Abstract
Micropyles in insects are small openings that allow sperm entry into, and the number was usually decreased on unfertilized and (or) undeveloped eggs. However, reports showed that Harmonia axyridis, a reproductive success model, deposited similar number of micropyles on undeveloped and developing eggs. Thus, it was confusing whether micropyles in H. axyridis were unaffected. To solve this confusion, two experiments were conducted here. Firstly, virgin female and four different days delayed mating (DDM) experiments were conducted to reveal the effects of fertilization stimulus and delayed-fertilization. Secondly, intercrosses between a light-colored mutant (HAM, an adaptive deficiency) and wild type (HAW) were conducted to further reveal whether there were female and male interactions. We found that (1) eggs produced by virgin and DDM females had significantly less micropyles than control. Even so, more than 18 micropyles were observed on eggs following fertilization and, consequently, egg production as well as hatch rate was not negatively affected by mating delay; (2) number of micropyles was significantly varied among the four reciprocal crosses and virgin HAW female. Specifically, the heterozygous eggs (Cross-D) and wild-type homozygous eggs (Cross-B) respectively had the least and maximum micropyles, and eggs from virgin HAW female had significantly less micropyles compared to those from HAW female (Cross-B or Cross-C), but the number was significantly higher than those from HAM female (Cross-A or Cross-D). These results informed us that the number of micropyles in H. axyridis is plastic but maintaining a high-quantity that offers many benefits, which should contribute to its reproduction success.
Similar content being viewed by others
Introduction
In insects, there were one or more small openings locating on the eggshell that called micropyles1. Each micropyle was a channel that extended from the external gateway through chorion and ended in vitelline membrane, serving as the route of sperm entry into a mature oocyte2,3. In addition, for some species, there were special accessory structures located in micropyle region facilitating sperm penetration4. Thus, the number of micropyles as well as their structures had crucial roles in the fertilization of eggs5. At the functional level, eggs equipped with multiple micropyles would suggest greater potential for multiple sperm entry into an egg, which might offer several benefits, e.g. increase the opportunity for post-copulatory choice of female within the egg environment6.
However, the formation of micropyles was a considerable material and (or) energetic cost process7. Thus, for some insect species, the number of micropyles could be modulated on females’ own accord, which acted as a critical adaptive mechanism. For some species laying both viable and inviable eggs, the number of micropyles was vastly different between these two types8. Among which, the subsocial burrower bug, Adomerus triguttulus (Heteroptera: Cydnidae) and the stingless bee, Trigona (Tetragonisca) angustula (Hymenoptera: Apidae), were two typical examples. Their viable eggs were presented with micropyles, whereas their inviable eggs (trophic eggs) were by and large devoid of such structures7,9. The above cases might be caused by the fact that inviable eggs were usually produced as food for newly hatched larvae, which could ensure the offspring have siblings to eat10. In addition, the number of micropyles also showed to be influenced by the experience of copulation (or called fertilization). For example, virgin female workers of the ant, Gnamptogenys menadensis (Hymenoptera: Formicidae) and virgin queen of the subterranean termite, Reticulitermes chinensis (Isoptera: Rhinotermitidae) can only lay small number of eggs lacking micropyle; while those females following mating produced a mass number of eggs presenting micropyles11,12. Queens of the termite, Reticulitermes speratus (Isoptera; Rhinotermitidae), in particular, could close the gate of micropyle to switch from sexual to asexual reproduction when the kings were presented13. The results from above studies indicated that the number of micropyles in some insect species is plastic. However, most studies only focused on the viable and inviable eggs that might be too limited to elucidate the variation as well as function of micropyles.
