Abstract
The internal anal sphincter (IAS) generates phasic contractions and tone. Slow waves (SWs) produced by interstitial cells of Cajal (ICC) underlie phasic contractions in other gastrointestinal regions. SWs are also present in the IAS where only intramuscular ICC (ICC-IM) are found, however the evidence linking ICC-IM to SWs is limited. This study examined the possible relationship between ICC-IM and SWs by recording Ca2+ transients in mice expressing a genetically-encoded Ca2+-indicator in ICC (Kit-Cre-GCaMP6f). A role for L-type Ca2+ channels (CavL) and anoctamin 1 (ANO1) was tested since each is essential for SW and tone generation. Two distinct ICC-IM populations were identified. Type I cells (36% of total) displayed localised asynchronous Ca2+ transients not dependent on CavL or ANO1; properties typical of ICC-IM mediating neural responses in other gastrointestinal regions. A second novel sub-type, i.e., Type II cells (64% of total) generated rhythmic, global Ca2+ transients at the SW frequency that were synchronised with neighbouring Type II cells and were abolished following blockade of either CavL or ANO1. Thus, the spatiotemporal characteristics of Type II cells and their dependence upon CavL and ANO1 all suggest that these cells are viable candidates for the generation of SWs and tone in the IAS.
Similar content being viewed by others
Introduction
The internal anal sphincter (IAS) is responsible for approximately 70% of resting anal pressure; an important property for maintaining faecal continence1,2. Reports suggest that approximately 43% of faecal incontinence cases may be related to disturbances in IAS motility3. Unlike the majority of the gastrointestinal (GI) tract, the IAS spontaneously develops tone; a critical feature for raising pressure in the anal canal. While others have suggested that the IAS is a “purely tonic muscle” that develops tone as a result of enhanced myofilament sensitivity to Ca2+ 4,5, we have found that the IAS is fundamentally a phasic smooth muscle that generates tone as a result of the summation of phasic contractions6,7,8,9,10. The IAS exhibits slow wave (SW) activity, the electrophysiological events that give rise to phasic contractions in many regions of the GI tract11. SWs, phasic contractions and tone rely upon Ca2+ influx via voltage-gated L-type Ca2+ channels (CavL)8,12,13,14. Thus, mechanisms regulating Ca2+ entry are clearly fundamental for tone development in the IAS.
Interstitial cells of Cajal (ICC) are present throughout the GI tract and studies of ICC in non-sphincter muscles indicate that SWs are generated by specialised ICC located predominantly at the myenteric (ICC-MY) and/or submucosal (ICC-SM) edges of the circular muscle layer11. Pacemaker ICC in these regions are coupled electrically to one another and to adjacent smooth muscle cells (SMCs) via gap junctions allowing conduction of SWs from ICC to SMCs where excitation-contraction coupling occurs15. These cells are typically highly-branched stellate-shaped cells16,17. In contrast, another population of spindle-shaped intramuscular ICC (ICC-IM) are involved in neuromuscular transmission16,18. ICC are also present in the IAS of various species but their distribution and morphology differs significantly from that of non-sphincteric muscles19,20,21,22,23. Importantly, the density of ICC-MY and ICC-SM declines from rectum to IAS with only ICC-IM present in the distal IAS21,23. SW amplitude and frequency are greatest in the distal IAS8,23,24 and thus we hypothesise that ICC-IM could be the pacemaker cells that generate SWs in the IAS.
IAS-SWs differ significantly from intestinal SWs. Intestinal SWs persist in the presence of antagonists of CavL25, but IAS-SWs are inhibited by these antagonists, suggesting an essential role for CavL in these events in the IAS7,8,14,26. In addition, intestinal SWs have a more rapid upstroke due to the opening of T-type Ca2+ channels (CavT) which activate and deactivate at a more negative membrane potential (Em) than CavL27,28. The predominance of CavL as a mediator of SWs in the IAS is not surprising because “resting” Em in the IAS (−43 to −49 mV mouse IAS7,23,26) is more depolarised than intestine (−65 mV mouse jejunum29) and thus CavT channels would be substantially inactivated7,8,30.
SWs in the IAS and other GI muscles are dependent upon the Ca2+-activated Cl- channel, Anoctamin-1 (ANO1, encoded in mice by Ano1). ANO1 is highly expressed in ICC throughout the GI tract including the IAS14,29,31,32, but this conductance is not resolved in SMCs or the other type of interstitial cell found in GI muscles, platelet-derived growth factor receptor alpha-positive (PDGFRα+) cells. The cell-specific expression of ANO1 is important because antagonists of this conductance or genetic deactivation of Ano1 can be used to examine the functional role of ICC in intact muscles. ANO1 antagonists greatly reduce or abolish SWs14,29, and SWs fail to develop in ANO1 deficient mice29,33,34,35. Localised intracellular Ca2+ transients in ICC activate ANO1 channels, causing depolarisation, activation of voltage-gated Ca2+ channels and SW generation29,31,36,37,38. Since CavL and ANO1 antagonists block SWs as well as tone in the IAS we have proposed that IAS-SWs are important for tone generation7,8,13,14,26.
The current study utilised transgenic mice that express the genetically encoded Ca2+ indicator, GCaMP6f, in a cell-specific manner to visualise intracellular Ca2+ events in ICC-IM in the distal IAS in situ. The spatiotemporal properties of Ca2+ transients were measured in order to determine if ICC-IM generate pacemaker activity in the IAS. The effects of inhibition of CavL, hyperpolarisation of the Em and blockade of ANO1, were examined as these characteristics have previously been shown to alter the electrical activity, phasic contractions and tone in the IAS.
Results
Two functionally distinct subtypes of ICC-IM are present in the IAS
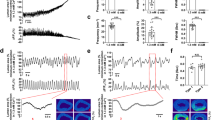
Ca2+ transients in ICC-IM in the distal IAS were imaged in situ using a spinning-disk confocal microscope. Initial experiments revealed two distinct patterns of Ca2+ transients in different spindle-shaped cell populations within the same field of view (FOV; Fig. 1A). Cell types were distinguished based upon differences in the characteristics of Ca2+ transients. Type I cells generated asynchronous Ca2+ transients that originated from multiple active sites and spread only short distances within the cell (Fig. 1A; cells i, ii and iii and 1B). In contrast, Type II cells generated synchronised, rhythmic Ca2+ transients that spread globally throughout the whole cell (Fig. 1A; cells iv, v and vi and 1C). By superimposing the plot profiles of Ca2+ transients from adjacent cells it is apparent that Type I cell activity was not synchronised within or between cells (Fig. 1B,D) whereas Type II cell activity was highly synchronised between neighbouring cells (Fig. 1C,E).
