fitness of stored product insect pests. For example, clove and cinnamon essential oils have reported as toxic as the pyrethroid-based insecticide deltamethrin against C. maculatus and severely decreased adult emergence and egg number16. Host shift effects from kidney beans to cranberry beans have been studied in the bean weevil Acanthoscelides obtectus, and reproductive performance has been evaluated after exposure to clove and cinnamon essential oils. The results indicated that clove essential oil was more effective when insects were reared on cranberry bean masses and caused higher mortality17.
With the highest concentration (227.1 µl/lair), the flower essential oil took the shortest time (LT50 = 4.4 h) to cause 50% mortality compared to leaves (LT50 = 9.6 h) (Supplementary Table 2).
Flower essential oil reached 100% mortality after 24 h at the highest concentration (227.1 µl/lair), while leaf essential oil caused 70% mortality at the same concentration and time point (Supplementary Fig. 1). Median lethal time (LT50) significantly decreased with the leaf and flower increased oil concentrations (Fig. 2).
The trade-off between reproduction and longevity
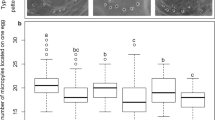
We observed that both essential oils were toxic to eggs of C. maculatus and significantly decreased hatch rate by about 50% (56.6 ± 3.3% for flower essential oil and 50 ± 1.5% for leaf essential oil). The control egg hatch rate was 83.33 ± 3.3% (Fig. 3a). Interestingly, survivors could compensate harmful effects of embryo exposure to essential oils, and hence, control and treatments had statistically the same larval duration (Fig. 3b). Insects have developed evolutionary strategies to compensate for unfavorable situations encountered during different life stages. For example, insects that experience a period of lack of food and nutrition grow faster than those who were in a normal situation to compensate for initial failure18.
Effect of eucalyptus leaf and flower essential oils (EO) on different biology parameters of C. maculatus F0 generation. (a) F0 egg hatch (%); (b) F0 larval duration (day); (c) F0 adult emergence (%); (d) F0 adult longevity (day). Essential oils did not influence F0 larval duration and adult longevity. However, decreased F0 egg hatch (%) and adult emergence. Different letters indicate significant differences between the treatments (P < 0.05).
However, a significantly remarkable reduction in adult emergence was observed in essential oil-treated insects (56.6 ± 3.3% for flower essential oil and 50 ± 1.5% for leaf essential oil) when compared to the control (83.33 ± 3.3%) (Fig. 3c). Adult emergence reduction was due to egg mortality because it was the same as egg hatch, and no larval mortality was recorded.
Embryo short time exposure to the essential oils influenced female adult longevity (Fig. 3d). The longevity was 10.8 days for control females and 8.0 and 8.1 days for leaf and flower essential oil-treated females, respectively. Treated and untreated adult males had statistically the same longevity. Male longevity was shorter than that of females (Fig. 3d).
It is worth noting that F0 embryo exposure to flower essential oil significantly reduced F1 egg number (10 ± 0.7) compared to control (15 ± 1.1) and leaf essential oil (14.4 ± 0.7) group (Fig. 4a). Therefore, there was a fitness consequence of essential oil stress by a trade-off between reproduction and female longevity in leaf essential oil-treated embryo. Thus, the improvement in reproduction was costly in terms of longevity decrease to avoid further reduction of egg numbers.
Effect of eucalyptus leaf and flower essential oils (EO) on different biology parameters of C. maculatus F1 generation. (a) F1 egg number; (b) F1 egg hath (%); (c) F1 larval duration (day); (d) F1 sex ratio; (e) F1 adult emergence (%); (f) F1 adult longevity (day). Flower essential oil treatment decreased F1 egg numbers, and both essential oils caused a decrease in F1 adult emergence and female longevity. Different letters indicate significant differences between the treatments (P < 0.05). Essential oils had no significant effects on F1 egg hatch (%), larval duration, and sex ratio.
A trade-off occurs when an improvement in one life-history trait (improving fitness) is combined with a decrease in another life-history feature (reducing fitness). So that the fitness value is balanced against fitness costs by increasing one trait by decreasing another trait. Trade-offs are typically defined by negative genetic or phenotypic correlations between individual fitness components within a population19. In Bemisia tabaci, a trade-off between longevity and egg numbers has been reported20.
