Abstract
A rapid and early diagnostic test to identify the encephalopathic babies at risk of adverse outcome may accelerate the development of neuroprotectants. We examined if a whole blood transcriptomic signature measured soon after birth, predicts adverse neurodevelopmental outcome eighteen months after neonatal encephalopathy. We performed next generation sequencing on whole blood ribonucleic acid obtained within six hours of birth from the first 47 encephalopathic babies recruited to the Hypothermia for Encephalopathy in Low and middle-income countries (HELIX) trial. Two infants with blood culture positive sepsis were excluded, and the data from remaining 45 were analysed. A total of 855 genes were significantly differentially expressed between the good and adverse outcome groups, of which RGS1 and SMC4 were the most significant. Biological pathway analysis adjusted for gender, trial randomisation allocation (cooling therapy versus usual care) and estimated blood leukocyte proportions revealed over-representation of genes from pathways related to melatonin and polo-like kinase in babies with adverse outcome. These preliminary data suggest that transcriptomic profiling may be a promising tool for rapid risk stratification in neonatal encephalopathy. It may provide insights into biological mechanisms and identify novel therapeutic targets for neuroprotection.
Similar content being viewed by others
Introduction
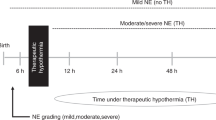
Neonatal encephalopathy (NE) related to perinatal asphyxia is the most common cause of death and neurodisability in term babies with an incidence of 2 to 3 per 1,000 live births in high-income countries, and 10 to 20 per 1,000 livebirths in low and middle-income countries1,2. In high income countries, therapeutic hypothermia partially improves outcomes, although adverse outcomes still occur in up to 30% of the cooled infants3.
As the underlying brain injury evolves over hours and days after birth, early identification of at-risk encephalopathic infants is challenging and often inaccurate, particularly in cooled infants4. Furthermore, NE is heterogenous condition resulting from a multitude of aetiologies including acute or sub-acute perinatal hypoxia, inflammation, infection, metabolic and genetic causes, although these may be clinically indistinguishable5. These difficulties have hindered the development of novel neuroprotective strategies. A better understanding of molecular mechanisms of hypoxia-induced brain injury and of the different underlying pathologies has translational potential that may lead to future discoveries and novel neuroprotective therapies.
The use of gene expression for disease stratification and for elucidation of underlying disease mechanisms has previously been shown in sepsis, paediatric tuberculosis and Kawasaki Disease6,7,8. More recently, we have reported that babies with NE have a unique gene expression profile when compared with healthy controls and septic babies, and have an upregulation of the hypoxia inducible transcription factor 1α (HIF1α)9. Here, we examined if babies who developed adverse neurodevelopmental outcome after NE, had a unique host blood gene expression profile at birth.
Results
We analysed the blood samples from the first 47 neonates recruited to a randomised controlled trial of whole body cooling versus usual care—HELIX (Hypothermia for Encephalopathy in Low and Middle-Income Countries) trial10. Two neonates with positive blood culture sepsis (Klebsiella pneumoniae and Pseudomonas aeruginosa) were excluded and therefore 45 infants were included in the analysis. The adverse outcome group was defined as death or moderate or severe disability (motor disability and Bayley III). Neurodevelopmental outcome data, based on Bayley assessments carried-out between 18 to 22 months of age, were available in all infants, of which 23 (51%) had adverse outcome and 22 (49%) had good outcome. No significant demographic differences were found between NE infants with adverse and good outcome (Table 1).
As the trial is ongoing, authors were masked to the treatment (therapeutic hypothermia) allocation, which was provided as A or B. There was no statistically significant difference in outcome between treatment groups A and B, and the clinical severity of NE in two groups was balanced at the time of randomisation allocation (Supplementary Fig. 1). Differential gene expression analyses comparing infants with adverse and good outcome revealed 855 significant differentially expressed genes with a false discovery rate (FDR) value < 0.05 and an absolute log2 fold change ≥ 0.4, after adjusting for the randomisation allocation and gender (Fig. 1A). Of these genes, 523 (61%) were over-expressed and 332 (38%) under-expressed in NE infants with adverse outcome as shown in the volcano plot.