Two distinct populations of intramuscular interstitial cells of Cajal (ICC-IM) are present in the internal anal sphincter (IAS). (A) Frame of movie showing Ca2+ transients in two populations of GCaMP6f+ cells within the distal IAS (left, see also Supplemental Fig. S1). (B,C) Representative spatio-temporal (ST) maps created from cell i and iv comparing Ca2+ transients in Type I (B) and Type II cells (C). (D,E) Superimposed plot profiles of Ca2+ activity in adjacent cells highlighted in A, demonstrating the asynchrony of Type I cells (D) and the synchrony of Type II cells (E). (F) Scatter plots comparing (i) amplitude (P = 0.008), (ii) time to peak (P = 0.0057), (iii) spatial spread (P < 0.0001) and (iv) modal interval (P = 0.4413) of Ca2+ transients in Type I (○) and Type II ICC (○). Unpaired t test; Type I, n = 24, N = 15; Type II, n = 30, N = 18.
Detailed analysis from all recordings indicated that there was a significantly greater mean amplitude (P = 0.008), mean time to peak (P = 0.006), and mean spatial spread (P = 0.0001) of Ca2+ transients in Type II cells whereas the modal interval between Ca2+ transients was not significantly different (P = 0.441) between Type I and Type II cells (Fig. 1F). However, with regard to the modal interval between Ca2+ transients these were clustered tightly around the mean in Type II cells. This property is in keeping with their synchronised, rhythmic behaviour. In contrast, Type I cell intervals were distributed over a much wider range, commensurate with the generation of localised, asynchronous Ca2+ transients.
The rhythmic, highly synchronised behaviour of Type II cells makes it possible for these cells to be the pacemaker cells that generate IAS-SWs. To provide further insight into this relationship we determined the average frequency of Type II cell Ca2+ transients from the modal interval between Ca2+ transients. This analysis revealed an average Ca2+ transient frequency for Type II cells of 66.8 ± 1.6 cycles per min (cpm; n = 30, N = 18); a value that is well within the range reported for SWs at the distal end of the IAS (69.7 ± 3.2 cpm23). These data are consistent with the hypothesis that a relationship exists between Ca2+ transients in ICC-IM and SWs in the IAS.
Distribution of Type I and Type II cells in the rectoanal region
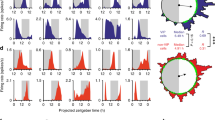
The distribution of Type I and Type II cells across the thickness of the distal IAS was determined by counting cell numbers exhibiting either Type I or Type II behaviour within the myenteric, middle or submucosal third of the muscle layer (Fig. 2A). Both cell types were present through the thickness of the muscle layer, however significantly greater numbers of Type II cells (63.7%) were observed than Type I cells (36.3%). Type II cells were greatest by more than a factor of two within the middle portion of circular muscle layer (P = 0.0001), which represents a novel distribution for pacemaker cells compared to other regions of the GI tract, i.e., ICC-MY and ICC-SM form networks along the margins of the circular muscle layer in other GI regions11. Although this study focused upon the distal end of the IAS, additional measurements were also undertaken to evaluate whether cell numbers within the muscle interior changed in the proximal direction (Fig. 2B). Type II cells declined orally, reaching undetectable levels 2–3 mm from the distal edge whereas Type I cells increased. These data add further support to our proposal that Type II cells represent a unique population of ICC-IM that is restricted to the IAS.
Distribution of Type I and Type II GCaMP6f+ cells in the internal anal sphincter (IAS) and rectum. (A) Bar graphs showing the distribution of Type I (black) and Type II cells (red) within the submucosal third, middle third and myenteric third of the circular muscle layer at the distal end of the IAS. *P = 0.034, ****P = 0.0001, N = 27, Two-way ANOVA with Šidák post-hoc test. (B) Distribution of Type I and Type II cells in the middle third of the muscle layer at increasing distance from the distal end of the IAS. Paired t test; 1 mm: **P = 0.0086, N = 7, 2 mm: ***P = 0.0007, N = 7, 3 mm: ***P = 0.0001, N = 6, 5 mm: ***P = 0.0002, N = 6.
Dependence of Ca2+ transients on extracellular Ca2+ and release of Ca2+ from stores
The dependence upon extracellular Ca2+ for the distinct behaviours of Type I and Type II cells was evaluated. Ca2+ transients were recorded from Type I and Type II cells before and during superfusion of Ca2+ free KRBS plus 0.5 mM EGTA. In Type I cells Ca2+ transients ceased 10.2 ± 0.6 min after beginning perfusion with Ca2+ free KRBS whereas Ca2+ transients in Type II cells were abolished after only 6.4 ± 0.7 min of Ca2+ free KRBS. These data indicate that both cell types are dependent to some extent on extracellular Ca2+ but that Type I cells are significantly more resistant to Ca2+ removal (P = 0.003; Supplemental Fig. S2). Additional experiments were undertaken to determine whether Ca2+ transients are due to Ca2+ release from the endoplasmic reticulum. The Ca2+-ATPase inhibitor CPA (10 µM) completely abolished Ca2+ transients in both cell types indicating their dependence on Ca2+ release from the endoplasmic reticulum (n = 12, N = 5, data not shown).
Dependence of Ca2+ transients on CavL
IAS-SWs and tone are dependent upon CavL7,8,13,14,26. Thus, we compared the sensitivity of Ca2+ transients in Type I and Type II cells to blockade of CavL. Ca2+ transients were recorded from Type I and Type II cells before and during superfusion with the CavL antagonist nifedipine (1 µM). Nifedipine caused a small but significant decrease in the time to peak (P = 0.015) of Ca2+ transients in Type I cells but did not significantly change the amplitude (P = 0.204), spatial spread (P = 0.279) or the mean modal interval (P = 0.999) between Ca2+ transients (Fig. 3A–C).
