Abstract
Exposure to stress during early life affects subsequent behaviors and increases the vulnerability to adult pathologies, a phenomenon that has been well documented in humans and rodents. In this study, we introduce a chronic unpredictable stress protocol adapted to young zebrafish, which is an increasingly popular vertebrate model in neuroscience research. We exposed zebrafish to a series of intermittent and unpredictable mild stressors from day 10 to 17 post-fertilization. The stressed fish showed a reduced exploration of a novel environment one day post-stress and an increased responsiveness to dark-light transition two days post-stress, indicative of heightened anxiety-related behaviors. The stress-induced decrease in exploration lasted for at least three days and returned to control levels within one week. Moreover, stressed fish were on average 8% smaller than their control siblings two days post-stress and returned to control levels within one week. All together, our results demonstrate that young zebrafish exposed to chronic unpredictable stress develop growth and behavioral alterations akin to those observed in rodent models.
Similar content being viewed by others
Introduction
The stress response is a combination of neural, endocrine and autonomic reactions that prepares an organism to cope with adverse events (stressors) that threaten its homeostasis. It results in an immediate release of catecholamines via the sympathetic nervous system followed by the rapid production of glucocorticoids via the hypothalamic-pituitary-adrenal (HPA) axis1. While the acute stress response is crucial for an animal’s adaptation to a changing environment, the repeated activation of the stress response within a short timeframe (chronic stress) contributes to a variety of adverse health effects including growth impairments, as well as mood and anxiety disorders2,3. In particular, animal and human studies have indicated that chronic stress exposure during early life increases the vulnerability to anxiety and depressive disorders via HPA axis dysregulation4,5,6,7,8. Since early-life adversity is an important risk factor for developing subsequent pathologies, understanding the neurobiological mechanisms underlying its adverse effects on the developing brain and behaviors is of utmost importance.
The zebrafish is rapidly becoming a popular model organism to study stress-induced changes in behaviors and neural circuits at early life stages9,10,11. Firstly, the main molecular and cellular components of the human stress response are conserved in zebrafish, including cortisol release12,13,14. Secondly, anxiety-related behavioral tests in zebrafish are relevant to understand anxiety disorders in mammals, including humans; for example, stressed zebrafish avoid the center of an openfield15, similar to center avoidance reported in humans with high anxiety sensitivity16. In addition, zebrafish larvae are optically transparent, which allows for rapid measurement of brain-wide neural activity at single-cell resolution during the first weeks of life17,18. Lastly, during this period, zebrafish already swim freely, feed externally and develop complex sensorimotor and social behaviors19,20,21, which are modulated by stress in adults15,22,23 and therefore enable to investigate the outcomes of chronic stress.
Leveraging these advantages, several recent studies have investigated how activation of the stress axis during early life affects zebrafish behavior. In post-hatch zebrafish, brief activation of the stress response by physiological stressors (acidic pH, hyperosmotic medium) or via optogenetic hypercortisolemia, resulted in transiently increased locomotion and stimulus responsiveness13,24 as well as temporary suppression of feeding behavior25. Upon longer exposure to inescapable mild electric shocks, young zebrafish initially attempted to escape before rapidly reducing their locomotor activity26, which is an example of a transition from active to passive coping strategies also found in other vertebrates27. Additionally, exposure to prolonged stressors such as 24 h exogenous cortisol or 9 h forced swimming, caused a heightened locomotor activity28,29 and a blunted stress-induced cortisol release29 in the following days. All together, these studies illustrate that the behavioral outcomes of early-life adversity vary depending on the nature, intensity, duration and timing of the stressors, and motivate further investigation of how early-life stress affects zebrafish behavior.
Despite a growing understanding of early-life stress in zebrafish, little is known about how chronic stress affects zebrafish during this sensitive developmental period. Therefore, our study aimed at establishing a chronic unpredictable stress protocol suitable for early life stages and at assessing how chronic stress exposure affects zebrafish development and anxiety-related behaviors.
Results
Repeated stressor exposure affects fish development
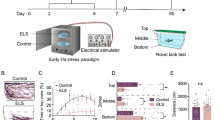
We adapted a chronic unpredictable stress (CUS) paradigm previously used in adult zebrafish22,30, and exposed 10 days-post-fertilization (dpf) fish to intermittent stressors twice a day for eight consecutive days (see Methods). As previous studies have demonstrated that early life stress slows down development in fish31,32, we compared body length between groups of fish two days post-CUS and found that CUS-exposed fish were 8% smaller on average than their control siblings (Fig. 1A). No such difference was observed at eight days post-CUS (Fig. 1B). Therefore, CUS exposure temporarily slowed the young zebrafish development.
Stressed zebrafish are smaller two days post-CUS, but return to normal size within a week. (A) Fish size at 19 dpf (control: n = 99 fish; stressed: n = 94 fish). (B) Fish size at 25 dpf in a different set of fish (control: n = 27 fish; stressed: n = 26 fish). Each dot represents a fish. Data are represented as mean ± SEM. **p < 0.01, Kruskal-Wallis test.