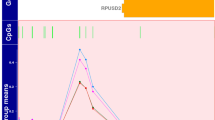
(A) Volcano plot showing the significant genes identified in the comparison of neonates with adverse versus good outcome, plotted according to log2 fold-change (x axis) and log10 p value (y axis). In green are genes with false discovery rate (FDR) < 0.05 and log2 fold change < 0.4 in red are genes with FDR < 0.05 and log2 fold change > 0.4. (B) Box plot (median, IQR) of gene count values expressed as Fragments Per Kilobase of transcript per Million mapped reads (FPKM) (y axis) of the 6 most significant genes for children with normal (blue) compared with abnormal neurodevelopmental outcome at 2 years (orange). (C) Brain hypoxia leads to Ca2+ influx with activation of the Ca2+/calmodulin dependent protein kinase IV (CaMK-IV) cascade. CaMK-IV in the cytosol has a proapoptotic effect and is responsible for hypoxic neural cell death both through activation of MAP kinases signalling in the cytosol and through phosphorylation of cAMP response element-binding protein (CREB) in the nucleus, which enhances the expression of pro-apoptotic proteins. Melatonin binds to its plasma membrane receptor MTNR1, to calmodulin and to nuclear receptor retinoid-related receptor alpha (RORA) increasing its expression. RORA is also considered a downstream target of HIF-1α and its levels have been found upregulated in the cellular response to hypoxia. MTNR1A and MTNR1B activation increases PKC activity through activation of Gαq, which stimulates the PLC signalling cascade and leads to inhibition of Ca2+/calmodulin dependent protein kinase (CAMK). Both MTNR1A and MTNR1B activation by melatonin inhibits cAMP formation. Furthermore, activation of MTNR1B decreases the expression of the glucose transporter GLUT4, which in turn decreases glucose uptake. The upregulated genes in our analysis are shown in red, while the downregulated genes are shown in green. CALM Calmodulin, CAMK4 calcium/calmodulin dependent protein kinase 4, CAMKII calcium/calmodulin dependent protein kinase II, CREB cAMP-response element binding protein, DAG diacylglycerol, ERK extracellularly regulated kinase, Gαq Gq protein alpha subunit, Gαi α subunit of the heterotrimeric G protein complex, GLUT4 Glucose transporter type 4, Hif-1 alpha Hypoxia-inducible factor 1-alpha, PIP2 Phosphatidylinositol biphosphate, PKA protein kinase A, PKC Protein Kinase C signalling, MAPK mitogen-activated protein kinases, MTNR1A Melatonin receptor type 1A, MTNR1B Melatonin receptor type 1B, IP3 Inositol trisphosphate, NMDAR N-methyl-D-aspartate receptor, RORA Retinoid-related receptor alpha. A,B were created using R (version 4.0.0) (https://cran.r-project.org/). C Was created through the use of Ingenuity pathway software (QIAGEN Inc., https://www.qiagenbioinformatics.com/products/ingenuity-pathway-analysis).
The most significant gene ordered by FDR was regulator of G-protein signalling 1 (RGS1) (FDR value < 0.001, log2 fold change 2.28) followed by structural maintenance of chromosomes protein 4 (SMC4) (FDR value < 0.001, log2 fold change 0.63) (Fig. 1B).
Finally, we re-analysed the data after adjusting for the randomisation allocation, gender and blood cell type proportions (neutrophils and lymphocyte cell fractions) and identified 660 significant differentially expressed genes with an FDR value < 0.05 and an absolute log2 fold change ≥ 0.4 (Supplementary Fig. 2). Of these genes, 389 (59%) were over-expressed and 271 (41%) under-expressed. The most significant gene remained RGS1 (FDR value < 0.001, log2 fold change 2.28) together with SMC4 (FDR value < 0.001, log2 fold change 0.63). We then applied pathway enrichment analysis (Ingenuity Pathway Analysis—www.Ingenuity.com) to all the differentially expressed genes (FDR < 0.01 and absolute log2 fold change ≥ 0.4). The most statistically significant pathways based on the FDR and ratio values were Mitotic Roles of Polo-Like Kinase (FDR = 0.002, ratio = 0.15) and Melatonin (FDR = 0.003, ratio = 0.11) (Fig. 1C).