Ca2+ transients in Type I intramuscular interstitial cells of Cajal (ICC-IM) are not dependent on Ca2+ influx via CavL, but rhythmic, global Ca2+ transients in Type II ICC-IM are. (A) Spatio-temporal (ST) map of a Type I ICC-IM under control conditions. (B) ST map of the same cell as (A) in the presence of 1 µM nifedipine. (C) Scatter plots summarising the effect of nifedipine (Nifed) on (i) amplitude (P = 0.2041), (ii) time to peak (P = 0.0146), (iii) spatial spread (P = 0.2793) and (iv) modal interval (P = 0.9999), in Type I cells (paired t test; n = 9, N = 4). (D,E) Representative ST maps of the same cell before (D) and after (E) application of 1 µM nifedipine. (F) Scatter plots summarising the effect of nifedipine on (i) amplitude (P = 0.1166), (ii) time to peak (P = 0.0912), (iii) spatial spread (P = 0.0001) and (iv) modal interval (P = 0.0013) in Type II cells. Control= ○, nifedipine = ○ (paired t test; n = 13, N = 6).
In contrast, the rhythmic, synchronised Ca2+ transients in Type II cells (Fig. 3D) were blocked by nifedipine, with activity similar to the Ca2+ transients in Type I cells persisting (Fig. 3E). Specifically, there was a significant decrease in the spatial spread (P = 0.0001) of Ca2+ transients and a significant increase in the mean modal interval (P = 0.001) between Ca2+ transients (Fig. 3F). These changes indicate a shift in behaviour of Type II cells to a more ‘Type I-like’ pattern when CavL are blocked and suggest that the rhythmic Ca2+ transients in Type II cells are organised into global, or propagated, Ca2+ transients when a mechanism for voltage-dependent Ca2+ entry is available. Similar observations occurred with ICC-MY, the pacemaker cells of the small intestine, although the voltage-dependent conductance required in those cells was CavT39.
Dependence of Ca2+ transients on membrane potential
IAS-SWs are abolished by membrane hyperpolarisation10, whereas Ca2+ transients in ICC-IM of the mouse colon are not40. We investigated whether Ca2+ transients in Type I or Type II cells are modified by membrane hyperpolarisation with the KATP channel agonist, pinacidil (Fig. 4). This compound has been shown to hyperpolarise Em and decrease phasic contractions and tone in the IAS10. Pinacidil (10 µM) had no significant effect on any of the Ca2+ transient parameters measured in Type I cells in the IAS (Fig. 4C), but pinacidil blocked the global, rhythmic Ca2+ transients in Type II cells (Fig. 4D,E). The global Ca2+ transients characteristic of Type II cells were replaced by Ca2+ transients similar to those in Type I cells (Fig. 4E), and a significant decrease in amplitude (P = 0.014) and spatial spread (P = 0.0001) was noted (Fig. 4F). There was also a significant increase in the mean modal interval (P = 0.035) between Ca2+ transients (Fig. 4F). These data further support the conclusion that global, rhythmic Ca2+ transients in Type II cells require voltage-dependent Ca2+ entry.
Type I cell Ca2+ transients are insensitive to changes in Em, whereas Type II cell Ca2+ transients are sensitive to changes in Em. (A) Spatio-temporal (ST) map of a Type I cell under control conditions. (B) ST map of the same cell as (A) in the presence of 10 µM pinacidil. (C) Scatter plots summarising the effect of pinacidil (Pinac) on (i) amplitude (P = 0.1245), (ii) time to peak (P = 0.9969), (iii) spatial spread (P = 0.83) and (iv) modal interval (P = 0.7318), in Type I cells (paired t test; n = 8, N = 3). (D) ST map of a Type II cell under control conditions. (E) ST map of the same cell as (D) in the presence of 10 µM pinacidil. Type II cell rhythmic Ca2+ transients are suppressed by changes in Em. (F) Scatter plots summarising the effect of pinacidil on (i) amplitude (P = 0.0138), (ii) time to peak (P = 0.85), (iii) spatial spread (P = 0.0001) and (iv) modal interval (P = 0.0353) in Type II cells. Control= ○, pinacidil = ○ (Paired t test; n = 9, N = 6).
Dependence of Ca2+ transients on ANO1
ANO1 plays a pivotal role in the generation of SWs in the GI tract including IAS-SWs14. For this reason, we examined the actions of the ANO1 blocker CaCCinh-A01 (10 µM) on Type I and Type II cells. Previously we have shown that 10 µM CaCCinh-A01 reduces spontaneous contractions in the mouse IAS by 83% while the contraction elicited by raising [K+]o to 66 mM is reduced by only 14%14. Since the K+-induced contraction is entirely abolished by nifedipine but is largely intact following CaCCinh-A01, this concentration of CaCCinh-A01 appears to have limited direct effects upon CavL. CaCCinh-A01 (10 µM) had no effect on Type I cells (Fig. 5A–C), but this compound shifted Ca2+ activity in Type II cells from rhythmic, global Ca2+ transients (Fig. 5D) to asynchronous Ca2+ transients similar to those observed in Type I cells (Fig. 5E). A significant decrease in the spatial spread of Ca2+ transients was also noted after exposure to CaCCinh-A01 (P = 0.0001; Fig. 5F). These data indicate that rhythmic, global Ca2+ transients in Type II cells depend on the availability of ANO1 channels.
Inhibition of anoctamin 1 (ANO1) does not abolish Ca2+ transients in Type I cells, but it does abolish Type II rhythmic global cell Ca2+ transients. (A) Spatio-temporal (ST) map of Type I cell Ca2+ transients under control conditions. (B) ST map of Ca2+ activity in the same cell as (A) after the application of 10 µM CaCCinh-A01. (C) Scatter plots summarising the effect of CaCCinh-A01 (10 µM) on (i) amplitude (P = 0.46), (ii) time to peak (P = 0.7818), (iii) spatial spread (P = 0.8007) and (iv) modal interval (P = 0.103), in Type I cells (Paired t test; n = 8, N = 4). CaCCinh-A01 inhibits rhythmic Ca2+ transients in Type II cells. (D) ST map from a Type II cell under control conditions. (E) ST map from the same cell after the application of CaCCinh-A01 (10 µM). (F) Scatter plots summarising the effect of CaCCinh-A01 on (i) amplitude (P = 0.2191), (ii) time to peak (P = 0.7768), (iii) spatial spread (P = 0.0001) and (iv) modal interval (P = 0.0639) in Type II cells. Control= ○, CaCCinh-A01 = ○. (paired t test; n = 8, N = 6).