Increased stimulus responsiveness in stressed zebrafish
To determine the effect of CUS on zebrafish behavior, we adapted assays classically used to measure anxiety-like behaviors in vertebrates. These consisted of an open field and a dark-light preference test that quantify anxiety in animal models based on the thigmotaxis and phototaxis behaviors respectively15. In the open field test, the fish trajectory was recorded as they swam in a round petri dish (Fig. 2A). We calculated a thigmotaxis index, reflecting the relative time spent along the walls of the arena, which is a common measure of anxiety-like behavior33. There was no difference in thigmotaxis between CUS-exposed and control fish (Fig. 2B). Basal locomotion, assessed via velocity and total distance swam, was similar across groups (Fig. 2C,D).
CUS exposure does not affect anxiety-like behaviors two days post-CUS in the open field test. (A) Representative swimming trajectory of a fish (in red) during the open field test lasting for ten minutes. The outer circle is indicated by two concentric black circles. (B) Thigmotaxis index that corresponds to the percentage of time spent in the outer circle. (C) Average swimming velocity in body length/second. The dot signaled by a red square was out of range (value = 2.78). (D) Total distance travelled for ten minutes in body length. The dot signaled by a red square was out of range (value = 1669). Each dot represents one fish (control: n = 99 fish; stressed: n = 94 fish). Data are represented as mean ± SEM.
We next used a combination of two assays; the dark-light transition, which measures the change in locomotion resulting from sudden illumination, and the dark-light preference test, which measures the fish preference for the light over the dark compartment (Fig. 3A, see Methods). There was no difference in the number of freezing events (control: 13.1 ± 1.2; stressed: 9.8 ± 0.1; p value = 0.08; Kruskal-Wallis test) or in the duration of freezing events (in minute, control: 1.9 ± 0.2; stressed: 1.3 ± 0.2; p value = 0.06; Kruskal-Wallis test) between stressed and control groups. In both groups, fish exhibited a biphasic response following the transition from dark to light phase, which consisted in a short-term decrease in locomotion immediately after light onset (Fig. 3B), followed by an increase in distance swam in both groups (Fig. 3C). Interestingly, this light-induced locomotor response was more prominent in CUS-exposed fish than in control fish (Fig. 3E,F). We calculated the locomotion parameters in body length in order to account for the difference in size between stressed and control fish at 19 dpf (Fig. 1A). Measuring locomotion in mm showed a similar increase in light-induced response in CUS-exposed fish compared with control fish (velocity (mm/s); control: 1.5 ± 0.1; stressed: 1.7 ± 0.1; p value = 0.03; Kruskal-Wallis test; distance (mm), control: 651.5 ± 46.5; stressed: 728.6 ± 42.8; p value = 0.03; Kruskal-Wallis test). We also compared dark-light preference during the light phase of the assay. Fish avoided the dark compartment, as reflected by the negative average preference index that was similar in both groups (Fig. 3D,G).
No change in light preference, but increased light-induced locomotion two days post-CUS, in CUS-exposed fish. (A) Representative swimming trajectory (in red) of a 19 dpf fish during the dark-light test. Fish were initially maintained in the dark for two minutes (dark phase). Upon the start of illumination (light phase), they had the choice between a dark and a light compartment for eight minutes. (B) Average velocity during the dark and light phases, per time bins of twelve seconds, in body length/second. (C) Average distance travelled during the dark and light phases, per time bins of twelve seconds, in body length. (D) Preference index over time, during the dark and light phases, calculated per time bins of twelve seconds. (E) Average velocity of the fish during the light phase. The dot signaled by a red square was out of range (value = 2.07). (F) Total distance travelled by the fish during the light phase. (G) Preference index for the dark or the light compartments during the light phase. Values of −1 indicate 100% time spent in the light compartment and values of 1 indicate 100% time spent in the dark compartment. Each dot represents one fish (control: n = 99 fish; stressed: n = 94 fish). Data are represented as mean ± SEM. **p < 0.01, Kruskal-Wallis test.
CUS exposure reduces exploration of a novel tank
To further characterize stress-induced anxiety-like behaviors, groups of fish were introduced to a novel tank (Fig. 4A,B). There was no difference in the number of freezing events (control: 0.6 ± 0.1; stressed: 0.6 ± 0.14; p value = 1; Kruskal-Wallis test) or in the duration of freezing events (in second; control: 11.9 ± 4.9; stressed: 14.3 ± 3.7; p value = 0.27; Kruskal-Wallis test) between stressed and control groups. We also measured their exploration of this new environment, a parameter that is consistently reduced in anxious fish23,34,35. Chronic stressor exposure resulted in a significantly lower vertical position in the tank (Fig. 4C) and a higher proportion of fish swimming at the bottom of the tank (Fig. 4D).
Decreased exploration of the novel tank in stressed fish tested one day post-CUS. (A) Representative image showing control fish’s position in the arena at the beginning of the novel tank test. The arena is vertically positioned and the dashed line at the top indicates the water surface. The red arrows indicate the fish, and the the bottom third of the tank is indicated. (B) Representative image showing stressed fish’s position at the beginning of the novel tank test. (C) Average vertical position of all fish during the early phase of the novel tank test (see Methods). (D) Ratio of fish in the bottom third of the tank. (E) Representative image showing control fish’s position relative to each other in the late phase (from three to ten minutes) of the novel tank test, in the same group as in A. The black line indicates the distance from a fish to its nearest neighbor (NND). The grey lines indicate the distance between one fish to the other fish in the tank that are used for interindividual distance (IID) calculations. (F) Representative image showing stressed fish’s position in the late phase of the novel tank test, in the same group as in (B). (G) Average nearest neighbor distance for all fish in the arena from three to ten minutes. (H) Average interindividual distance for all fish in the arena from three to ten minutes. Each dot represents the average value for one group of fish (control: n = 9 groups; stressed: n = 10 groups). Data are represented as mean ± SEM. *p < 0.05, Student’s t-test.