The multicolored Asian ladybird beetle, Harmonia axyridis (Coleoptera: Coccinellidae), native to Asia, is an important biological control agent and has been introduced to many areas. However, this ladybird beetle quickly became invasive and spread rapidly worldwide due to its excellent reproductive success14,15. Previous studies showed that female H. axyridis could also lay developing and undeveloped eggs (trophic eggs) synchronously, and mother ladybird beetle could adjust the proportion of undeveloped trophic eggs to mitigate the starvation risk of offspring16. However, similar number of micropyles (about 19.8) was detected on the developing and undeveloped eggs5. Thus, it was confusing whether the number of micropyles in H. axyridis was unaffected, and, if the number was plastic, what degree could be affected. In this study, we desinged two experiments to evaluate the variation of micropyles in H. axyridis. (1) Delayed mating is common in nature due to many factors, e.g. temperature, rain or wind17, and studies showed that delayed mating usually caused detrimental effects on female reproduction18,19,20. For H. axyridis, the first copulation has been confirmed to be the stimulus for normal oviposition. The virgin females could only lay a few scattered eggs, whereas they laid many eggs in batches soon after the first copulation21. Here, we hypothesized that the number of micropyles as well as the reproductive output in H. axyridis could be affected by the delay of first copulation/fertilization. (2) In insects, the mutations usually had vastly different biological and physiological characteristics compared to the wild type. Studies on Bombyx mori (Lepidoptera: Bombycidae) have shown that the eggs of mutations (e.g. emi, Ge, Se, bd and bdsw) usually had vastly different number of micropylar channels compared to those of normal strain22,23,24. For H. axyridis, a light-colored mutant gr (HAM) was found in our laboratory and displayed obvious different elytra coloration (grey-dark spots) compared to the wild type (HAW, deep-dark spots), and also showed to be an adaptive deficiency25. Thus, we hypothesized that the number of micropyles in HAM might be different from that in HAW. In addition, phenotype-dependent mate choice has been confirmed to be obvious in H. axyridis. For example, in a Chinese population, both succinic and melanic females preferred to mate with succinic males and resulted in relatively higher fertility, which showed that melanism in H. axyridis might result in pleiotropic effects on male fertility26. Here, we doubt if there were any significant differences of the number of micropyles among the crosses of HAM and HAW. Actually, studies conducted before have shown that mysterious interaction effects of HAM and HAW were detected for egg production as well as hatch rate25.
In this study, a series of delayed mating experiments were conducted to confirm whether fertilization stimulus as well as mating delay would affect the number of micropyles as well as reproductive output. After that, a reciprocal cross experiments of HAM and HAW were conducted to confirm whether there was any significance of the number of micropyles between HAM and HAW, in particular, focused on the effects of male partner. The aim of this study was to elucidate the reproductive characters of H. axyridis from the perspective of micropyle variation, especially under delayed mating conditions.
Results
Delayed mating experiment
Number of micropyles
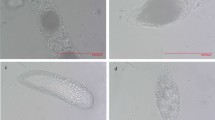
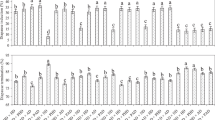
Typical distribution patterns of the micropyles located on an egg were shown in Fig. 1a. Eggs produced by females in control had an average number of 21.0 ± 0.4 micropyles, which was significantly higher than that in treatment virgin female (17.6 ± 0.3) and those in the four delayed mating treatments (DDM-A (18.6 ± 0.3), DDM-B (19.6 ± 0.3), DDM-C (18.0 ± 0.4) or DDM-D (19.6 ± 0.3)) (Kruskal-Wallis χ2 = 67.358, p < 0.0001). Number of micropyles deposited on eggshell in treatments DDM-B and DDM-D was significantly higher than that in treatment virgin female; while, no significant difference was detected among the four delayed mating treatments, except that DDM-C had significantly less micropyles compared to DDM-B or DDM-D (Kruskal-Wallis χ2 = 67.358, p < 0.001) (Fig. 1b).
Typical distribution patterns of micropyles (a), and the average number of micropyles located on an egg produced by virgin female and delayed mating females (b). In the box plots, horizontal line within the box is the median; box indicates the lower and upper quartiles; capped vertical lines are 95% confidence limits, and white dots are outliers. Different letters indicate significant differences (Kruskal-Wallis test, p < 0.05). DDM-A, DDM-B, DDM-C, and DDM-D respectively represented treatment 2, 7, 12, and 17 days of mating delay.
Egg production
Virgin female of H. axyridis can only lay small number of eggs on each day; while the oviposition greatly increased at the third day following virgin female mated with a male, irrespective of the female age at mating (Fig. 2a). For the 10 days’ post-mating oviposition, the average number of eggs produced by female was similar in treatment DDM-A (43.2 ± 2.3), DDM-B (37.7 ± 2.6), DDM-C (47.4 ± 2.9), and DDM-D (43.3 ± 3.1), and all were not significantly different from that in control (42.3 ± 2.3) (F4, 45 = 1.672, p = 0.173) (Fig. 2b).
Dynamic trend of the egg production of virgin female and four delayed mating treatments (a), and the average number of eggs produced by the delayed mating and normally mated (control) females (b). a: the arrow indicated the date that provided a male for virgin female in treatments DDM-A, DDM-B, DDM-C, and DDM-D. b: in the box plots, horizontal line within the box is the median; box indicates the lower and upper quartiles; capped vertical lines are 95% confidence limits, and white dots are outliers. No significant differences were detected among the treatments (Tukey HSD test, p > 0.05). DDM-A, DDM-B, DDM-C, and DDM-D respectively represented treatment 2, 7, 12, and 17 days of mating delay.