Immunohistochemical examination of the Kit-Cre-GCaMP6f mouse IAS
We tested whether GCaMP6f is expressed selectively in ICC using dual labelling immunohistochemistry. In the GI tract, SMCs, ICC and PDGFRα+ cells form an electrical syncytium known as the “SIP” syncytium11. Therefore, we also examined expression in the other two cell types, i.e., SMCs and PDGFRα+ cells. Selective labelling of ICC, PDGFRα+ cells and SMCs was achieved using antibodies for c-Kit, PDGFRα and smMHC, respectively, while GCaMP6f+ cells were labelled with an antibody for GFP. GFP labelling did not overlap with either PDGFRα (Fig. 6B) or smMHC (Fig. 6C) indicating that GFP+ cells were neither PDGFRα+ cells nor SMCs. In contrast, the majority of labelled cells (60.4 ± 2.4%, n = 11) were GFP+/c-Kit+ while 34% were GFP+/c-Kit− (34.2 ± 2.7%, n = 11; Fig. 6A). A final small percentage was GFP−/c-Kit+ (5.4 ± 1.0%, n = 11). We reasoned that the reduced expression of c-Kit in GFP+ cells may occur as a result of the methodology used to achieve specific GCaMP6f expression in ICC, i.e., reduction of Kit alleles from two to one (see methods). Therefore, we compared the density of c-Kit+ cells in wildtype and Kit-Cre-GCaMP6f mice (Fig. 6D). The cell density of c-Kit+ cells in Kit-Cre-GCaMP6f mice was found to be significantly less (P = 0.001) than that of wildtype mice whereas GFP+ density in Kit-Cre-GCaMP6f mice was similar (P = 0.957) to c-Kit density in wildtype mice. In summary, (1) GCaMP6f expression is coupled to c-Kit, (2) GCaMP6f cells are neither SMCs nor PDGFRα+ cells, and (3) GCaMP6f cells have a similar distribution and density as c-Kit+ cells in wildtype mice. These data suggest that GCaMP6f cells are ICC-IM even though c-Kit expression in some cells is low.
Immunohistochemical characterisation of GCaMP6f cells in the Kit-Cre-GCaMP6f mouse IAS. (A–C) Dual labelling images of GCaMP6f cells in the internal anal sphincter (IAS) labelled with an anti-GFP antibody in combination with either c-Kit, platelet-derived growth factor receptor alpha (PDGFRα) or smooth muscle myosin heavy chain (smMHC) antibodies. Higher magnification images are shown in the bottom panels (Ad, Bd, Cd). GFP+ cells were neither PDGFRα+ (B) nor smMHC+ (C) but the majority (61.6 ± 2.6%, N = 11) were c-Kit+ (A). (D) Comparison of c-Kit+ cell density in wildtype mice (first bar) versus the density of c-Kit+ cells (second bar), GFP+ cells (third bar) and ‘all cells’ which includes all GFP+ cells plus cells that were c-Kit+/GFP− (fourth bar) in Kit-Cre-GCaMP6f mice. The density of c-Kit+ cells in wildtype mice (N = 11) was significantly greater than in Kit-Cre-GCaMP6f mice (**P = 0.001) while wildtype c-Kit+ cell density was not different from the density of GFP+ cells (P = 0.727) or ‘all cells’ (P = 0.957) in Kit-Cre-GCaMP6f mice. Comparison of cell densities in Kit-Cre-GCaMP6f mice indicated that c-Kit+ cell density was significantly less than the densities of GFP+ cells (*P = 0.023) and ‘all cells’ (**P = 0.006). One-way ANOVA, post-hoc Tukey’s, N = 11.
Discussion
The IAS generates SWs that initiate phasic contractions which can summate to produce tone10. The present study examined the possible link between ICC-IM and pacemaker activity (i.e., SWs) in the IAS. To this end, Cre-lox methodologies were used to generate mice containing the genetically encoded Ca2+ indicator GCaMP6f in ICC (Kit-Cre-GCaMP6f). With the aid of these mice Ca2+ transients were examined in ICC-IM in situ within the IAS. An interesting outcome of this study was that two distinct sub-populations of cells were present. The first population (Type I ICC-IM) exhibited asynchronous Ca2+ transients similar to the ICC-IM that are involved in neuromuscular transmission in other GI muscles11,16,18,40,41,42,43,44,45,46,47,48. The second population (Type II ICC-IM) was novel and displayed spatiotemporal and pharmacological properties consistent with these cells generating pacemaker activity in the IAS.
GCaMP6f is expressed in c-Kit+ spindle-shaped cells of the IAS
Immunohistochemical studies of the mouse rectoanal region have shown that ICC-MY and ICC-SM populations decline aborally reaching undetectable levels at the distal IAS whereas spindle-shaped ICC-IM are found throughout23. The present study supports this assessment since GCaMP6f expression in the distal IAS was limited to spindle-shaped intramuscular cells. Immunohistochemical examination of Kit-Cre-GCaMP6f tissues revealed that GCaMP6f (GFP+) cells. were smMHC−/PDGFRα− indicating they were neither SMCs nor PDGFRα+ cells. As described in Methods, the Kit-CreERT2 mice used to produce Kit-Cre-GCaMP6f mice were generated by inserting CreERT2 in exon 1 of the Kit gene on a single allele46. Since, CreERT2 is expressed in place of Kit on one allele, Kit-Cre-GCaMP6f mice may have reduced Kit gene expression. None-the-less, the majority of GCaMP6f cells were c-Kit+ and the density of GCaMP6f cells in Kit-Cre-GCaMP6f mice was equivalent to the density of c-Kit+ cells in wildtype mice. Taken together, these data suggest that GCaMP6f expressing cells are ICC-IM.