Since zebrafish tend to swim in tighter shoals when exposed to direct or perceived threats36, we quantified shoaling by measuring the average distance between individuals as well as nearest-neighbor distance during the late part of the novel tank test (Fig. 4E,F). We found no difference in either of these parameters between the stressed and control fish (Fig. 4G,H). Since displacements constrained to the vertical dimension could affect social behavior, we also assessed shoaling in a horizontal arena, whose dimensions matched those used for larval fish in previous studies37,38 (Fig. 5A,B). There was no difference in distance to nearest-neighbor (Fig. 5C), or in average distance between fish (Fig. 5D) between the stressed and control groups.
CUS exposure does not affect shoaling. Shoaling was measured for twenty minutes in a horizontal arena. (A) Representative image of the position of control fish relative to each other. (B) Representative image of the position of stressed fish relative to each other. Red arrows indicate the fish’s positions. (C) Average nearest neighbor distance for all fish in the arena. (D) Average interindividual distance for all fish in the arena. Each dot represents the average value for a group of fish (control: n = 7 groups; stressed: n = 7 groups). Data are represented as mean ± SEM.
Altogether, our results indicate that repeated stressor exposure for eight consecutive days induced an anxiety-like phenotype, which consists of heightened responsiveness to dark-light transition and decreased exploration in the novel tank test. To determine the duration of the anxious phenotype in CUS-exposed fish, we assessed their exploratory behavior two or three days, as well as eight days, post-CUS. We found that decreased exploration persisted in stressed fish at least three days after the last stressor was applied, as shown by the significantly higher proportion of stressed fish swimming at the bottom (Fig. 6B) and a tendency to swim lower in the tank (Fig. 6A). After one week, the anxious phenotype was no longer observed, as the exploration levels were similar in stressed and control fish (Fig. 6C,D).
Decreased exploration of the novel tank persists three days post-CUS and returns to control level within a week. (A) Average vertical position in the novel tank test at two-three days post-CUS. (B) Ratio of fish in the bottom third at two-three days post-CUS (control: n = 11 groups; stressed: n = 11 groups). (C) Average vertical position in the novel tank test eight days post-CUS. (D) Ratio of fish in the bottom third eight days post-CUS. Each dot represents the average value for a group of fish (Control: n = 6 groups; Stressed: n = 6 groups). Data are represented as mean ± SEM. *p < 0.05, Student’s t-test.
Discussion
The neurobiological consequences of prolonged stress have long been studied using chronic unpredictable stress (CUS) exposure in rodents39,40,41 and recently in adult zebrafish14,22,30,42. Adding to this, we present an adapted CUS protocol to investigate how chronic stress affects zebrafish during early-life stages. We used psychological and physiological aversive stimuli previously documented to activate the zebrafish stress response22,24. We found that repeated stressor exposure from 10 to 17 dpf significantly altered young zebrafish’s growth, as well as some aspects of anxiety-like behaviors.
Zebrafish larvae exposed to eight days of CUS were significantly smaller than their unstressed siblings. This observation was consistent with the decreased growth rates and reduced weight gain, reported in a variety of vertebrates during or shortly after prolonged stress exposure31,42,43. Fish in the control and stressed groups were fed equal amounts of larval food several times a day, thus the difference in growth cannot be explained by variation in food availability. However, it is well documented that reduced food intake is a common consequence of many types of stress and is partly mediated by the HPA axis44,45,46. Numerous studies have reported decreased appetite in adult fish following chronic stress47,48, and food intake was temporarily inhibited after exposure to strong stressors in developing zebrafish25. Therefore, the cumulative stress-mediated anorexia periods could account for the smaller size of CUS fish. Besides, cortisol-mediated proteolysis can directly affect muscle mass32, providing an additional mechanism through which CUS could have hampered fish growth. The decreased growth in stressed fish was temporary; stressed fish were the same size as age-matched unstressed siblings one week after the stressor had ceased. This is consistent with a previous report of body mass recovery after CUS exposure in developing salmon31 and has been attributed to post-stress compensatory feeding as observed in several fish species31,49,50.
Stressed fish showed reduced exploration of a novel environment in our study, which is in line with many other reports of acutely23,51 and chronically22,30,42 stressed fish. The novel tank test is a robust and widely used assay characterizing adult fish anxiety phenotypes35,52, that exploits the fish’s natural diving response when placed in a new environment. Despite the extensive use of this assay, little is known regarding the ontogeny of the zebrafish diving response. A recent study demonstrating larval preference for deeper waters suggests that this behavior could already emerge during the first week of life53. Here, we observed a robust diving response in groups of 18–20 dpf larvae, which lasted around two minutes (data not shown) and was consistently heightened by prior stress (Figs. 4C,D, 6B). Therefore, we show that the novel tank test is a suitable assay for measuring stress-induced anxious states in young zebrafish.