Egg hatch rate
Egg hatch rates were also not significantly affected by delayed mating. No significant differences were detected among the four delayed mating treatments (DDM-A (45.2 ± 5.2%), DDM-B (34.1 ± 3.8%), DDM-C (45.4 ± 6.8%), and DDM-D (53.2 ± 5.1%)), and all were similar to that in control (43.0 ± 3.1%) (Kruskal-Wallis χ2 = 8. 889, p = 0.064) (Fig. 3).
Box plots showing differences in egg hatch rates among the four delayed mating treatments and control. In the box plots, horizontal line within the box is the median; box indicates the lower and upper quartiles; capped vertical lines are 95% confidence limits, and white dots are outliers. No significant differences were detected among the treatments (Kruskal-Wallis test, p > 0.05). DDM-A, DDM-B, DDM-C, and DDM-D respectively represented treatment 2, 7, 12, and 17 days of mating delay.
Crossing experiments of the mutant and wild phenotype
The average number of micropyles located on an egg varied significantly among the four crosses, and they all were significantly different from that of virgin HAW female (Kruskal-Wallis χ2 = 187.09, p < 0.001). Specifically, among the four crosses, number of micropyles located on wild-type homozygous eggs from Cross-B (22.1 ± 0.5) was significantly higher than those on eggs from the other three crosses, and the least number was detected on heterozygous eggs from Cross-D (13.6 ± 0.3); the mutant homozygous eggs from Cross-A (15.2 ± 0.3) had significantly higher number of micropyles compared to the heterozygous eggs from Cross-D, but the number was significantly smaller than those on heterozygous eggs from Cross-C (19.1 ± 0.4). Eggs from virgin HAW female had significantly smaller number of micropyles (17.4 ± 0.3) compared to those from HAW female in Cross-B or Cross-C, but the number was significantly higher than those from HAM female in Cross-A or Cross-D (Kruskal-Wallis χ2 = 187.09, p < 0.001) (Fig. 4).
Box plots showing differences in average number of micropyles among the four crosses of light-colored mutant of H. axyridis (HAM) and wild type H. axyridis (HAW). In the box plots, horizontal line within the box is the median; box indicates the lower and upper quartiles; capped vertical lines are 95% confidence limits, and white dots are outliers. Asterisk indicates a significant difference (Kruskal-Wallis test, p < 0.05). Cross-A, Cross-B, Cross-C, and Cross-D respectively represented the cross ♀(HAM) × ♂(HAM), ♀(HAW) × ♂(HAW), ♀(HAW) × ♂(HAM), and ♀(HAM) × ♂(HAW).
Discussion
In this study, we found that the number of micropyles in H. axyridis was greatly affected by the fertilization stimulus of female, and delayed mating (or fertilization) can also result in the variation of micropyles. Based on the mutation HAM, we further found that female as well as its interaction with male partner can determine the number of micropyles. All these results proved that the number of micropyles in H. axyridis was plastic.
In delayed mating experiments, virgin female of H. axyridis can lay small number of eggs that were also presented with micropyles, but the average number was significantly smaller than that in control. These results indicated that the stimulus of copulation (or special substances) might affect the formation of micropyle on eggshell. The possible mechanisms might be listed as following. Firstly and most importantly, the formation of micropyles was a considerable material and (or) energetic cost process7, while for virgin female, the micropyles serving as the route of sperm entry into a mature oocyte was not functional necessary2,3. Thus, decreasing the number of micropyles on eggs produced by virgin female might be a material and (or) energetic saving strategy. In addition, in the process of internal fertilization, females obtained a cocktail of molecules which could affect virtually all aspects of the female’s reproductive activity, e.g. leading to the increasing production of yolk protein by follicle cells27,28,29,30, and, during insect oogenesis, micropyles has been confirmed to be deposited by the follicle cells31. Thus, virgin female that not obtain such molecules from male might affect the formation of micropyles. The typical examples were observed in many social insect species (e.g. ant or termite) that the micropyle structure disappeared on eggs produced by virgin workers11,12. Here, for H. axyridis, almost 17 micropyles were still presented on eggs from virgin female. Results from delayed mating experiments partly revealed the significance of this specialty and the crucial roles of timely fertilization in normal formation of micropyles.