Two distinct populations of GCaMP6f cells are present in the IAS
Ca2+ imaging of GCaMP6f cells in the distal IAS revealed two distinct functional populations, i.e., Type I and Type II cells. Type I cells exhibited localised Ca2+ transients originating from multiple active sites each spreading a short distance. These Ca2+ transients were asynchronous both within and between neighbouring cells. Ca2+ transients in Type I cells were also smaller in amplitude and reached peak intensity faster than Ca2+ transients in Type II cells. The asynchronous activity described here for Type I cells is similar to that previously described for ICC-IM in the mouse colon and small intestine40,47. A large body of evidence from other GI muscles suggests that ICC-IM (and ICC-DMP in small intestine) serve as post-junctional targets for excitatory cholinergic and inhibitory nitrergic neuromuscular transmission11,16,18,41,42,43,44,45,46,47,48. It is likely that the Type I cells described here serve a similar role in the IAS. Indeed, functional nitrergic and cholinergic pathways are present in the mouse IAS7,13 and we have previously identified expression of guanylate cyclase and cGMP-dependent protein kinase in ICC-IM49. Ca2+ release events were independent of ANO1 but it is still possible that neurotransmitter-induced changes in Ca2+ release modify ANO1 activity giving rise to junction potentials (i.e., changes in Em) that change the contractile behaviour of adjacent SMCs.
In contrast, the activity of Type II cells consisted of rhythmic, global Ca2+ transients. Furthermore, the activity of neighbouring Type II cells was synchronised. In other GI regions, pacemaker ICC form networks at the myenteric or submucosal edges of the circular muscle, and SWs spread actively through these networks conducting to coupled SMCs as the wave of activity progresses11. In the IAS, synchronisation of Type II cells occurs in spite of the fact that these cells do not form a network with one another. However, ICC-IM are electrically coupled to SMCs via gap junctions11,50,51. Thus, SMCs may act as a bridge in the propagation of SW activity between ICC-IM. This is a novel model of SW propagation that may be unique to the IAS.
Type I and Type II cells were distributed across the thickness of the distal IAS but the changes occurring in their density in the oral direction differed. Type I cell density increased moderately in the proximal direction. In contrast, Type II cell density declined proximally reaching negligible levels 2 to 3 mm from the distal edge indicating that these cells are unique to the IAS. In like manner, previous functional studies have shown that the amplitude and frequency of SWs23 and SMC Ca2+ transients52 are greatest at the distal IAS and decline proximally. Thus, the anatomical location of Type II cells in the anorectum coincides with the functional distribution of SWs; an observation consistent with a role for Type II cells in the generation of SWs.
CavL is critical for rhythmic Ca2+ events in Type II cells
CavL plays a critical role in the generation of SWs and tone in the IAS8,12,13,14,26. In a similar manner, rhythmic, global Ca2+ transients in Type II cells were abolished by blocking CavL with nifedipine or by hyperpolarisation of the Em with the KATP agonist pinacidil. In contrast, asynchronous, localised Ca2+ transients of Type I cells persisted following both procedures. Interestingly, following CavL blockade, the rhythmic, global Ca2+ transients observed in Type II cells were replaced by smaller amplitude asynchronous, localised Ca2+ transients similar in time course and amplitude to those of Type I cells indicating a common underlying feature of Type I and Type II cells.
As mentioned in the introduction, “resting” Em (i.e., the level of Em between SWs) is more depolarised in the IAS than intestine making CavL the primary mediator of SWs rather than CavT7,8,30. The depolarised status of Em is likely the result of low resting K+ conductance (GK) since increasing GK with pinacidil hyperpolarises Em by more than 20 mV, blocking SWs10,53 and global Ca2+ transients in ICC (present study). In like manner, purinergic neuromuscular transmission, which activates small conductance Ca2+-activated K+ channels on PDGFRα+ cells54,55,56,57, also hyperpolarises Em in the mouse IAS by more than 20 mV, again abolishing SWs7,26. Although nerve stimulation can dramatically alter the electrical and contractile activity of the in vitro mouse IAS7,13,26,49,58, blockers of cholinergic, purinergic and nitrergic transmission as well as the neurotoxin TTX have little or no effect upon the basal electrical and contractile activity in the mouse IAS7,14,49,58. Hence, the properties of Ca2+ transients described in this study are intrinsic to the Type I and Type II ICC-IM examined rather than to neural inputs.
Relationship of Type I and Type II cell Ca2+ events to Ca2+ release and ANO1
Substantial evidence indicates that SW generation in ICC begins with localised release of Ca2+ from stores followed by activation of ANO1, depolarisation, activation of voltage-gated Ca2+ channels and finally a fully developed SW36,59,60,61. To examine the role of Ca2+ release in Type I and Type II cells, extracellular Ca2+ was removed or the Ca2+-ATPase inhibitor CPA was added. Ca2+ removal abolished all Ca2+ transients in Type I and Type II cells although the time required to produce full inhibition was greater for Type I cells (10.2 min) than Type II cells (6.4 min). This difference in time course may reflect the greater ongoing entry of Ca2+ into Type II cells via CavL and/or difference(s) in the mechanism(s) contributing to the uptake and storage of Ca2+ by the endoplasmic reticulum (ER). Asynchronous Ca2+ transients in Type I and Type II cells were also abolished by addition of the SERCA pump inhibitor CPA providing evidence they are due to Ca2+ release from the ER. The characteristics observed for asynchronous Ca2+ transients in Type I and Type II cells in the IAS are similar to those described for ICC-IM and ICC-DMP in the mouse colon and small intestine40,47. Furthermore, both studies conclude that Ca2+ release mechanisms involve inositol triphosphate receptors (InsP3Rs) as well as ryanodine receptors (RyRs) with ongoing refilling of the endoplasmic reticulum via Ca2+-ATPase.