In contrast to prior studies using a single stressor exposure28, we found no difference in basal locomotion between CUS and control fish (Fig. 2C,D). However, stressed fish swam faster and over greater distances than their unstressed siblings after sudden illumination of the arena in the dark-light transition assay (Fig. 3B,C,E,F). Zebrafish larvae aged under one week react to sudden increased illumination by reducing locomotion54. We observed the opposite response pattern in three-weeks old fish, which exhibited a light-induced increase in locomotor activity. Since few prior studies have investigated the response to dark-light transitions in three-weeks old zebrafish, one needs to be cautious in interpreting this increased reactivity in terms of anxiety-related behavior. Interestingly, this effect is reminiscent of the enhanced behavioral reactivity induced by acute stress in response to changes in relevant environmental stimuli such as water temperature or vibrations13. Therefore, our data suggest that CUS specifically exacerbates the locomotor response to dark-light transitions, potentially reflecting an adaptive response to optimize coping with aversive environment changes.
Time spent close to the walls and dark-light preference are commonly used to assess fish anxiety-like behaviors at larval and adult stages15,55. Shoaling has also been used as an indicator of fear or anxiety states since adult zebrafish tend to group into tighter shoals when exposed to anxiogenic situations22,56. Although we observed significant effects of CUS on size, stimulus responsiveness and exploration of a novel environment, we found no effect on thigmotaxis, dark-light preference and shoaling. This could reflect the lower sensitivity of these assays at that developmental stage. Indeed, the three weeks-old zebrafish lie in a key dark-light preference transitory phase between phototactic larvae and negative phototactic adults57. In accordance with this, we found that 19 dpf zebrafish showed on average a moderate preference for the light compartment. However, the interindividual variability was very prominent (Fig. 3G), which could have masked subtle differences between the CUS and control fish. Regarding shoaling, fish were separated on average by 10 body lengths in the vertical novel tank test (Fig. 4H) and by 15 body lengths in an horizontal arena (Fig. 5D). This is similar to values reported for that developmental stage, but larger than the 4 body lengths between older shoaling fish38. Although preference for conspecifics is already strong in three-weeks old zebrafish21,58,59, our data confirm that shoaling, and by extension its modulation by stress22, develops at later stages. All together, our results underscore the importance of using age-appropriate and diverse behavioral assays. Moreover, it adds to the growing evidence documenting the differences in sensitivity and aspects of the anxiety-like response detected by different approaches53,60.
The behavioral outcomes of chronic stress exposure are most often assessed within 24 h after the last stressor22,29,30. We found that stressed fish displayed a decreased exploration of the novel tank for at least three days post-CUS and returned to normal levels after a week. The relatively short duration of the anxious phenotype must be placed in perspective with the mild stress protocol used here, to avoid deleterious effects on fish survival during the sensitive developmental period. Moreover, since we tested fish in groups in the novel tank test, the anxiolytic presence of conspecifics61 might have alleviated subtle anxiety phenotypes during the late post-CUS assessments. Early life stress can produce delayed effects on fish behaviors later in life62,63,64. Therefore, it would be interesting to compare the individual diving response and shoaling behavior of CUS and control fish during adulthood to determine the long-term persistence of the anxious phenotype.
Methods
Animals and housing
We used 548 zebrafish of following lines: nacre (mitfa)65, and Tg (elavl3: GCaMP6s)66. Eggs were obtained from crosses of adult fish raised in the facility. Eggs were collected between 10–12 am and kept until hatching at a density of 50 eggs/50 mL petridishes filled with artificial fish water. Fish were then transferred to new water tanks and raised at a constant temperature of 28 °C, under a 12/12 h light cycle and fed twice a day with commercial flakes (Tetra). All experiments were performed on zebrafish aged under 25 days post-fertilization (dpf) and the procedures followed the 2010/63/EU directive and were approved by the Norwegian Animal Research Authority (Food and Safety Authority; Permit number: 17127).
Experimental design
Fish in the stressed group were exposed to chronic unpredictable stress (CUS) from 10 to 17 dpf (see Fig. 7). At that age, zebrafish possess a functional stress axis in response to external stressors12,67. Moreover, they are sufficiently small and transparent to permit in vivo functional brain imaging68,69 to investigate the effects of early life stress on neural activity in future studies. Control groups were raised in the same conditions without stressor exposure. Fish from both control and stressed groups were tested for social behaviour at 18 dpf. Another set of fish were tested for anxiety-like behaviors in the novel tank diving test (at 18 dpf; 81 control fish; 83 stressed fish), followed by the open field and dark-light tests (at 19 dpf; 99 control fish; 94 stressed fish). Note that all fish tested at 19 dpf had performed the novel tank test at 18 dpf. However, the video files for three groups (two control groups, n = 18 fish; and one stressed group, n = 11 fish) were overwritten, which prevented the inclusion of novel tank test results for these fish and explains the difference in animal numbers between 18 and 19 dpf. Additionally, fish that were left undisturbed after the end of the CUS were tested in the novel tank test at 19, 20 or 25 dpf to assess the persistence of the anxiety-like phenotype.
Experimental design. Fish in the stressed group were exposed to stressors for eight days. Fish in the control group were raised in the same conditions without stressor exposure. Social interaction was assessed in groups of fish at 18dpf (control: n = 59; stressed: n = 58). Early assessment of anxiety-like behaviors was done using the novel tank test at 18 dpf (control: n = 81; stressed: n = 83). The same fish were then tested in the open field and dark-light tests at 19 dpf (control: n = 99; stressed: n = 94). A separate group of fish was then tested in a novel tank for intermediate (19 or 20 dpf, (control: n = 95; stressed: n = 92)) or late (25dpf, (control: n = 24; stressed: n = 27)) assessment of anxiety-like behaviors.