A large number of studies have demonstrated that sublethal concentrations of essential oils influence insect biology and inhibit insect oviposition by reduction of egg number or hatchability. For example, lemongrass, rosemary, Vanillosmopsis arborea, Eucalyptus camaldulensis, and Heracleum persicum essential oils caused egg number reduction in C. maculatus10,21,22,23. It is noteworthy that in the all mentioned experiments, sexually mature adults were exposed to essential oils. However, we used the embryo, and the effect of essential oil exposure persisted until adulthood and F1 generation.
F1 egg quality, larval duration, and sex ratio were not affected by F0 embryo exposure to essential oils; hence egg hatch rate was statistically equal in the treatments and control. Sex ratio was 1:1 (female/male) in control and treatments (Fig. 4b–d).
Although leaf essential oil-treated embryos attempted to deposit the same egg number, with the same hatch rate as control in adulthood, all larvae could not make it until adulthood, and F1 adult emergence rate was significantly lower than control (84 ± 4%) in both essential oils group offspring (57.9 ± 6% and 48.3 ± 7% for leaf and flower essential oils, respectively) (Fig. 4e).
Interestingly, F1 females that did not directly expose to essential oil fumigation exhibited significant reductions in their longevity like their parents (10.3, 8.5 and 8.4 days for control, leaf, and flower essential oils, respectively) (Fig. 4f).
Sublethal doses or short-term exposure of insects to essential oils have been found to affect insect fertility, vitality, and longevity, even in the F1 generation. Carlina acaulis root essential oil topical application reduced female Musca domestica longevity and egg number. Mortality of F1 larvae and pupae were increased, and F1 adult emergence were decreased24,25. Besides, M. domestica adult exposure to thyme oil sublethal doses negatively impacted adult longevity, F1 vitality, and F1 fecundity26.
The maize weevil, Sitophilus zeamais sublethal exposure to clove and cinnamon essential oils caused similar insecticidal toxicity and reduced respiratory rate and parent longevity and influenced progeny fitness by accelerating offspring emergence and producing heavier progeny27,28.
We recorded the F2 generation egg number and hatch rate to realize if the effects of short-time exposure of the embryo to essential oil could be passed on to future generations after the F1 generation. The F2 generation could completely concur with the deleterious effects of essential oils, and; thus, egg numbers and hatching rates were statistically the same in treatments and control (Supplementary Fig. 3).
Reproductive behavior fitness
The environment can affect reproductive behavior in males and females, including mate recognition, courtship, mating, and post-mating behavioral changes. Mating behavior in insects is a significant reproductive process29.
We demonstrated that leaf essential oil-exposed male embryos improved their reproductive behavior in adulthood and achieved more copulation success (70%) and less mating latency (85.9 s) compared to control (55% and 149.3 s) and flower essential oil group (45% and 120.8 s) (Fig. 5). Some studies have documented that exposure to the aroma of essential oil or particular plant compounds increases male insect mating competitiveness30,31,32. For example, ginger root oil, tea tree oil, and orange oil are involved in male competitiveness of the Mediterranean fruit fly, Ceratitis capitata32,33, and grapefruit oil enhanced Anastrepha ludens male mating success34. A single Plant volatile exposure increased the mating tendency of both sexes in the adult olive fruit flies Bactrocera oleae35.
Our results showed that flower essential oil-exposed embryos struggled to fight against future deleterious effects of essential oil on their potential reproductive success and finally were significantly more successful in increasing copulation duration time (264.4 s) compared to control (207.1) and leaf essential oil group (209.3) (Fig. 6d). C. maculatus females prefer short copulations due to physical injuries to their reproductive tracts36, whereas it was shown that in insects, the duration of copulation increases the reproductive success of males37. Some studies have shown that copulation behavior and duration can directly affect insect reproductive fitness and ability. Prolonged copulations increased male fertilization success in the damselfly, Ceriagrion tenellum, and two aphidophagous ladybirds38,39. Based on another study40, prolonged copulations not only did not hurt C. maculatus females but also could increase lifetime fecundity and material benefit that females derive from the increased ejaculate size. Females with long copulation duration deposited more eggs than females with shorter copulations.