When the virgin female was provided with a male at different periods post the first oviposition, the average number of micropyles located on an egg was still significantly less than that in control. For H. axyridis, Obata21 deduced that their mating acceptance is coincides with the start of oocyte development, and studies showed that the formation of egg shell and other associated structures is occurred at around the 6–7th day post-emergence under 25 °C32. Under similar developmental temperature, first mating of H. axyridis occurred at the 3rd day post-emergence and all individuals have mated at least one time at the 5th day33. Thus, mating occurring of H. axyridis seemed to be a little earlier than eggshell formation, and timely copulation/fertilization might be the stimulus for normal formation of micropyles on eggshell. However, in a social insect, the honey bee, Apis mellifera (Hymenoptera: Apidae), the change in micropylar chorionic morphology was reported to be fertilization-independent34. In this study, we also found that eggs produced by females with specific periods of delayed mating (e.g. DDM-B and DDM-D) had significantly more micropyles than that in virgin female. Osawa35 reported that the ovarian development of H. axyridis was selection pressure-dependent and showed to be dynamic in ways that permit populations to cope with exotic variations (e.g. host and food resources). In our delayed mating experiments, the great increasing of egg production occurred two days later after the virgin female was mated. Thus, the differences of micropyles between DDM-B (or DDM-D) and virgin female might be caused by the come across of copulation/fertilization and special ovarian development stage of virgin female that following 7th or 17th day of asexual oviposition, but the real mechanisms remain still unclear.
In the intercross experiments of HAM and HAW, we found that the phenotype (or called quality) of female and male can also greatly affect the number of micropyles. Results showed that significantly less micropyles was detected on the mutant eggs from Cross-A compared to homozygous wild-type eggs from Cross-B. More importantly, mutant homozygous eggs from Cross-A or heterozygous eggs from Cross-D that produced by female HAM even had significantly smaller number of micropyles than the unfertilized eggs from virgin HAW female. These results indicated that the function of special genetic process might be dominated over that of fertilization. In insects, exochorion formation is governed by the genetic processes occurring within the follicle cells36. For example, the gene hemipterous (hep) is required for morphogenesis of micropyles in Drosophila37; and in the German cockroach, Blattella germanica (Blattaria: Blattellidae), the gene Brownie is necessary for the formation of micropyle-like structures38. Here, HAM is a single gene mutation that related to body pigmentation and how such pleiotropic effects occurring remain unknown. More importantly, the significant variances detected among the four crosses indicated a mysterious female × male interaction between HAM and HAW. For many insect species, female usually exhibit different reproductive investments in response to male quality39. HAM has been confirmed to be an adaptive deficiency25, and thus selective investments in reproduction might be occurred in the intercross of HAM and HAW. Actually, similar phenomenon was also found in egg production and hatch rate25.
However, in any case, a large quantity of micropyles were detected on eggs that produced by female H. axyridis. At a functional level, more micropyles would suggest greater potential for multiple sperm entry into an egg, which might offer several benefits. Firstly, the presence of multiple micropyles could increase the opportunity for post-copulatory choice of female within the egg environment6. For example, in the polyspermic ctenophore, Beroe ovata, the female pronucleus migrates among male pronuclei within the egg before fusing with one40, and studies conducted in Lepidoptera have also confirmed that the micropyle number was positively correlated with female promiscuity6. Actually, for H. axyridis, multiple mating is prevalent41, and the sperm stored by a female was from multiple males42. Secondly, polyspermy might be important for embryonic development. Studies conducted in both domestic fowl and the zebra finch, Taeniopygia guttata have confirmed that polyspermy is essential for their early embryonic development43. However, whether such deduced benefits are all available for an insect H. axyridis requires further studies to confirm, and other possible benefits also need to be explored.
In this study, mating delay, at least, did not cause any negative effects on the reproductive performance (egg production and hatch rate) of female H. axyridis; while, on the contrary, almost all studies conducted before have reported that delayed mating would result in reduced fecundity and fertility20,44,45. Maintaining high reproductive capability under mating delay conditions might be important characters contribute to the reproduction success of H. axyridis. Firstly, it can ensure this ladybird beetle overcome the negative effects caused by wind, rain or unfavorable temperatures during mating stage17. In addition, studies showed that H. axyridis females mostly overwintered (up to several months) as unmated individuals46,47, and the physiological status of non-mate could also greatly increase the survival of post-storage adults48. The characteristic that maintaining high reproductive output under mating delay could help them to quickly establish populations after winter season. In practice, based on these specialties, non-mated individuals of H. axyridis would be suitable for cold storage, and thus could further promote their application in biological control program in the native regions.
In conclusion, our results confirmed that the number of micropyles in H. axyridis is plastic, but maintaining a high-quantity that would offer many benefits. In perspective of micropyle, the results revealed another possible mechanism that contributes to the reproduction success of H. axyridis. In addition, these results informed us new knowledge of factors that could influence the formation of micropyle in insects, which might encourage more studies to elucidate the molecular mechanisms.