Blockade of ANO1 with CaCCinh-A01 had similar effects on Type I and Type II cells to those observed with nifedipine or pinacidil. CaCCinh-A01 failed to block asynchronous localised Ca2+ transients in Type I cells while rhythmic, global Ca2+ transients in Type II cells were replaced by asynchronous localised Ca2+ transients. These data indicate that CavL, Ca2+ release and ANO1 each contribute to the generation of synchronised rhythmic Ca2+ transients in Type II cells; observations consistent with SW generation in ICC (see Fig. 7). The very minimal effect of ANO1 inhibition on Ca2+ transients in Type I cells was interesting since there is evidence that spontaneous transient inward currents (STICs) initiated by ANO1 in ICC-IM contribute to regulating resting Em and modulation of Em by nerves62,63. However, since nifedipine, pinacidil and CaCCinh-A01 all have minimal effects on Ca2+ transients in Type I cells it supports the general consensus that Ca2+ transients in ICC-IM (other than Type II cells in the IAS) are voltage-independent events.
Proposed mechanism for the generation of pacemaker activity by Type II intramuscular interstitial cells of Cajal (Type II ICC-IM). In Type II ICC-IM, Ca2+ release from the endoplasmic reticulum activates the Ca2+-activated Cl− channel ANO1 causing Cl− efflux and membrane depolarisation. This in turn activates L-type voltage-dependent Ca2+ channels (CavL) on ICC-IM resulting in Ca2+ influx and further activation of ANO1 which in turn leads to slow wave (SW) generation (observed as a rhythmic, global Ca2+ transient). Depolarisation is conducted to adjacent smooth muscle cells (SMCs) via gap junctions leading to Ca2+ influx in SMCs via CavL and thus, contraction. SWs, rhythmic, global Ca2+ transients and tone are all abolished by the ANO1 blocker CaCCinh-A01 (10 µM) and the CavL blocker nifedipine (1 µM) indicating that Type II ICC-IM represent a viable candidate for generating pacemaker activity in the IAS and that these cells may therefore play an important role in tone generation. This cartoon was drawn using CorelDraw 2019 Software.
Relationship of SWs to tone development in the IAS
The IAS differs from the majority of GI muscles in that it generates tone to raise pressure in the anal canal thereby facilitating faecal continence1,2,10. CavL channels play a fundamental role in this process since tone is blocked by manoeuvres which inhibit CavL activity12,13,14,64,65,66. A common feature of the IAS in all animal species studied is SWs6,7,8,9,10. Like tone, SWs are abolished by inhibitors of CavL activity8,9,14,26,67 indicating an important link between SWs and tone. One possible way in which phasic SWs can give rise to tone is via a partial (incomplete) tetanus type mechanism like that first described for skeletal muscle. Partial tetanus occurs as stimulus frequency is increased beyond a certain level, leading to an inability of Ca2+ removal to keep up with Ca2+ delivery and thus resulting in increased basal cytoplasmic [Ca2+]68. This “partial tetanus” consists of phasic contractions superimposed upon tone. SW frequency in the mouse IAS is quite high (i.e., around 70 cpm) and we have recently provided evidence for a partial tetanus type mechanism in studies of the SM-GCaMP mouse IAS52. In larger animal species (e.g., dog, monkey) the IAS is composed of numerous muscle bundles separated by wide connective tissue septa19,21. The degree to which these muscle bundles are electrically coupled is still unclear. Thus, an additional mechanism by which SWs may give rise to tone in larger animals is via summation of asynchronous phasic events arising from poorly coupled muscle bundles. In like manner, skeletal postural muscles can maintain tone through the asynchronous firing of motor units69. Finally, the overall depolarised level of Em in the IAS likely results in some Ca2+ entry via CavL that is time-independent (i.e., window current)10.
In summary, Ca2+ transients in ICC-IM were imaged in situ in Kit-Cre-GCaMP6f mice to examine the possibility that these cells are responsible for SW generation in the IAS. Two distinct sub-populations of ICC-IM were identified. The first, Type I ICC-IM, exhibited asynchronous Ca2+ transients with properties resembling those of ICC-IM in other GI muscles that participate in enteric neuromuscular transmission. The second, Type II ICC-IM, exhibited Ca2+ transients with spatiotemporal and pharmacological properties novel for ICC-IM but very consistent with the behaviour of pacemaker ICC. Type II ICC-IM exhibited rhythmic, global Ca2+ transients that were synchronised with adjacent Type II cells. These events were dependent upon CavL as well as ANO1; a Ca2+-activated Cl− channel that is highly expressed in ICC-IM of the mouse IAS14. From these data we conclude that Type II ICC-IM are a viable candidate for the generation of pacemaker activity in the mouse IAS. Since tone in the IAS is also dependent upon CavL and ANO114, it follows that Type II ICC-IM may also play an important physiological role in the regulation of anal pressure and maintenance of faecal continence.
Methods
Animals
In order to study intracellular Ca2+ activity in ICC, the genetically encoded Ca2+ indicator GCaMP6f was expressed specifically in ICC. GCaMP6f-lox+ mice (B6;129S-Gt(ROSA)26S°rtm95.1(CAG-GCaMP6f)Hze/J; The Jackson Laboratory; strain number: 024105) were crossed with Kit+/Cre-ERT2 mice (gift from Dr. D. Saur; Technical University of Munich, Munich, Germany) to generate Kit-Cre-GCaMP6f mice. As previously described, Kit-CreERT2 mice were generated by inserting CreERT2 into exon 1 of the Kit gene on a single allele46 thus confining Kit expression to one allele. To activate Cre-recombinase and induce GCaMP6f expression, mice were injected with tamoxifen at 6–8 weeks old (2 mg for three consecutive days) as previously described47. Animals were sacrificed 10–30 days after tamoxifen injection. For cell density studies, wildtype C57Bl/6 mice (6–8 weeks old, The Jackson Laboratory) were also used. Mice were euthanised with isoflurane (Baxter) inhalation and cervical dislocation. All procedures were approved by the Institutional Animal Use and Care Committee at the University of Nevada, Reno. Animals used and experiments performed were also in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Tissue preparation
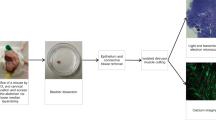
Rectoanal tissues were removed and placed in a Sylgard-lined dissection dish with ice cold Krebs-Ringer bicarbonate solution (KRBS) of the following composition (in mM): 118 NaCl, 4.7 KCl, 2.5 CaCl2, 23.8 NaHCO3, 1.2 KH2PO4, 11 dextrose. KRBS had a pH of 7.4 after bubbling to equilibrium with 95% O2/5% CO2. Flat sheet preparations of the rectoanal region were prepared for Ca2+ imaging experiments by clearing the tissue of surrounding skeletal muscle including the external anal sphincter and fat, cutting the tissue open from rectum to anus and removing the mucosal layer to expose the inner circular layer of the muscularis externa. Tissues were pinned flat, with the circular muscle facing upwards in a 60 mm Sylgard/charcoal-lined dish. To rule out the possible excitatory effects of cholinergic motor neurons all experiments were carried out in the presence of atropine (1 µM).