We assessed the relationship of genes of interest with different blood cell types (neutrophils and lymphocytes)11 and found that RGS1, SMC4, as well as the differentially expressed genes of the melatonin pathway were most positively correlated with the lymphocytes fraction (Supplementary Table 1).
Discussion
Our study shows that infants who will go on to develop adverse outcome after NE have a specific gene expression profile, when compared to those who have good outcome. Our data also suggest that polo like kinase and melatonin pathways play a key role in those babies with NE who later develop adverse outcomes. Melatonin is a powerful free radical scavenger, which controls cell death, free radical production, ATP consumption and integrity of the electron transport chain12,13. Polo-like kinases instead, are activated by hypoxia/reoxygenation insult and result in a decreasing survival and increasing cell apoptosis14.
Despite the widespread use of therapeutic hypothermia as standard treatment in NE, up to 55% of infants with moderate/severe NE are reported to have adverse long-term outcome, in high-income countries15. Therefore, there is need to identify adjunct neuroprotective strategies to improve the outcome of infants with moderate or severe NE. Establishing the biological function of differentially expressed genes associated with adverse outcome after neonatal encephalopathy may enable better understanding of brain injury, permitting the identification of new therapeutic targets.
Different pre-clinical studies suggest the potential of melatonin as a neuroprotectant16. Melatonin counteracts the effects of hypoxia firstly through the inhibition of ERK/MAK signalling with down regulation of c-AMP response element binding (CREB) protein, secondly through the inhibition Ca2+/calmodulin-dependent protein kinases and finally through the upregulation of RORα, which causes a decreased expression of pro-apoptotic proteins and anti-inflammatory effects17,18,19. RORA is also considered a HIF-1 target gene and its levels have been found upregulated in the cellular response to hypoxia (Fig. 1C)20.
Animal evidence has shown that after hypoxia, there is an increased nuclear Ca2+ influx and nuclear Ca2+/calmodulin kinase IV (CaMKIV) activity, which enhances the expression of different transcription factors responsible for programmed cell death through the upregulation of CREB protein21,22. This latter is responsible for the neuronal apoptosis following cerebral hypoxia and is a crucial point of action of therapeutic hypothermia neuroprotective effects21,22,23. A recent study has underlined the importance of this cascade, showing that its synergic downregulation together with therapeutic hypothermia, potentiates neuroprotection with reduced cell death and improved neuropathology in a piglet hypoxia model24. Of note, CaMKIV levels have been found to increase with the increase in the degree of cerebral tissue hypoxia assessed by cerebral tissue high energy phosphates25. Therefore, the present upregulation of CaMKIV in our study, may reflect that infants with adverse outcome were those exposed to more severe degrees of cerebral hypoxia.
The polo-like kinase family are cell cycle process regulators, which are important mediators of the cellular responses to hypoxia and reactive oxygen species exposure26. Hypoxia-induced HIF-1α expression is strongly associated with a significant down-regulation of polo-like kinases expression in HeLa cells leading to increased cell survival and proliferation27. HIF-1 in fact, has as an important role under hypoxic conditions in regulating cell proliferation and metabolism. Taken together, our findings stress the leading role of HIF-1α in NE, which is involved in both the melatonin and polo-like kinase cascades27,28,29,30 and interacts directly with both RGS1 and SMC4, which are downstream targets of HIF-1α30,31. These results are also consistent with our previous data, comparing healthy infants and NE, which showed an upregulation of HIF1A and MALAT1 in NE9. MALAT1 inhibits HIF-1α ubiquitination and in this way, enhances its activity and increases anaerobic glycolysis.