Materials and Methods
Insects
Colonies of the pea aphid (Acyrthosiphon pisum (Hemiptera: Aphididae)) and the peach aphid (Myzus persicae (Hemiptera: Aphididae)) were respectively established on broad bean (Vicia faba, var. “Jinnong”) and pepper (Capsicum annum fasciculatum, var. “Changfeng”) seedlings in nylon mesh cages (60 × 45 × 40 cm). The wild type of H. axyridis (indicated as HAW) were from continuous laboratory rearing colonies established on A. pisum; while, the colony of light-colored mutant of H. axyridis (HAM) was established on M. persicae due to their poor reproductive performance on A. pisum (unpublished data). All these insects were reared in an insectary (24 ± 1 °C, 65% RH and 14:10 h L: D) at Gansu Agricultural University.
In order to prepare newly emerged adults for subsequent experiments, two pairs of HAW and HAM were respectively kept in a plastic Petri dish (9 cm in diameter) and supplemented with sufficient aphids as food. In addition, two host plant leaves were provided as the oviposition substrate. The egg production was checked once a day and the eggs were incubated in a new plastic Petri dish (3 cm in diameter) with an immersed cotton ball for keeping moisture. Newly hatched larvae (25 to 30) were reared in plastic Petri dishes (9 cm in diameter, 1.5 cm in height) equipping with barriers, which was confirmed to be effective in reducing larval cannibalism (unpublished data). Specifically, the barriers were made with transparent plastic bands (1.5 cm in width), and one circular band (4.5 cm in diameter) was connected with eight short bands (4.5 cm long) with equal distance (one end of them were jointed together in the center area of the Petri dish). Thus, the Petri dish area was divided into 16 partitions, while the larvae could move freely through a small gap located in the walls of each partition. The larvae were daily supplied with sufficient A. pisum (for HAW) or M. persicae (for HAM) (ca 120 mg) as food until pupation. Adult emergence was monitored and the newly emerged adults were used for different measurements. The following experiments were all conducted in bioclimatic chambers set at 24 ± 1 °C, 65% RH and 14:10 h L:D.
Experimental design and sample collection
Delayed mating experiments
Newly emerged HAW adults were sexed and equally divided into six groups. Group 1, female was immediately paired with a male after emergence and served as control. Group 2, continuous egg production by virgin females, in which the female adults were individually reared throughout the experiments. The rest adults were individually reared in plastic Petri dishes (9 cm in diameter) for treatments of mating delay. Egg production of all females was carefully checked once a day. We found that females in control as well as the virgin females started to lay eggs at almost the 7th day post-emergence. Once egg production initiated, the virgin females were divided into four groups. Group 3–6, different days delayed mating (DDM), each female was provided with a male (no mating experience) at the 2nd, 7th, 12th, or 17th day post the first oviposition and respectively named DDM-A, DDM-B, DDM-C, and DDM-D. Eggs from each pair were counted and incubated in one Petri dish (3 cm in diameter) with an immersed cotton ball for keeping moisture. Specially, at the 2nd, 5th, and 10th day post-mating, 6–8 eggs were randomly selected from the egg cluster of each pair and used for determination of the number of micropyles. After hatching, the inactive infant larvae were counted and immediately transferred out from the Petri dish to avoid cannibalizing on the eggs. Egg hatch rate was calculated following the equation: egg hatch rate = number of hatched larvae/number of eggs incubated × 100%. In total, 10 females were used for each treatment, and their post-mating oviposition were recorded for 10 days.
Crossing experiments of HAM and HAW
Newly emerged adults of HAM and HAW were paired as four groups with the design of reciprocal crosses, and each pair were kept in a plastic Petri dish and supplied with sufficient M. persicae (ca 100 mg) infesting on pepper leaves. The crosses ♀(HAM) × ♂(HAM), ♀(HAW) × ♂(HAW), ♀(HAW) × ♂(HAM), and ♀(HAM) × ♂(HAW) were respectively named as Cross-A, Cross-B, Cross-C, and Cross-D. Their oviposition were checked once a day and, at the 5–6th day post-oviposition, 15 eggs were randomly selected from the egg cluster of each pair for determination of the number of micropyles. In total, twelve pairs were used for each group.
Determination of the number of micropyles
After collection, the eggs from each pair were immediately transferred to a 1.5 ml centrifuge tube containing 10% ethanol. Then, they were dehydrated in a graded series of 20%, 30%, 50% ethanol for 20 min each and maintained in 70% ethanol before measurement. Number of micropyles located on the egg shell was determined by photographing the top area with scanning electron microscope (SEM).