Chronic unpredictable stress
At 10 dpf, fish were randomly assigned to control or stressed groups and housed in nursery tank with a meshed bottom that enabled easier transfer to the holding tank where CUS was applied. From 10 to 17 dpf, the fish in the stressed group were exposed to two stressors per day, applied at random times between 8 am and 8 pm to maintain unpredictability. The five following stressors were used according to previously published work24,55: (1) chasing (using a small net or a pipette for five minutes); (2) turbulences (tank water replacements followed by increased air bubbling for three minutes, repeated three times); (3) hyperosmotic shock (100 mM NaCl for ten minutes); (4) pH drop (pH = 4 for three minutes); (5) light flashes exposure (6 mW/cm2 light flashes at 5 Hz for ten minutes). The light flashes were produced by a custom-made panel of white LEDs controlled by a microcontroller board (Arduino Uno). Fish in the control group remained undisturbed except for routine feeding and tank cleaning.
Behavioral assays
All recording sessions took place in the afternoon from 12–6 pm in a temperature-controlled room (28 °C) with limited experimenter intervention.
Novel tank diving test
Fish in groups of 5–11 (early and intermediate assessment groups) or 3–5 (late assessment group) were kept for one hour in temporary holding tanks, before being transferred to the novel arena (length × width × height: 13 ×1.4 × 6.4 cm). The fish movement was then recorded for ten minutes at 1 Hz using a webcam (Logitech 720p) placed in front of the arena and connected to a computer. The fish position was manually detected using the ImageJ ROI manager plugin70 (every five seconds for the first two minutes then every twenty seconds for the next eight minutes) and exported in Matlab (MathWorks 2017) to calculate the behavioral response indices. Vertical position for a group of fish was calculated by averaging all fish’s normalized vertical positions (bottom = 0 and water surface = 1). The first half a minute was not included in the analysis to avoid variations due to fish delivery. The fish vertical position and the ratio of fish in the bottom third were calculated for the following one minute. The ratio of fish in the bottom third is the average proportion of fish present in the bottom third of the tank during the specified period. The nearest-neighbor distance (NND) was calculated by averaging the distance to the closest neighbor for each fish within the group after the initial diving response took place (minutes three to ten). The interindividual distance (IID) was calculated as the average of all pairwise distances between individual fish within the group during the same period. Freezing episodes were defined as periods of immobility lasting for more than three seconds and were measured manually for the first three minutes of the assay by an experimenter blind to the group identity. The total number of freezing events and cumulative duration of freezing events were normalized to the number of fish within a group.
Shoaling test
Fish were transferred to the behavior testing room at least one hour prior to the experiments. Fish (6–10 per group) were gently transferred from the home tank to a centrifuge tube (Falcon, 50 ml) containing 15 ml of fish water and were poured into the center of a square petri dish (length × width × height: 12 × 12 × 1.5 cm) containing 60 mL of water. The petri dish arena had linear dimensions over 28 times the average fish body length, as recommended in previous studies of shoaling behavior37,38. The fish movement was then recorded for 20 minutes at 1 Hz using a webcam (Logitech 720p) placed above the arena and connected to a computer. The individual fish position was manually detected using the ImageJ ROI manager plugin (every 10 seconds during minutes 3, 5, 7, 9, 15 and 20) and exported in Matlab to calculate NND and IID as described above.
Open field and dark-light tests
Fish were transferred in individual petridishes (radius = 2 cm, depth = 0.4 cm; Falcon) and habituated for 1 h. The dishes were then positioned on a transparent platform illuminated from below using visible and infrared LEDs. The walls of the dishes were sanded to prevent visual and social interaction between fish. A camera (AVT Manta G-235B; Allied Vision) equipped with an infrared high-pass filter (cut-off wavelength 750 nm; ePlastics) was positioned above the platform and used to simultaneously record the behavior of sixteen fish at 2 Hz. An enclosure positioned around the setup prevented visual interference. A recording of 10 minutes was taken for the open field test. Then the visible-light LEDs were switched off and the walls and bottom of halves of each petridishes were covered with black infrared-transparent material (cut-off wavelength 750 nm; ePlastics). After five minutes of habituation to the new settings in the dark, the fish position in the dark-light partitioned dish was recorded for ten minutes: two minutes in the dark (dark phase) followed by eight minutes with visible light illumination (light phase). This allowed us to first test the response to change in background illumination (dark-light transition) and then the fish preference for the dark or the light compartment (dark-light preference).
To reconstruct the fish swimming trajectory, the video recordings were analyzed using custom-written Matlab scripts71. The following behavior indices were then calculated based on fish position. Freezing episodes were defined as periods of immobility lasting more than three seconds, during which the fish’s speed was inferior to a fifth of its body length. Velocity was quantified in body length/sec after excluding freezing episodes. Distance travelled corresponded to the cumulative displacement of the fish. For the calculation of the open field thigmotaxis index, the petridish was divided into three equal-area concentric circles. The relative time spent by fish in the outermost circle during the test was then calculated, with a value close to 100% indicating a strong preference for the border of the dish, whereas values close to 0% indicated a preference for the center. The preference index in the dark-light test was calculated as follows57:
Values close to −1 indicated a strong preference for the light compartment; values close to 1 indicated a strong preference for the dark compartment and values close to 0 indicated no preference.