Effect of eucalyptus leaf and flower essential oils (EO) on different copulation parameters of C. maculatus F0 generation. (a) F0 mating latency (s); (b) F0 kicking phase start; (c) F0 kicking duration (s); (d) F0 copulation duration (s). Both essential oils significantly decreased male mating latency, and flower essential oil significantly increased copulation duration (P < 0.05). Essential oils did not impact female kicking phase start and duration.
Reproductive behavioral compensation mechanisms have been reported in the butterfly Pararge aegeria. Males that experienced diet with low nutritional quality during larval development could not monopolize an energetically costly territory similar to well-provided males. However, they compensated this weakness with a patrolling tactic to maximize reproductive success41. In insects and other animals, lower-quality individuals that influenced by environmental conditions try to make the best of an unfavorable situation and maximize their reproductive success by adopting alternative tactics42.
We did not observe significant differences in further examined reproductive behaviors among control and treated groups including, the number of males with no tendency to copulation, number of male with the struggle to start mating but rejected by females, and kicking phase duration of females to terminate copulations (Fig. 6).
In F1 generation, both essential oil groups were still affected by their parents early-life exposure to essential oils; hence, both improved fitness by reproductive behavioral changes and male copulation success rate increased (85.7% and 71.42% for flower and leaf essential oils, respectively) in contrast to control (50%) (Fig. 7). There was no significant difference in other reproductive behaviors in the F1 generation (Fig. 8).
Effect of eucalyptus leaf and flower essential oils (EO) on different copulation parameters of C. maculatus F0 generation. (a) F0 mating latency (s); (b) F0 kicking phase start; (c) F0 kicking duration (s); (d) F0 copulation duration (s). There was no significant difference in the evaluated parameters (P > 0.05).
External stresses such as exposure to some substances or chemical pollutants can indeed have long-lasting effects on metabolism, development, and gene expression, sometimes even in subsequent generations43. Exposure to different volatile plant compounds can have long-term consequences on insect physiology as well as evolutionary adaptation44.
Epigenetic changes during early embryonic development will be amplified during development by cell division, and therefore influence a high amount of cells in the fully grown organism. However, when epigenetic changes arise in adult cells, they remain limited to those cells or a specific tissue45.
Conclusion
In conclusion, our study demonstrates that C. maculatus embryo, after exposure to an environmental stressor like essential oils, struggles to compensate deleterious effects even by changing reproductive behavior to increase fitness and guarantee progeny production or trade-off between longevity and fecundity. Epigenetic is the mechanism that can transfer gene expression alteration template during different life stages and even to the later generations. It can be an evolutionary mechanism to save the species from possible extinction in deleterious environmental situations.
Also, our findings showed that botanical insecticides like eucalyptus leaf and flower essential oils could be used in C. maculatus control programs, especially in warehouses, as a substitute for conventional pesticides after further investigation.
Materials and methods
Insect colony
The strain of the cowpea weevil, C. maculatus, was maintained on black-eyed peas, Vigna unguiculata under laboratory conditions of 30 ± 1 °C and 50 ± 5% RH under 16L: 8D photoperiod. All experiments were accomplished under the same conditions. The newly emerged (< 24 h-old) adults and eggs (< 24 h-old) were chosen to set up the experiments.
Plant materials
Flowers and leaves of Eucalyptus camaldulensis were collected from Zahedan, Sistan and Baluchestan province, Iran (Latitude: 29.4519, Longitude: 60.8842 and Elevation above sea level: 1,352 m). The plant samples were air-dried at room temperature for one week and then were hydrodistilled to extract the essential oils.
Essential oil extraction
Dried flowers and leaves of E. camaldulensis (300 g) were grounded, and then essential oils were extracted by hydrodistillation in a Clevenger apparatus for 3 h. After extraction, water was removed by anhydrous sodium sulfate, and the extracted oil was stored in a dark box in a refrigerator at 4 °C.
Fumigation bioassay
We used the newly emerged (< 24 h-old) adults of C. maculatus to set up the fumigation bioassay. We deposited each ten freshly emerged adults in a Petri dish (diameter 60 mm), which its top covered with filter paper. Based on an initial dose-setting experiment, 2, 3, 5, and 6 µl of E. camaldulensis leaf and flower essential oils (corresponding to essential oil concentrations of 75.7, 113.55, 189.25 and 227.1 µl/lair) were applied to the filter paper pieces. We used the same concentrations for both leaf and flower essential oils to be able to compare different effects of essential oils. Then, the Petri dishes' edges were sealed with parafilm. Each concentration and control (without essential oil) was replicated four times. Mortality was recorded at 6, 12, 24, 48, and 72 h after treatments. Insects with no movement of leg or antenna were considered as dead36.