For SEM photographing, the sample preparations were following the methods described by Hao et al.49. Samples were dehydrated in a graded series of 80%, 85%, 90%, 95% ethanol for 20 min each and 99.9% ethanol for 30 min twice before being transferred to a mixed solution of ethanol and tert-butanol (3:1, 1:1, and 1:3, by volume) for 15 min each, and finally in 100% tert-butanol for 30 min. After removal from the tert-butanol, the specimens were transferred into a freeze-drier (VFD-21S, SHINKKU VD, Japan) for 3 h. The dried specimens were mounted on aluminum stubs using double-sided copper sticky tape and coated with gold/palladium (40/60) in a high-resolution sputter coater (MSP-1S, SHINKKU VD, Japan). The samples were subsequently photographed with a Hitachi S-3400N SEM (Hitachi, Tokyo, Japan) operated at 15 kV. After then, number of micropyle located on the top area of each egg was counted from the images.
Data analysis
Before analysis, all data were performed with Bartlett’s test and Shapiro-Wilk normality test. For a given parameter, one-way analysis of variance (ANOVA) was applied for comparing the differences among different treatments, while the non-parametric Kruskal-Wallis test was used under non-normal and heteroscedastic conditions. Specially, in delayed mating experiments, at first, the number of micropyles located on eggs collected at the 2nd, 5th, and 10th day post the first oviposition were compared, and no significant variance was detected among the three sampling periods, with one exception of DDM-C (Control: Kruskal-Wallis χ2 = 3.282, p = 0.1938; Virgin female: F2, 89 = 1.323, p = 0.272; DDM-A: Kruskal-Wallis χ2 = 1.487, p = 0.475; DDM-B: F2, 89 = 1.882, p = 0.158; DDM-C: Kruskal-Wallis χ2 = 6.574, p = 0.0374; DDM-D: F2, 89 = 0.430, p = 0.652), thus these data were pooled together for analysis; in crossing experiments of HAM and HAW, the differences of number of micropyle among the four groups and virgin HAW female (eggs collected at the 5th day post first oviposition) were compared. Means were separated with Tukey HSD test (p < 0.05). For analyzing the differences of micropyles, almost 90 available photographs were used in each treatment. All data analyses were conducted with R version 3.2.1 software50.
Data Availability
Te datasets generated and analyzed during the current study are available in the fgshare repository, https://figshare.com/s/ae454eb19c3092e68992.
References
Hinton, H. E. Biology of insect eggs: in 3 vol (Oxford, UK: Pergamon Press, 1981).
Kubrakiewicz, J., Jędrzejowska, I., Szymańska, B. & Biliński, S. M. Micropyle in neuropterid insects. Structure and late stages of morphogenesis. Arthropod Struct. Dev. 34, 179–188 (2005).
Yamauchi, H. & Yoshitake, N. Formation and ultrastructure of the micropylar apparatus in Bombyx mori ovarian follicles. J. Morphol. 179, 47–58 (1984).
Gullan, P. & Cranston, P. The insects: an outline of entomology (Oxford, UK: Blackwell Publishing, 2005).
Osawa, N. & Yoshinaga, A. The presence of micropyles in the shells of developing and undeveloped eggs of the ladybird beetle Harmonia axyridis (Coleoptera: Coccinellidae). Eur. J. Entomol. 106, 607–610 (2009).
Iossa, G., Gage, M. J. & Eady, P. E. Micropyle number is associated with elevated female promiscuity in Lepidoptera. Biol. Letters 12, 20160782 (2016).
Kudo, S.-I., Nakahira, T. & Saito, Y. Morphology of trophic eggs and ovarian dynamics in the subsocial bug Adomerus triguttulus (Heteroptera: Cydnidae). Can. J. Zool. 84, 723–728 (2006).
Chapman, R. F. The insects: structure and function, fourth ed. (Cambridge, UK: Cambridge University Press, 1998).
Koedam, D., Velthausz, P., Dohmen, M. & Sommeijer, M. Morphology of reproductive and trophic eggs and their controlled release by workers in Trigona (Tetragonisca) angustula llliger (Apidae, Meliponinae). Physiol. Entomol. 21, 289–296 (1996).
Alexander, R. D. The evolution of social behavior. Annu. Rev. Ecol. Syst. 5, 325–383 (1974).
Gobin, B., Peeters, C. & Billen, J. Production of trophic eggs by virgin workers in the ponerine ant Gnamptogenys menadensis. Physiol. Entomol. 23, 329–336 (1998).
Li, G. H. et al. Physiological profiles associated with ceasing growth of unfertilized eggs produced by unmated queens in the subterranean termite Reticulitermes chinensis. Biol. Open 5, 756–763 (2016).