Measurement of fish size
At 19 dpf, the fish body length was calculated in Matlab for each fish by averaging snout-to-tail measurements on five frames randomly selected from the open field test recordings. At 25 dpf, fish were euthanized using an overdose of 222 mg/L buffered tricaine methane sulfonate (MS222). They were placed laterally on a petridish and snout-to-tail measurements were made on pictures taken using a microscope camera (Axiocam 506, ZEISS).
Statistical analysis
Data were evaluated using SPSS Statistics 25 (IBM). Each data set was first tested for normality with descriptive statistics for mean, skewness and kurtosis. Group effects were then tested using Student’s t-test or non-parametric Kruskal-Wallis test. Data are reported as mean ± standard error of the mean (SEM) in the text and figure legends. A p-value < 0.05 was considered significant.
References
McCarty, R. Learning about stress: neural, endocrine and behavioral adaptations. Stress 19, 449–475 (2016).
McEwen, B. S. Stress, adaptation, and disease. Allostasis and allostatic load. Ann. N. Y. Acad. Sci. 840, 33–44 (1998).
Calhoon, G. G. & Tye, K. M. Resolving the neural circuits of anxiety. Nat. Neurosci. 18, 1394–1404 (2015).
Pechtel, P. & Pizzagalli, D. A. Effects of early life stress on cognitive and affective function: an integrated review of human literature. Psychopharmacology (Berl.) 214, 55–70 (2011).
van Bodegom, M., Homberg, J. R. & Henckens, M. J. A. G. Modulation of the Hypothalamic-Pituitary-Adrenal Axis by Early Life Stress Exposure. Front Cell Neurosci 11, 87 (2017).
Nugent, N. R., Tyrka, A. R., Carpenter, L. L. & Price, L. H. Gene-environment interactions: early life stress and risk for depressive and anxiety disorders. Psychopharmacology (Berl.) 214, 175–196 (2011).
McEwen, B. S. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol. Rev. 87, 873–904 (2007).
Danese, A. & McEwen, B. S. Adverse childhood experiences, allostasis, allostatic load, and age-related disease. Physiol. Behav. 106, 29–39 (2012).
Löhr, H. & Hammerschmidt, M. Zebrafish in endocrine systems: recent advances and implications for human disease. Annu. Rev. Physiol. 73, 183–211 (2011).
Stewart, A. M. et al. Molecular psychiatry of zebrafish. Molecular Psychiatry 20, 2–17 (2015).
Stewart, A. M., Braubach, O., Spitsbergen, J., Gerlai, R. & Kalueff, A. V. Zebrafish models for translational neuroscience research: from tank to bedside. Trends Neurosci. 37, 264–278 (2014).
Alsop, D. & Vijayan, M. M. Development of the corticosteroid stress axis and receptor expression in zebrafish. Am. J. Physiol. Regul. Integr. Comp. Physiol. 294, R711–719 (2008).
De Marco, R. J., Thiemann, T., Groneberg, A. H., Herget, U. & Ryu, S. Optogenetically enhanced pituitary corticotroph cell activity post-stress onset causes rapid organizing effects on behaviour. Nat Commun 7, 12620 (2016).
Pavlidis, M., Theodoridi, A. & Tsalafouta, A. Neuroendocrine regulation of the stress response in adult zebrafish, Danio rerio. Prog. Neuropsychopharmacol. Biol. Psychiatry 60, 121–131 (2015).
Champagne, D. L., Hoefnagels, C. C. M., de Kloet, R. E. & Richardson, M. K. Translating rodent behavioral repertoire to zebrafish (Danio rerio): relevance for stress research. Behav. Brain Res. 214, 332–342 (2010).
Walz, N., Mühlberger, A. & Pauli, P. A Human Open Field Test Reveals Thigmotaxis Related to Agoraphobic Fear. Biological Psychiatry 80, 390–397 (2016).
Ahrens, M. B., Orger, M. B., Robson, D. N., Li, J. M. & Keller, P. J. Whole-brain functional imaging at cellular resolution using light-sheet microscopy. Nat. Methods 10, 413–420 (2013).
Randlett, O. et al. Whole-brain activity mapping onto a zebrafish brain atlas. Nat. Methods 12, 1039–1046 (2015).
Kalueff, A. V. et al. Towards a comprehensive catalog of zebrafish behavior 1.0 and beyond. Zebrafish 10, 70–86 (2013).
Orger, M. B. & de Polavieja, G. G. Zebrafish Behavior: Opportunities and Challenges. Annu. Rev. Neurosci. 40, 125–147 (2017).
Dreosti, E., Lopes, G., Kampff, A. R. & Wilson, S. W. Development of social behavior in young zebrafish. Front Neural Circuits 9, 39 (2015).
Piato, Â. L. et al. Unpredictable chronic stress model in zebrafish (Danio rerio): behavioral and physiological responses. Prog. Neuropsychopharmacol. Biol. Psychiatry 35, 561–567 (2011).
Egan, R. J. et al. Understanding behavioral and physiological phenotypes of stress and anxiety in zebrafish. Behav. Brain Res. 205, 38–44 (2009).
Ryu, S. & De Marco, R. J. Performance on innate behaviour during early development as a function of stress level. Sci Rep 7, 7840 (2017).