In our study a marked differential expression was found in RGS1 and SMC4. SMC4 is responsible for regulating chromosome organization and dynamics32. Recent studies demonstrated also that SMC4 is associated with tumor progression and invasion and the expression of SMC4 is positively correlated with HIF-1a30. RGS1 protein is the most abundant RGS protein in the microglia and is a key gene of the immunomodulatory response to neuroinflammation as well as a key target of different immunological and neurodegenerative diseases such as multiple sclerosis, inflammatory bowel and Parkinson’s disease33,34. Recent evidence suggests that RGS1 protein is involved in the hypoxia-dependent inflammatory response35,36, through activation of the AKT signalling pathway, which is switched-on after chronic moderate hypoxia. A leading role of neuroinflammation is now increasingly recognized in neonatal brain damage following NE37,38. Therefore, the high expression levels of this gene around birth in babies with adverse outcome later in life, may reflect the increased hypoxia-dependent neuroinflammation in babies with an adverse outcome.
When we assessed the gene expression signature of different blood cell types, we found that the top differentially expressed genes were expressed the most in lymphocytes. This may reflect the immunomodulatory effect of HIF signal, which has been shown to increase the potency of regulatory T cells by affecting the expression of their transcriptional activator Foxp339.
Our study has several limitations. Firstly, the sample size of the study was small and these findings need to be validated in a larger cohort. Secondly, we examined gene expression at only one timepoint, within six hours after birth. Gene expression profiles are likely to change over time in the post-natal period and therefore our findings may differ if gene expression profiles are examined beyond this time window. Finally, this study was conducted in low-middle income countries and therefore, caution is needed before translating these findings to different settings.
Our research was exploratory without a predefined hypothesis on hypoxic-ischemic injury induced gene expression changes and was intended to examine the early host gene expression profile associated with later adverse outcomes. Our preliminary data suggest that babies with adverse outcome after NE had a characteristic gene expression profile that can be measured in whole blood soon after birth, and that selected transcripts correlated better with final clinical outcome than immediate clinical assessment of NE severity. If these findings can be replicated in larger cohort of babies with NE, it may open new therapeutic avenues and to develop new neuroprotection therapies in the future.
Materials and methods
Study design and subjects, samples
The HELIX trial was a multi-country randomised controlled trial of whole-body cooling versus usual care in NE and recruited 408 babies from seven tertiary neonatal units in India, Sri Lanka and Bangladesh between Aug 2016 and Feb 201910. The study was approved by the research ethics committee of Imperial College London, and recruiting hospital sites, and informed parental consent was obtained prior to recruitment. All methods were carried out in accordance with relevant guidelines and regulations.
All babies had a structured neurological examination based on the Eunice Kennedy National Institute of Child health and Human Development (NICHD) system by a certified examiner within six hours of birth and the babies were classified as having moderate or severe encephalopathy40,41. We collected 0.5 ml of blood (venous or arterial) from the recruited infants within six hours of birth and prior to initiation of cooling therapy, if randomised to the cooling arm. The blood was gently mixed with 1.4 mL RNA stabilizing solution (PreAnalytiX BD/QIAgen) and subsequently stored in a − 80 °C freezer until analysis. The blood samples from the first 47 infants recruited from three Indian sites were included in this work. We excluded babies with culture positive sepsis from the analysis. A flow-chart of the study is in supplementary Fig. 1.
Between 18 to 22 months of age, a certified examiner performed neurodevelopmental evaluation using Bayley scales of Infant development Version III. The primary outcome was death or moderate or severe disability. We defined severe disability as any of the following: a Bayley III cognitive score < 70; a Gross Motor Function Classification System (GMFCS) level of 3 to 5; blindness; or hearing loss (inability to understand commands despite amplification)41. We defined moderate disability as a Bayley III cognitive score of 70 to 84 and either a GMFCS level of 2, seizure disorder or a hearing deficit requiring amplification to understand commands41.