Yashiro, T. & Matsuura, K. Termite queens close the sperm gates of eggs to switch from sexual to asexual reproduction. P. Natl. A. Sci. 111, 17212–17217 (2014).
Koch, R. L. The multicolored Asian lady beetle, Harmonia axyridis: a review of its biology, uses in biological control, and non-target impacts. J. Insect Sci. 3, 1–16 (2003).
Laugier, G. J. M. et al. Increase in male reproductive success and female reproductive investment in invasive populations of the harlequin ladybird Harmonia axyridis. PLoS One 8, e77083, https://doi.org/10.1371/journal.pone.0077083 (2013).
Perry, J. C. & Roitberg, B. D. Ladybird mothers mitigate offspring starvation risk by laying trophic eggs. Behav. Ecol. Sociobiol. 58, 578–586 (2005).
Carde, R. T. & Minks, A. K. Control of moth pests by mating disruption: successes and constraints. Annu. Rev. Entomol. 40, 559–585 (1995).
Torres-Vila, L. M., Rodríguez-Molina, M. C. & Stockel, J. Delayed mating reduces reproductive output of female European grapevine moth, Lobesia botrana (Lepidoptera: Tortricidae). B. Entomol. Res. 92, 241–249 (2002).
Wang, X. P., Fang, Y. L. & Zhang, Z. N. Effects of delayed mating on the fecundity, fertility and longevity of females of diamondback moth, Plutella xylostella. Insect Sci. 18, 305–310 (2011).
Wenninger, E. J. & Averill, A. L. Effects of delayed mating on reproductive output of female oriental beetle Anomala orientalis (Coleoptera: Scarabaeidae). Agr. Forest Entomol. 8, 221–231 (2006).
Obata, S. Mating refusal and its significance in females of the ladybird beetle Harmonia axyridis. Physiol. Entomol. 13, 193–199 (1988).
Kawaguchi, Y., Kusakabe, T. & Koga, K. Morphological variation of micropylar apparatus in Bombyx mori eggs. J. Insect Biotechnol. Sericol. 71, 49–54 (2002).
Kawaguchi, Y., Kusakabe, T., Lee, J. M., Nakajima, Y. & Koga, K. Micropylar structure of chorion of the female sterile mutation, bd, in Bombyx mori. J. Insect Biotechnol. Sericol. 75, 9–14 (2006).
Kawaguchi, Y., Yoshida, H., Kusakabe, T., Lee, J. M. & Koga, K. Characteristics of Se, the white-sided egg mutation in Bombyx mori. J. Insect Biotechnol. Sericol. 76, 71–77 (2007).
Sun, Y. X. et al. Morphological and biological characterization of a light-colored mutant in the multicolored Asian lady beetle, Harmonia axyridis. Ecol. Evol. 8, 9975–9985 (2018).
Wang, S., Michaud, J. P., Tan, X. L., Murray, L. & Zhang, F. Melanism in a Chinese population of Harmonia axyridis, (Coleoptera: Coccinellidae): a criterion for male investment with pleiotropic effects on behavior and fertility. J. Insect Behav. 26, 679–689 (2013).
Gillott, C. Male accessory gland secretions: modulators of female reproductive physiology and behavior. Annu. Rev. Entomol. 48, 163–184 (2003).
Ravi, R. K. & Wolfner, M. F. Seminal influences: Drosophila Acps and the molecular interplay between males and females during reproduction. Integr. Comp. Biol. 47, 427–445 (2007).
Soller, M., Bownes, M. & Kubli, E. Mating and sex peptide stimulate the accumulation of yolk in oocytes of Drosophila melanogaster. Eur. J. Bioch. 243, 732–738 (1997).
Soller, M., Bownes, M. & Kubli, E. Control of oocyte maturation in sexually mature Drosophila females. Dev. Biol. 208, 337–351 (1999).
Rezende, G. L., Vargas, H. C. M., Moussian, B. & Cohen, E. Composite eggshell matrices: chorionic layers and sub-chorionic cuticular envelopes. In Extracellular composite matrices in arthropods. 325–366 (New York: Springer, 2016).
Chen, J. et al. Ovarian development and oogenesis of Harmonia axyridis Pallas. J. Plant Prot. 42, 237–243 (2015).
Stathas, G. J., Eliopoulos, P. A., Kontodimas, D. C. & Giannopapas, J. Parameters of reproductive activity in females of Harmonia axyridis (Coleoptera: Coccinellidae). Eur. J. Entomol. 98, 547–549 (2001).
Williams, J. L. Metamorphosis of the micropylar chorion of the honey bee (Hymenoptera: Apidae) egg. Ann. Entomol. Soc. Am. 79, 971–974 (1986).