De Marco, R. J., Groneberg, A. H., Yeh, C.-M., Treviño, M. & Ryu, S. The behavior of larval zebrafish reveals stressor-mediated anorexia during early vertebrate development. Front Behav Neurosci 8, 367 (2014).
Andalman, A. S. et al. Neuronal Dynamics Regulating Brain and Behavioral State Transitions. Cell 177, 970–985.e20 (2019).
Maier, S. F. & Seligman, M. E. Learned helplessness: Theory and evidence. Journal of Experimental Psychology: General 105, 3–46 (1976).
Best, C. & Vijayan, M. M. Cortisol elevation post-hatch affects behavioural performance in zebrafish larvae. Gen. Comp. Endocrinol. 257, 220–226 (2018).
Castillo-Ramírez, L. A., Ryu, S. & De Marco, R. J. Active behaviour during early development shapes glucocorticoid reactivity. Sci Rep 9, 12796 (2019).
Chakravarty, S. et al. Chronic unpredictable stress (CUS)-induced anxiety and related mood disorders in a zebrafish model: altered brain proteome profile implicates mitochondrial dysfunction. PLoS ONE 8, e63302 (2013).
Vindas, M. A. et al. Coping with a changing environment: the effects of early life stress. R Soc Open Sci 3, 160382 (2016).
Faught, E. & Vijayan, M. M. Glucocorticoid and mineralocorticoid receptor activation modulates postnatal growth. J. Endocrinol., https://doi.org/10.1530/JOE-19-0358 (2019).
Schnörr, S. J., Steenbergen, P. J., Richardson, M. K. & Champagne, D. L. Measuring thigmotaxis in larval zebrafish. Behav. Brain Res. 228, 367–374 (2012).
Bencan, Z., Sledge, D. & Levin, E. D. Buspirone, chlordiazepoxide and diazepam effects in a zebrafish model of anxiety. Pharmacol. Biochem. Behav. 94, 75–80 (2009).
Levin, E. D., Bencan, Z. & Cerutti, D. T. Anxiolytic effects of nicotine in zebrafish. Physiol. Behav. 90, 54–58 (2007).
Kleinhappel, T. K., Pike, T. W. & Burman, O. H. P. Stress-induced changes in group behaviour. Scientific Reports 9, 17200 (2019).
Mahabir, S., Chatterjee, D., Buske, C. & Gerlai, R. Maturation of shoaling in two zebrafish strains: A behavioral and neurochemical analysis. Behavioural Brain Research 247, 1–8 (2013).
Buske, C. & Gerlai, R. Shoaling develops with age in Zebrafish (Danio rerio). Prog. Neuropsychopharmacol. Biol. Psychiatry 35, 1409–1415 (2011).
Nollet, M., Le Guisquet, A.-M. & Belzung, C. Models of depression: unpredictable chronic mild stress in mice. Curr Protoc Pharmacol Chapter 5, Unit 5.65 (2013).
Matuszewich, L. et al. The delayed effects of chronic unpredictable stress on anxiety measures. Physiol. Behav. 90, 674–681 (2007).
Antoniuk, S., Bijata, M., Ponimaskin, E. & Wlodarczyk, J. Chronic unpredictable mild stress for modeling depression in rodents: Meta-analysis of model reliability. Neurosci Biobehav Rev 99, 101–116 (2019).
Fulcher, N., Tran, S., Shams, S., Chatterjee, D. & Gerlai, R. Neurochemical and Behavioral Responses to Unpredictable Chronic Mild Stress Following Developmental Isolation: The Zebrafish as a Model for Major Depression. Zebrafish 14, 23–34 (2017).
Harris, R. B. S. Chronic and acute effects of stress on energy balance: are there appropriate animal models? Am. J. Physiol. Regul. Integr. Comp. Physiol. 308, R250–265 (2015).
Morton, G. J., Meek, T. H. & Schwartz, M. W. Neurobiology of food intake in health and disease. Nat. Rev. Neurosci. 15, 367–378 (2014).
Bernier, N. J. & Peter, R. E. The hypothalamic-pituitary-interrenal axis and the control of food intake in teleost fish. Comp. Biochem. Physiol. B, Biochem. Mol. Biol. 129, 639–644 (2001).
Pickering, A. D. Growth and stress in fish production. Aquaculture 111, 51–63 (1993).
Barton, B., Schreck, C. & Barton, L. Effects of chronic Cortisol administration and daily acute stress on growth, physiological conditions, and stress responses in juvenile rainbow trout. Diseases of Aquatic Organisms 2, 173–185 (1986).
Leal, E., Fernández-Durán, B., Guillot, R., Ríos, D. & Cerdá-Reverter, J. M. Stress-induced effects on feeding behavior and growth performance of the sea bass (Dicentrarchus labrax): a self-feeding approach. Journal of Comparative Physiology B 181, 1035–1044 (2011).
Ali, M., Nicieza, A. & Wootton, R. J. Compensatory growth in fishes: a response to growth depression. Fish and Fisheries 4, 147–190 (2003).
Azodi, M., Ebrahimi, E., Farhadian, O., Mahboobi-Soofiani, N. & Morshedi, V. Compensatory growth response of rainbow trout Oncorhynchus mykiss Walbaum following short starvation periods. Chinese Journal of Oceanology and Limnology 33, 928–933 (2015).