RNA extraction, alignment and next generation sequencing
We extracted total RNA from whole blood according to the manufacturer’s instructions and removed ribosomal RNA and globin mRNA from 4 microg of total RNA. RNA extracts were sequenced using Illumina HiSeq2500 to generate 30 M, 2 × 100 bp reads/sample (Medgenome Labs Ltd, Bangalore, India). Based on the quality report of fastq files, high quality sequence (Q ≥ 30) was retained for further analysis and the low-quality sequence reads were excluded from the analysis. Adapter trimming was performed using fastq-mcf (version—1.04.676) and cutadapt (version 1.8dev). Contamination removal was performed using Bowtie2 (version—2.2.4) and the paired end reads were aligned to the reference human genome (GRCh37/hg19) using “STAR-v2.4.1d”. Coverage analysis for genes was performed with “HTSeq-v0.6.1” (reference gtf: GRCh37 version 75) and “bedtools-v2.17.0”. The aligned reads were used for estimating expression of the genes and transcripts using the cufflinks program.
Data analysis
Data analysis was performed using R (version 4.0.0). For differential expression analysis we used DESeq2 (v1.17.18)42. We corrected all the p values for multiple testing using the Benjamini–Hochberg FDR method to control for type I error. The comparison of babies with adverse and good outcomes was performed first unadjusted and then adjusted for the randomisation allocation (masked as ‘A’ and ‘B’), gender and neutrophils and total lymphocyte cell counts, estimated by using CIBERSORT (deconvolution)11,43. The differentially expressed genes were subjected to pathway analysis (Ingenuity Pathway Analysis) and Fisher's Exact was used to assess the significance of the association between the differentially significant genes identified and the canonical pathways. Additional details of analysis are provided in the Supplementary Methods.
References
Kruse, M. et al. Lifetime costs of cerebral palsy. Dev. Med. Child Neurol. 51, 622–628. https://doi.org/10.1111/j.1469-8749.2008.03190.x (2009).
Montaldo, P., Pauliah, S. S., Lally, P. J., Olson, L. & Thayyil, S. Cooling in a low-resource environment: Lost in translation. Semin. Fetal Neonatal Med. 20, 72–79. https://doi.org/10.1016/j.siny.2014.10.004 (2015).
Jenkins, D. D. et al. Serum cytokines in a clinical trial of hypothermia for neonatal hypoxic-ischemic encephalopathy. J. Cereb. Blood Flow Metab. 32, 1888–1896. https://doi.org/10.1038/jcbfm.2012.83 (2012).
Murray, D. M. et al. The predictive value of early neurological examination in neonatal hypoxic-ischaemic encephalopathy and neurodevelopmental outcome at 24 months. Dev. Med. Child Neurol. 52, e55-59. https://doi.org/10.1111/j.1469-8749.2009.03550.x (2010).
Aslam, S., Strickland, T. & Molloy, E. J. Neonatal encephalopathy: Need for recognition of multiple etiologies for optimal management. Front. Pediatr. 7, 142. https://doi.org/10.3389/fped.2019.00142 (2019).
Anderson, S. T. et al. Diagnosis of childhood tuberculosis and host RNA expression in Africa. New Engl. J. Med. 370, 1712–1723. https://doi.org/10.1056/NEJMoa1303657 (2014).
Wright, V. J. et al. Diagnosis of Kawasaki disease using a minimal whole-blood gene expression signature. JAMA Pediatr. https://doi.org/10.1001/jamapediatrics.2018.2293 (2018).
Smith, C. L. et al. Identification of a human neonatal immune-metabolic network associated with bacterial infection. Nat. Commun. 5, 4649. https://doi.org/10.1038/ncomms5649 (2014).
Montaldo, P. et al. Whole blood gene expression reveals specific transcriptome changes in neonatal encephalopathy. Neonatology 115, 68–76. https://doi.org/10.1159/000492420 (2019).
Thayyil, S. et al. Hypothermia for encephalopathy in low and middle-income countries (HELIX): Study protocol for a randomised controlled trial. Trials 18, 432. https://doi.org/10.1186/s13063-017-2165-3 (2017).
Newman, A. M., Liu, C. L. & Green, M. R. Robust enumeration of cell subsets from tissue expression profiles. Nat. Methods 12, 453–457. https://doi.org/10.1038/nmeth.3337 (2015).