Osawa, N. The effect of prey availability on ovarian development and oosorption in the ladybird beetle Harmonia axyridis (Coleoptera: coccinellidae). Eur. J. Entomol. 102, 503–511 (2005).
Chasan, R. & Anderson, K. V. Maternal control of dorsal-ventral polarity and pattern in the embryo. In The development of Drosophila melanogaster (eds Bate, M. & Martinez-Arias, A.) 387–424 (New York: Cold Spring Harbor Laboratory Press, 1993).
Suzanne, M., Perrimon, N. & Noselli, S. The Drosophila JNK pathway controls the morphogenesis of the egg dorsal appendages and micropyle. Dev. Biol. 237, 282–294 (2001).
Irles, P., Bellés, X. & Piulachs, M. D. Brownie, a gene involved in building complex respiratory devices in insect eggshells. PLoS One 4, e8353, https://doi.org/10.1371/journal.pone.0008353 (2009).
Sheldon, B. C. Differential allocation: tests, mechanisms and implications. Trends Ecol. Evol. 15, 397–402 (2000).
Carre, D. & Sardet, C. Fertilization and early development in Beroe ovata. Dev. Biol. 105, 188–195 (1984).
Osawa, N. The occurrence of multiple mating in a wild population of the ladybird beetle Harmonia axyridis Pallas (Coleoptera: Coccinellidae). J. Ethol. 12, 63–66 (1994).
Ueno, H. Estimate of multiple insemination in a natural population of Harmonia axyridis (Coleoptera: Coccinellidae). Appl. Entomol. Zool. 31, 621–623 (1996).
Hemmings, N. & Birkhead, T. R. Polyspermy in birds: sperm numbers and embryo survival. Proc. R. Soc. B 282, 20151682, https://doi.org/10.1098/rspb.2015.1682 (2015).
Amoah, B. A., Mahroof, R. M., Gerken, A. R. & Campbell, J. F. Effect of delayed mating on longevity and reproductive performance of Lasioderma serricorne (Coleoptera: Anobiidae). J. Econ. Entomol. 112, 475–484 (2019).
Walker, P. W. & Allen, G. R. Delayed mating and reproduction in the autumn gum moth Mnesam pelaprivata. Agr. Forest Entomol. 13, 341–347 (2011).
Nalepa, C. A., Kidd, K. A. & Ahlstrom, K. R. Biology of Harmonia axyridis (Coleoptera: Coccinellidae) in winter aggregations. Ann. Entomol. Soc. Am. 89, 681–685 (1996).
Iperti, G. & Bertrand, E. Hibernation of Harmonia axyridis (Coleoptera:Coccinellidae) in Southern France. Acta Soc. Zool. Bohem. 65, 207–210 (2001).
Facon, B. et al. Mating status influences cold tolerance and subsequent reproduction in the invasive ladybird Harmonia axyridis. Front. Ecol. Evol. 5, 108, https://doi.org/10.3389/fevo.2017.00108 (2017).
Hao, Y. N., Dietrich, C. H. & Dai, W. Development of mouthparts in the cicada Meimuna mongolica (Distant): successive morphological patterning and sensilla differentiation from nymph to adult. Sci. Rep. 6, 38151, 38110.31038/srep38151 (2016).
R Development Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing (2015).
Acknowledgements
We are grateful for the assistance of all staff and students in the Biocontrol Engineering Laboratory of Crop Diseases and Pests of Gansu Province, College of Plant Protection, Gansu Agricultural University, Lanzhou, China. Funding of this research was partially supported by the following grants: Scientific Research Start-up Funds for Openly-recruited Doctors of Gansu Agricultural University (2017RCZX-09), the Lift-Project Funds for Young Scientific Talents of Gansu Province (GKXTJ20181126), the National Natural Science Foundation of China (31660522), Open Funds of Biocontrol Engineering Laboratory of Crop Diseases and Pests of Gansu Province (BELCDP-2018-01), and the National Key Research and Development Program of China (2018YFD0200405).
Author information
Authors and Affiliations
Contributions
Y.X.S. and C.Z.L. designed the research; Y.X.S. and Y.N.H. performed the research; and Y.X.S., Y.N.H., and S.S.W wrote the paper.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sun, YX., Hao, YN., Liu, CZ. et al. Investigating reproductive success of the ladybird beetle Harmonia axyridis from the perspective of micropyle variation. Sci Rep 9, 12742 (2019). https://doi.org/10.1038/s41598-019-49249-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-49249-z
This article is cited by
-
Reproductive behaviour of predaceous ladybirds (Coleoptera: Coccinellidae): A review
International Journal of Tropical Insect Science (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.