The embryo exposure to essential oils and insect biological parameters
Based on the bioassay, the embryo exposure to the concentration of 75.7 µl/lair caused almost 50% of hatching in both essential oils (Supplementary Fig. 2). Therefore, 150 eggs were fumigated with leaf essential oil and 150 eggs with flower essential oil for 24 h. Also, 75 eggs that achieved no treatment considered as control. Treated eggs were placed in Petri dishes individually. Total numbers of eggs hatched were counted after 7 days. The daily observation was done, and F0 larval duration, adult emergence, and longevity were monitored every day. Since adults emerged, males and females were paired and checked daily to record their survival and the numbers of laid eggs. The experiments continued until all of the individuals died. Insects were allowed to oviposit 24 h to obtain the F1 egg number and hatch. Then seeds with eggs were transferred to a separate Petri dish. The experiment was repeated three times, and F1 adult emergence and longevity were recorded daily. Egg number and hatch rate were documented for F2 generation, too.
Copulation test
We collected virgin males and females by removing adults as they emerged. We used 120 newly emerged (< 24 h-old) virgin adults to do copulation test. The male was placed in a Petri dish, and then immediately, a female was transferred. Pairs were given 5 min to mate. The pair's behavioral changes were monitored, and mating latency, the start of copulation, the start of a male kicking by female, and termination of copulation were recorded. If a male does nothing during this time, it was recorded as male without tendency. If a male tried to start copulation and female actively rejected males and prevent copulation, it was recorded as male rejection.
Statistical analysis
One-way ANOVA analysis was performed using SPSS version 26.0 to compare egg number, egg hatch rate, larval duration, and adult longevity followed by Tukey’s test for mean separation. Statistical significance was established as P < 0.05. All other comparisons between treatments were analyzed using student’s t-test at 5% level. The LC10, LC50 and LC90, LT10, LT50, and LT90, as well as their respective 95% confidence intervals, were calculated by probit analysis (Polo Plus software).
References
Clark, M. M. & Galef, B. G. Prenatal influences on reproductive life history strategies. Trends Ecol. Evol.10, 151–153 (1995).
Bradley, T. J. et al. Episodes in insect evolution. Integr. Comp. Biol.49, 590–606 (2009).
de Roode, J. C. & Lefèvre, T. Behavioral immunity in insects. Insects3(3), 789–820 (2012).
Gulyas, L. & Powell, J. R. Predicting the future: Parental progeny investment in response to environmental stress cues. Front Cell Dev. Biol.7, 115. https://doi.org/10.3389/fcell.2019.00115 (2019).
Deng, J. et al. Enhancement of attraction to sex pheromones of Spodoptera exigua by volatile compounds produced by host plants. J. Chem. Ecol.30, 2037–2045 (2004).
Schoonhoven, L. M., Van Loon, J. J. A. & Dicke, M. Insect-Plant Biology (Oxford University Press, Oxford, 2005).
Plata-Rueda, A. et al. Insecticidal activity of garlic essential oil and their constituents against the mealworm beetle, Tenebrio molitor Linnaeus (Coleoptera: Tenebrionidae). Sci. Rep.7, 46406. https://doi.org/10.1038/srep46406 (2017).
Pourya, M., Sadeghi, A., Ghobari, H., Nji Tizi Taning, C. & Smagghe, G. Bioactivity of Pistacia atlantica desf. Subsp. Kurdica (Zohary) Rech. F. and Pistacia khinjuk stocks essential oils against Callosobruchus maculatus (F, 1775) (Coloeptera: Bruchidae) under laboratory conditions. J. Stored Prod. Res.77, 96–105 (2018).
Gaire, S., Scharf, M. E. & Gondhalekar, A. D. Toxicity and neurophysiological impacts of plant essential oil components on bed bugs (Cimicidae: Hemiptera). Sci. Rep.9(1), 3961. https://doi.org/10.1038/s41598-019-40275-5 (2019).