Hardeland, R. Melatonin and the electron transport chain. Cell. Mol. Life Sci. CMLS 74, 3883–3896. https://doi.org/10.1007/s00018-017-2615-9 (2017).
Gitto, E., Pellegrino, S., Gitto, P., Barberi, I. & Reiter, R. J. Oxidative stress of the newborn in the pre- and postnatal period and the clinical utility of melatonin. J. Pineal Res. 46, 128–139. https://doi.org/10.1111/j.1600-079X.2008.00649.x (2009).
Wang, L., Gao, J., Dai, W. & Lu, L. Activation of Polo-like kinase 3 by hypoxic stresses. J. Biol. Chem. 283, 25928–25935. https://doi.org/10.1074/jbc.M801326200 (2008).
Shankaran, S. et al. Neonatal magnetic resonance imaging pattern of brain injury as a biomarker of childhood outcomes following a trial of hypothermia for neonatal hypoxic-ischemic encephalopathy. J. Pediatr. 167, 987-993.e983. https://doi.org/10.1016/j.jpeds.2015.08.013 (2015).
McAdams, R. M. & Juul, S. E. Neonatal encephalopathy: Update on therapeutic hypothermia and other novel therapeutics. Clin. Perinatol. 43, 485–500. https://doi.org/10.1016/j.clp.2016.04.007 (2016).
Pandi-Perumal, S. R. et al. Physiological effects of melatonin: role of melatonin receptors and signal transduction pathways. Prog. Neurobiol. 85, 335–353. https://doi.org/10.1016/j.pneurobio.2008.04.001 (2008).
He, B. et al. The nuclear melatonin receptor RORalpha is a novel endogenous defender against myocardial ischemia/reperfusion injury. J. Pineal Res. 60, 313–326. https://doi.org/10.1111/jpi.12312 (2016).
Soto-Vega, E., Meza, I., Ramirez-Rodriguez, G. & Benitez-King, G. Melatonin stimulates calmodulin phosphorylation by protein kinase C. J. Pineal Res. 37, 98–106. https://doi.org/10.1111/j.1600-079X.2004.00141.x (2004).
Chauvet, C., Bois-Joyeux, B., Berra, E., Pouyssegur, J. & Danan, J. L. The gene encoding human retinoic acid-receptor-related orphan receptor alpha is a target for hypoxia-inducible factor 1. Biochem. J. 384, 79–85. https://doi.org/10.1042/bj20040709 (2004).
Zubrow, A. B., Delivoria-Papadopoulos, M., Ashraf, Q. M., Fritz, K. I. & Mishra, O. P. Nitric oxide-mediated Ca2+/calmodulin-dependent protein kinase IV activity during hypoxia in neuronal nuclei from newborn piglets. Neurosci. Lett. 335, 5–8. https://doi.org/10.1016/s0304-3940(02)01138-2 (2002).
Maulik, D., Ashraf, Q. M., Mishra, O. P. & Delivoria-Papadopoulos, M. Effect of hypoxia on calcium influx and calcium/calmodulin-dependent kinase activity in cortical neuronal nuclei of the guinea pig fetus during development. Am. J. Obstet. Gynecol. 186, 658–662. https://doi.org/10.1067/mob.2002.122392 (2002).
Delivoria-Papadopoulos, M., Ashraf, Q. M. & Mishra, O. P. Mechanism of CaM kinase IV activation during hypoxia in neuronal nuclei of the cerebral cortex of newborn piglets: the role of Src kinase. Neurochem. Res. 36, 1512–1519. https://doi.org/10.1007/s11064-011-0477-3 (2011).
Kratimenos, P., Koutroulis, I., Jain, A., Malaeb, S. & Delivoria-Papadopoulos, M. Effect of concurrent src kinase inhibition with short-duration hypothermia on Ca2+/calmodulin kinase IV activity and neuropathology after hypoxia-ischemia in the newborn swine brain. Neonatology 113, 37–43. https://doi.org/10.1159/000480067 (2018).