Calcium imaging
Tissues were continually perfused with oxygenated KRBS at 37 °C and left to equilibrate for 1 hour. For experiments determining the reliance of each cell type on extracellular Ca2+ the tissue was perfused with Ca2+ free KRBS plus 0.5 mM EGTA. Images were obtained with an upright confocal microscope (Olympus) equipped with a spinning disc (CSU-X1, Yokogawa) and an Andor iXon EMCCD camera (Andor Technology, Belfast) to detect images. GCaMP6f was excited using a 488 laser (Coherent OBIS) and all recordings were acquired with a 20 × 1.00 NA XLUMPlanFL N lens (Olympus, Japan) at a frame rate of 30 frames per second. All recordings, unless otherwise indicated were made from GCaMP6f cells at the distal extremity of the IAS.
Calcium imaging analysis
Recordings of Ca2+ activity in ICC-IM of the IAS were imported into custom analysis software (Volumetry G8d, provided by Dr. Grant W. Hennig). If necessary, recordings were motion-stabilised to ensure accurate Ca2+ transient analysis. De-bleaching techniques were applied where appropriate in order to remove background fluorescence. Spatio-temporal (ST) maps were created for specific cells of interest by rotating image stacks so that ICC-IM were orientated vertically and drawing a region of interest (ROI) around the visible part of the cell. Further Ca2+ transient analysis was carried out by importing the ST maps into Image J (version 1.8, National Institutes of Health, MD, USA) as previously described39,70,71. Briefly, ST maps were normalised to obtain F/F0 by dividing the image by the mean intensity of the cell between Ca2+ transients. Active sites of Ca2+ activity, defined as sites that Ca2+ transients consistently originated from were analysed over a 10 s period (i.e. some cells had Ca2+ transients that originated from multiple active sites whereas others had Ca2+ transients that originated from only one active site, see descriptions of Type I and Type II cells in the results section). The amplitude (expressed as Δ F/F0), time to peak (time from trough to peak of a Ca2+ transient), spatial spread (distance Ca2+ transient spread along the cell, measured using the ‘wave speed’ command) and interval (time from one peak of a Ca2+ transient to the next, expressed as modal) were measured.
The cellular distribution within the circular muscle layer was calculated using confocal microscopy at 20x magnification (400 µm × 400 µm) and focusing upon either the myenteric, middle, or submucosal third of the circular muscle layer. All cells in focus within that region were then assigned according to established behaviours (see further description in the results section).
Immunohistochemistry
Specimen preparation and labelling
The distal GI tract was kept in a tube and the mucosa removed as described previously23. Tissues were fixed in ice-cold Zamboni’s fixative (2% paraformaldehyde) for 20 min at 20 °C before washing in 0.1 M PBS, dehydrated in graded sucrose solutions (5–20%), and frozen as previously described22. Frozen tissues were sectioned perpendicular to the circular muscle layer at a thickness of 10–12 µm with a Leica CM 3050 cryostat (Leica Microsystems, Wetzlar, Germany) and labelled with primary and secondary antibodies as previously described14. Sections were blocked using 1% bovine serum albumin (BSA) for 1 hr at 20 °C before incubation in the first primary antibody (anti-GFP, Abcam, Cambridge, MA, USA; 1:1000 dilution in Triton-X working solution) for 16 hours at 4 °C. Sections were washed with 0.1 M PBS before incubation in secondary antibody (Alexa Fluor anti-chicken 488, Invitrogen, Carlsbad, CA, USA; 1:1000 dilution). Subsequently, sections were washed in 0.1 M PBS prior to incubation with the second primary antibody (i.e., anti-PDGFRα, R&D Systems, Minneapolis, MN, USA; 1:1000 dilution, mSCFR, anti-c-Kit antibody, R&D Systems, Minneapolis, MN, USA; 1:1000 dilution or anti-smooth muscle myosin heavy chain (smMHC), Biomedical Technologies Inc., Stoughton, MA, USA; 1:100 dilution). Following incubation with the second primary antibody, sections were washed and incubated with secondary antibody (Alexa Fluor anti-goat 594 (PDGFRα, c-Kit) or Alexa Fluor anti-rabbit 594 (smMHC), Invitrogen, Carlsbad, CA, USA; 1:1000 dilution) as described above. After washing with 0.1 M PBS, slides were covered with coverslips using Aquamount mounting medium (Lerner Laboratories, Pittsburgh, PA). Wildtype mouse rectoanal tissues were dissected, fixed and labelled as described above. In this case, preparations were labelled with anti-c-Kit antibody (mSCFR) followed by Alexa Fluor anti-goat 594.
Imaging of Immunohistochemical Labelling and Determination of Cell Density
Confocal images of tissue sections were imaged using a Zeiss LSM 510 Meta confocal microscope (Carl Zeiss, Thornwood, NY, USA). Images shown in figures are composites of Z-series stacks taken through a depth of 1–13 µm. Figures were prepared using Zeiss LSM 510 Images Examiner Software, Adobe Photoshop CS5 Software and CorelDraw 2019 Software.
Cell density was determined from cross sections of the distal IAS circular muscle layer of wildtype (C57Bl/6) and Kit-Cre-GCaMP6f mice. A line was drawn around the edge of the circular muscle layer using CorelDraw 2019 Software and then all green, red and yellow cells were counted within this area. Total area was determined from images in Adobe Photoshop CS5 Software. Cell density was then expressed as number of cells per 100 µm2.