Moura, E. D. et al. Insecticidal activity of Vanillosmopsis arborea essential oil and of its major constituent α-bisabolol against Callosobruchus maculatus (Coleoptera: Chrysomelidae). Sci. Rep.9, 3723. https://doi.org/10.1038/s41598-019-40148-x (2019).
Conchou, L. et al. Insect odorscapes: From plant volatiles to natural olfactory scenes. Front. Physiol.10, 972. https://doi.org/10.3389/fphys.2019.00972 (2019).
Vilcinskas, A. The role of epigenetics in host–parasite coevolution: Lessons from the model host insects Galleria mellonella and Tribolium castaneum. Zoology (Jena).119, 273–280 (2016).
Knecht, A. L. et al. Transgenerational inheritance of neurobehavioral and physiological deficits from developmental exposure to benzo[a]pyrene in zebrafish. Toxicol. Appl. Pharmacol.329, 148–157 (2017).
Portnoy, D. S., Fields, A. T., Greer, J. B. & Schlenk, D. Genetics and oil: Transcriptomics, epigenetics, and population genomics as tools to understand animal responses to exposure across different time scales. In Deep Oil Spills (eds Murawski, S. et al.) (Springer, Berlin, 2020).
Pavela, R. & Sedlák, P. Post-application temperature as a factor influencing the insecticidal activity of essential oil from Thymus vulgaris. Ind. Crops Prod.113, 46–49 (2018).
Viteri Jumbo, L. O. et al. Toxicity to, oviposition and population growth impairments of Callosobruchus maculatus exposed to clove and cinnamon essential oils. PLoS ONE13, e0207618 (2018).
Haddi, K. et al. Changes in the insecticide susceptibility and physiological trade-offs associated with a host change in the bean weevil Acanthoscelides obtectus. J. Pest Sci.91, 459–468 (2018).
Metcalfe, N. B. & Monaghan, P. Compensation for a bad start: Grow now, pay later?. Trends Ecol. Evol.16, 254–260 (2001).
Fabian, D. & Flatt, T. Life history evolution. Nat. Educ. Knowl.3(10), 24 (2012).
Lü, Z. C. et al. Trade-offs between survival, longevity, and reproduction, and variation of survival tolerance in Mediterranean Bemisia tabaci after temperature stress. J. Insect Sci.14, 124. https://doi.org/10.1093/jis/14.1.124 (2014).
Campolo, O., Giunti, G., Russo, A., Palmeri, V. & Zappala, L. Essential oils in stored product insect pest control. J. Food Qual.2018, 1–18 (2018).
Alves, M. D. S. et al. Efficacy of lemongrass essential oil and citral in controlling Callosobruchus maculatus (Coleoptera: Chrysomelidae), a post-harvest cowpea insect pest. Crop Prot.119, 191–196 (2019).
Sabbour, M. M. A. Efficacy of natural oils against the biological activity on Callosobruchus maculatus and Callosobruchus chinensis (Coleoptera: Tenebrionidae). Bull. Natl. Res. Cent.43, 206. https://doi.org/10.1186/s42269-019-0252-1 (2019).
Benelli, G. et al. Carlina oxide from Carlina acaulis root essential oil acts as a potent mosquito larvicide. Ind. Crops Prod.137, 356–366 (2019).
Pavela, R. et al. Outstanding insecticidal activity and sublethal effects of Carlina acaulis root essential oil on the housefly, Musca domestica, with insights on its toxicity on human cells. Food Chem. Toxicol.136, 111037 (2020).
Pavela, R. Lethal and sublethal effects of thyme oil (Thymus vulgaris L.) on the house fly (Musca domestica Lin.). J. Essent. Oil Bear. Plants5, 346–356 (2007).
Haddi, K., Oliveira, E. E., Faroni, L. R. A., Guedes, D. C. & Miranda, N. N. S. Sublethal exposure to clove and cinnamon essential oils induces hormetic-like responses and disturbs behavioral and respiratory responses in Sitophilus zeamais (Coleoptera: Curculionidae). J. Econ. Entomol.108, 2815–2822 (2015).
Silva, S., Haddi, K., Viteri Jumbo, L. & Oliveira, E. Progeny of the maize weevil, Sitophilus zeamais, is affected by parental exposure to clove and cinnamon essential oils. Entomol. Exp. Appl.163, 220–228 (2017).