Delivoria-Papadopoulos, M., Ashraf, Q. M. & Mishra, O. P. Brain tissue energy dependence of CaM kinase IV cascade activation during hypoxia in the cerebral cortex of newborn piglets. Neurosci. Lett. 491, 113–117. https://doi.org/10.1016/j.neulet.2011.01.017 (2011).
Ward, A. & Hudson, J. W. p53-Dependent and cell specific epigenetic regulation of the polo-like kinases under oxidative stress. PLoS ONE 9, e87918. https://doi.org/10.1371/journal.pone.0087918 (2014).
Yang, Y. et al. Polo-like kinase 3 functions as a tumor suppressor and is a negative regulator of hypoxia-inducible factor-1 alpha under hypoxic conditions. Can. Res. 68, 4077–4085. https://doi.org/10.1158/0008-5472.can-07-6182 (2008).
Piovan, E. et al. Differential regulation of hypoxia-induced CXCR4 triggering during B-cell development and lymphomagenesis. Can. Res. 67, 8605–8614. https://doi.org/10.1158/0008-5472.can-06-4722 (2007).
Sharma, P., Bansal, A. & Sharma, P. C. RNA-seq-based transcriptome profiling reveals differential gene expression in the lungs of Sprague-Dawley rats during early-phase acute hypobaric hypoxia. Mol. Genet. Genom. MGG 290, 2225–2240. https://doi.org/10.1007/s00438-015-1064-0 (2015).
Chen, Y. et al. HIF-1-miR-219-SMC4 regulatory pathway promoting proliferation and migration of HCC under hypoxic condition. BioMed. Res. Int. 2019, 8983704. https://doi.org/10.1155/2019/8983704 (2019).
Wierenga, A. T., Vellenga, E. & Schuringa, J. J. Convergence of hypoxia and TGFβ pathways on cell cycle regulation in human hematopoietic stem/progenitor cells. PLoS ONE 9, e93494. https://doi.org/10.1371/journal.pone.0093494 (2014).
Losada, A. & Hirano, T. Dynamic molecular linkers of the genome: the first decade of SMC proteins. Genes Dev. 19, 1269–1287. https://doi.org/10.1101/gad.1320505 (2005).
Lee, J. K. & Bou, D. J. Regulator of G-protein signaling (RGS)1 and RGS10 proteins as potential drug targets for neuroinflammatory and neurodegenerative diseases. The AAPS J. 18, 545–549. https://doi.org/10.1208/s12248-016-9883-4 (2016).
Gibbons, D. L. et al. Cutting Edge: Regulator of G protein signaling-1 selectively regulates gut T cell trafficking and colitic potential. J. Immunol. (Baltimore, Md: 1950) 187, 2067–2071. https://doi.org/10.4049/jimmunol.1100833 (2011).
Sun, M. Y. et al. Critical role for nonGAP function of Galphas in RGS1mediated promotion of melanoma progression through AKT and ERK phosphorylation. Oncol. Rep. 39, 2673–2680. https://doi.org/10.3892/or.2018.6341 (2018).
Deguchi, J. O., Yamazaki, H., Aikawa, E. & Aikawa, M. Chronic hypoxia activates the Akt and beta-catenin pathways in human macrophages. Arterioscler. Thromb. Vasc. Biol. 29, 1664–1670. https://doi.org/10.1161/atvbaha.109.194043 (2009).
Ziemka-Nalecz, M., Jaworska, J. & Zalewska, T. Insights into the neuroinflammatory responses after neonatal hypoxia-ischemia. J. Neuropathol. Exp. Neurol. 76, 644–654. https://doi.org/10.1093/jnen/nlx046 (2017).
Donega, V., van Velthoven, C. T., Nijboer, C. H., Kavelaars, A. & Heijnen, C. J. The endogenous regenerative capacity of the damaged newborn brain: boosting neurogenesis with mesenchymal stem cell treatment. J. Cereb. Blood Flow Metab. 33, 625–634. https://doi.org/10.1038/jcbfm.2013.3 (2013).