Cachat, J. et al. Measuring behavioral and endocrine responses to novelty stress in adult zebrafish. Nat Protoc 5, 1786–1799 (2010).
Kysil, E. V. et al. Comparative Analyses of Zebrafish Anxiety-Like Behavior Using Conflict-Based Novelty Tests. Zebrafish 14, 197–208 (2017).
Varga, Z. K. et al. The swimming plus-maze test: a novel high-throughput model for assessment of anxiety-related behaviour in larval and juvenile zebrafish (Danio rerio). Scientific Reports 8 (2018).
Steele, W. B., Mole, R. A. & Brooks, B. W. Experimental Protocol for Examining Behavioral Response Profiles in Larval Fish: Application to the Neuro-stimulant Caffeine. Journal of Visualized Experiments, https://doi.org/10.3791/57938 (2018).
Bai, Y., Liu, H., Huang, B., Wagle, M. & Guo, S. Identification of environmental stressors and validation of light preference as a measure of anxiety in larval zebrafish. BMC Neurosci 17, 63 (2016).
Speedie, N. & Gerlai, R. Alarm substance induced behavioral responses in zebrafish (Danio rerio). Behav. Brain Res. 188, 168–177 (2008).
Lau, B. Y. B., Mathur, P., Gould, G. G. & Guo, S. Identification of a brain center whose activity discriminates a choice behavior in zebrafish. Proc Natl Acad Sci USA 108, 2581 (2011).
Hinz, R. C. & de Polavieja, G. G. Ontogeny of collective behavior reveals a simple attraction rule. Proc. Natl. Acad. Sci. USA 114, 2295–2300 (2017).
Larsch, J. & Baier, H. Biological Motion as an Innate Perceptual Mechanism Driving Social Affiliation. Curr. Biol. 28, 3523–3532.e4 (2018).
Maximino, C. et al. A comparison of the light/dark and novel tank tests in zebrafish. Behaviour 149, 1099–1123 (2012).
Faustino, A. I., Tacão-Monteiro, A. & Oliveira, R. F. Mechanisms of social buffering of fear in zebrafish. Scientific Reports 7 (2017).
Jonsson, B. & Jonsson, N. Early environment influences later performance in fishes: effects of early experiences. Journal of Fish Biology 85, 151–188 (2014).
Solomon-Lane, T. K. & Hofmann, H. A. Early-life social environment alters juvenile behavior and neuroendocrine function in a highly social cichlid fish. Hormones and Behavior 115, 104552 (2019).
Reyes-Contreras, M., Glauser, G., Rennison, D. J. & Taborsky, B. Early-life manipulation of cortisol and its receptor alters stress axis programming and social competence. Philosophical Transactions of the Royal Society B: Biological Sciences 374, 20180119 (2019).
Lister, J. A., Robertson, C. P., Lepage, T., Johnson, S. L. & Raible, D. W. nacre encodes a zebrafish microphthalmia-related protein that regulates neural-crest-derived pigment cell fate. Development 126, 3757–3767 (1999).
Dunn, T. W. et al. Brain-wide mapping of neural activity controlling zebrafish exploratory locomotion. eLife 5 (2016).
Alderman, S. L. & Bernier, N. J. Ontogeny of the corticotropin-releasing factor system in zebrafish. General and Comparative Endocrinology 164, 61–69 (2009).
Jetti, S. K., Vendrell-Llopis, N. & Yaksi, E. Spontaneous Activity Governs Olfactory Representations in Spatially Organized Habenular Microcircuits. Current Biology 24, 434–439 (2014).
Fore, S., Cosacak, M. I., Verdugo, C. D., Kizil, C. & Yaksi, E. Functional properties of habenular neurons are determined by developmental stage and sequential neurogenesis, https://doi.org/10.1101/722462 (2019).
Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682 (2012).
Kermen, F. et al. Investigating olfactory behaviors in adult zebrafish. bioRxiv, https://doi.org/10.1101/859033 (2019).
Acknowledgements
The authors wish to thank the members of the Animal Physiology section at the Department of Biology for scientific discussion, as well as Miriam Dørum and Jan Sand for fish care. This work was supported by the Research Council of Norway (FRIPRO grant 262698 to F.K.).
Author information
Authors and Affiliations
Contributions
F.K. & A.G. designed the study. A.G. collected experimental data with contribution from H.Ø. F.K. wrote the data analysis codes and A.G. analyzed the data and prepared the figures. A.G. & F.K. wrote the article with contribution from H.Ø. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Golla, A., Østby, H. & Kermen, F. Chronic unpredictable stress induces anxiety-like behaviors in young zebrafish. Sci Rep 10, 10339 (2020). https://doi.org/10.1038/s41598-020-67182-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-67182-4
This article is cited by
-
Systematic review and meta-analysis of 10 years of unpredictable chronic stress in zebrafish
Lab Animal (2023)
-
Zebrafish, a biological model for pharmaceutical research for the management of anxiety
Molecular Biology Reports (2023)
-
The Roles of Histamine Receptor 1 (hrh1) in Neurotransmitter System Regulation, Behavior, and Neurogenesis in Zebrafish
Molecular Neurobiology (2023)
-
Long lasting anxiety following early life stress is dependent on glucocorticoid signaling in zebrafish
Scientific Reports (2022)
-
Molecular and culture-based assessment of the microbiome in a zebrafish (Danio rerio) housing system during set-up and equilibration
Animal Microbiome (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.