Chang, M. M. et al. Effect of diallyl trisulfide on the reproductive behavior of the grain moth, Sitotroga cerealella (Lepidoptera: Gelechiidae). Insects11(1), E21. https://doi.org/10.3390/insects11010021 (2019).
Papadopoulos, N. T., Katsoyannos, B. I., Kouloussis, N. A. & Hendrichs, J. Effect of orange peel substances on mating competitiveness of male Ceratitis capitata. Entomol. Exp. Appl.99, 253–261 (2001).
Shelly, T. E. & Mclnnis, D. O. Exposure to ginger oil enchance mating success of irradiated, mass-reared males of Mediterranean fruit fly (Diptera: Tepritidae). J. Econ. Entomol.94, 1413–1418 (2001).
Papadopoulos, N., Shelly, T., Niyazi, N. & Jang, E. Olfactory and behavioral mechanisms underlying enhanced mating competitiveness following exposure to ginger root oil and orange oil in males of the Mediterranean fruit fly, Ceratitis capitata (Diptera: Tephritidae). J. Insect Behav.19, 1–16 (2006).
Shelly, T. E. & Epsky, N. D. Exposure to tea tree oil enhances the mating success of male mediterranean fruit flies (Diptera: Tephritidae). Fla. Entomol.98(4), 1127–1133 (2015).
Morató, S., Shelly, T., Rull, J. & Aluja, M. Sexual competitiveness of Anastrepha ludens (Diptera: Tephritidae): Males exposed to Citrus aurantium and Citrus paradisi essential oils. J. Econ. Entomol.108, 621–628 (2015).
Gerofotis, C. D., Ioannou, C. S. & Papadopoulos, N. T. Aromatized to find mates: α-Pinene aroma boosts the mating success of adult olive fruit flies. PLoS ONE8(11), e81336. https://doi.org/10.1371/journal.pone.0081336 (2013).
den Hollander, M. & Gwynne, D. T. Female fitness consequences of male harassment and copulation in seed beetles, Callosobruchus maculatus. Anim. Behav.78, 1061–1070 (2009).
Wolff, J. O. Biological functions and evolutionary aspects. In Attachment Structures and Adhesive Secretions in Arachnids. Biologically-Inspired Systems (ed. Gorb, S. N.) (Springer, Berlin, 2016).
Andrés, J. & Cordero Rivera, A. Copulation duration and fertilization success in a damselfly: An example of cryptic female choice?. Anim. Behav.59, 695–703 (2000).
Omkar Singh, K. & Pervez, A. Influence of mating duration on fecundity and fertility in two aphidophagous ladybirds. J. Appl. Entomol.130, 103–107 (2006).
Edvardsson, M. & Canal, D. The effects of copulation duration in the bruchid beetle Callosobruchus maculatus. Behav. Ecol.17, 430–434 (2006).
Vande Velde, L., Schtickzelle, N. & Van Dyck, H. Effect of larval food stress on male adult behavior, morphology and reproductive investment in the butterfly Pararge aegeria. Evol. Ecol.27, 221–234 (2013).
Brockmann, H. J. Alternative reproductive tactis in insects. In Alternative Reproductive Tactics: An Integrative Approach (eds Oliveira, R. F. et al.) 177–223 (Cambridge University Press, Cambridge, 2008).
Feil, R. & Fraga, M. Epigenetics and the environment: Emerging patterns and implications. Nat. Rev. Genet.13, 97–109 (2012).
Conchou, L. et al. Insect odorscapes: From plant volatiles to natural olfactory scenes. Front. Physiol.10, 972 (2019).
Robertson, J. L., Savin, N. E., Preisler, H. K. & Russell, R. M. Bioassays with Arthropods (CRC Press, Boca Raton, 2007).
Author information
Authors and Affiliations
Contributions
A.A and A.R.B conceived and designed the research. A.A performed the experiments. The manuscript was written by A.A with input and corrections from A.R.B.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Amiri, A., Bandani, A.R. Callosobruchus embryo struggle to guarantee progeny production. Sci Rep 10, 13269 (2020). https://doi.org/10.1038/s41598-020-70178-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-70178-9
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.