Ben-Shoshan, J., Maysel-Auslender, S., Mor, A., Keren, G. & George, J. Hypoxia controls CD4+CD25+ regulatory T-cell homeostasis via hypoxia-inducible factor-1alpha. Eur. J. Immunol. 38, 2412–2418. https://doi.org/10.1002/eji.200838318 (2008).
Laptook, A. R. et al. Effect of therapeutic hypothermia initiated after 6 hours of age on death or disability among newborns with hypoxic-ischemic encephalopathy: A randomized clinical trial. JAMA 318, 1550–1560. https://doi.org/10.1001/jama.2017.14972 (2017).
Shankaran, S. et al. Effect of depth and duration of cooling on death or disability at age 18 months among neonates with hypoxic-ischemic encephalopathy: A randomized clinical trial. JAMA 318, 57–67. https://doi.org/10.1001/jama.2017.7218 (2017).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550. https://doi.org/10.1186/s13059-014-0550-8 (2014).
Lukk, M. et al. A global map of human gene expression. Nat. Biotechnol. 28, 322–324. https://doi.org/10.1038/nbt0410-322 (2010).
Avila Cobos, F., Vandesompele, J., Mestdagh, P. & De Preter, K. Computational deconvolution of transcriptomics data from mixed cell populations. Bioinform. (Oxford Engl.) 34, 1969–1979. https://doi.org/10.1093/bioinformatics/bty019 (2018).
Lee, H. J. & Georgiadou, A. Integrated pathogen load and dual transcriptome analysis of systemic host-pathogen interactions in severe malaria. Sci. Transl. Med. https://doi.org/10.1126/scitranslmed.aar3619 (2018).
Gliddon, H. D. & Herberg, J. A. Genome-wide host RNA signatures of infectious diseases: Discovery and clinical translation. Natl. Lab. Med. 153, 171–178. https://doi.org/10.1111/imm.12841 (2018).
Flint, S. M. et al. Leucocyte subset-specific type 1 interferon signatures in SLE and other immune-mediated diseases. RMD open 2, e000183. https://doi.org/10.1136/rmdopen-2015-000183 (2016).
Montaldo, P. et al. Host gene expression in neonatal encephalopathy: Effect of deconvolution algorithms. PAS Philadelphia 20, 20 (2020).
Acknowledgements
This study was funded by the Gates and Melinda Foundation and Weston Garfield Foundation. The study was coordinated by the Centre for Perinatal Neuroscience at Imperial College London. PM is funded by a Medical Research Council doctoral fellowship (MR/R001375/1). VO is funded by the National Institute for Health Research doctoral fellowship (ICA-CDRF-2016-02-071). MK is funded by the Wellcome Trust (Sir Henry Wellcome Fellowship Grant 206508/Z/17/Z). ST is funded by a Weston Garfield Endowment Chair and an NIHR Advanced Fellowship. AC, MK and JH acknowledge support from the Imperial College BRC.
Author information
Authors and Affiliations
Contributions
S.T. and J.H. conceived the idea; P.M. performed bioinformatic analysis, under supervision of M.K., A.C., and J.H.; S.T., S.S., S.R. designed the HELIX trial; P.B., S.B., J.Me., M.B., I.J., S.M., M.S., R.A., S.K., M.C. and M.Se. recruited cases, and obtained the samples; G.A., I.Y., K.K., A.S., S.C.M., S.P., A.J.C., P.J.L., C.K., R.C., R.A,. S.M. and N.B. supported the data acquisition and interpretation. RS, RM, IT and SM performed neurodevelopmental outcome assessments; J.Mo., V.O., V.P., I.S., P.I. and M.Sa. provided methodology; P.M. wrote the original draft; all authors contributed to review and editing of the manuscript; S.T. obtained funding and is the guarantor of the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Montaldo, P., Cunnington, A., Oliveira, V. et al. Transcriptomic profile of adverse neurodevelopmental outcomes after neonatal encephalopathy. Sci Rep 10, 13100 (2020). https://doi.org/10.1038/s41598-020-70131-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-70131-w
This article is cited by
-
The use of gene expression as a disease stratification tool of neonatal encephalopathy
Pediatric Research (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.