Statistics
Data is expressed as the mean ± SD. Statistical analysis was carried out using GraphPad Prism (version 7.0). Student’s paired or unpaired two-tailed t-test was used to compare two groups, i.e., control conditions versus drug (paired) or various parameters in Type I versus Type II cells (unpaired). Two-way ANOVA with Šidák post-hoc test was used to compare Type I and Type II cell distribution in Ca2+ imaging studies. One-way ANOVA with Tukey’s post-hoc test was used in comparing c-Kit+ and GFP+ cell numbers in immunohistochemical studies. A P-value of <0.05 was considered statistically significant. n= number of cells, N = number of animals.
Drugs
Nifedipine, pinacidil, CPA (cyclopiazonic acid), EGTA (ethylene glycol-bis(2-aminoethylether)-N,N,N′,N′-tetraacetic acid), atropine and tamoxifen were purchased from Sigma-Aldrich (Saint Louis, MO, USA). CaCCinh-A01 was purchased from Tocris Bioscience (Minneapolis, MN, USA). Stock concentrations were made by dissolving in de-ionized water (pinacidil), ethanol (nifedipine) or DMSO (CaCCinh-A01). Final concentrations were achieved by further dilution in KRBS. EGTA was dissolved to final concentration in KRBS. Tamoxifen was first dissolved in ethanol before addition of safflower oil to make a final concentration of 20 mg ml−1.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Bharucha, A. In Pathophysiology of the enteric nervous system: A basis for understanding functional diseases. (eds. Spiller, R. & Grundy, D.) Ch. 13, 161–175 (Wiley Blackwell Publishing, 2004).
Rao, S. S. & Meduri, K. What is necessary to diagnose constipation? Best. Pract. Res. Clin. Gastroenterol 25, 127–140 (2011).
Thekkinkattil, D. K. et al. A classification system for faecal incontinence based on anorectal investigations. Br. J. Surg 95, 222–228 (2008).
Patel, C. A. & Rattan, S. Cellular regulation of basal tone in internal anal sphincter smooth muscle by RhoA/ROCK. Am. J. Physiol Gastrointest. Liver Physiol 292, G1747–G1756 (2007).
Rattan, S. & Singh, J. RhoA/ROCK pathway is the major molecular determinant of basal tone in intact human internal anal sphincter. Am. J. Physiol Gastrointest. Liver Physiol 302, G664–G675 (2012).
Papasova, M., Milousheva, E., Bonev, A. & Gachilova, S. Specific features in the electrical and contractile activities of the gastro-intestinal sphincters. Acta Physiol Pharmacol. Bulg 6, 19–27 (1980).
Duffy, A. M., Cobine, C. A. & Keef, K. D. Changes in neuromuscular transmission in the W/W(v) mouse internal anal sphincter. Neurogastroenterol. Motil 24, e41–e55 (2012).
Mutafova-Yambolieva, V. N., O’Driscoll, K., Farrelly, A., Ward, S. M. & Keef, K. D. Spatial localization and properties of pacemaker potentials in the canine rectoanal region. Am. J. Physiol Gastrointest. Liver Physiol 284, G748–G755 (2003).
Harvey, N., McDonnell, B., McKechnie, M. & Keef, K. Role of L-type calcium channels, membrane potential and nitric oxide in the control of myogenic activity in the primate internal anal sphincter. Gastroenterology 134, A63 (2008).
Keef, K. D. & Cobine, C. A. Control of Motility in the Internal Anal Sphincter. Journal of Neurogastroenterology and Motility 25, 189–204 (2019).
Sanders, K. M., Ward, S. M. & Koh, S. D. Interstitial cells: regulators of smooth muscle function. Physiol Rev 94, 859–907 (2014).
Cook, T. A., Brading, A. F. & Mortensen, N. J. Effects of nifedipine on anorectal smooth muscle in vitro. Dis. Colon Rectum 42, 782–787 (1999).
Cobine, C. A., Fong, M., Hamilton, R. & Keef, K. D. Species dependent differences in the actions of sympathetic nerves and noradrenaline in the internal anal sphincter. Neurogastroenterol. Motil 19, 937–945 (2007).
Cobine, C. A. et al. ANO1 in intramuscular interstitial cells of Cajal plays a key role in the generation of slow waves and tone in the internal anal sphincter. J. Physiol 595, 2021–2041 (2017).
Cousins, H. M., Edwards, F. R., Hickey, H., Hill, C. E. & Hirst, G. D. Electrical coupling between the myenteric interstitial cells of Cajal and adjacent muscle layers in the guinea-pig gastric antrum. J. Physiol 550, 829–844 (2003).
Burns, A. J., Lomax, A. E., Torihashi, S., Sanders, K. M. & Ward, S. M. Interstitial cells of Cajal mediate inhibitory neurotransmission in the stomach. Proc. Natl. Acad. Sci. U. S. A 93, 12008–12013 (1996).
Burns, A. J., Herbert, T. M., Ward, S. M. & Sanders, K. M. Interstitial cells of Cajal in the guinea-pig gastrointestinal tract as revealed by c-Kit immunohistochemistry. Cell Tissue Res 290, 11–20 (1997).
Ward, S. M., Sanders, K. M. & Hirst, G. D. Role of interstitial cells of Cajal in neural control of gastrointestinal smooth muscles. Neurogastroenterol. Motil 16(Suppl 1), 112–117 (2004).
Horiguchi, K., Keef, K. D. & Ward, S. M. Distribution of interstitial cells of Cajal in tunica muscularis of the canine rectoanal region. Am. J. Physiol Gastrointest. Liver Physiol 284, G756–G767 (2003).
Piotrowska, A. P., Solari, V. & Puri, P. Distribution of interstitial cells of Cajal in the internal anal sphincter of patients with internal anal sphincter achalasia and Hirschsprung disease. Arch. Pathol. Lab Med 127, 1192–1195 (2003).
Cobine, C. A. et al. Interstitial cells of Cajal in the cynomolgus monkey rectoanal region and their relationship to sympathetic and nitrergic nerves. Am. J. Physiol Gastrointest. Liver Physiol 298, G643–G656 (2010).
Cobine, C. A. et al. Relationship between interstitial cells of Cajal, fibroblast-like cells and inhibitory motor nerves in the internal anal sphincter. Cell Tissue Res 344, 17–30 (2011).
Hall, K. A., Ward, S. M., Cobine, C. A. & Keef, K. D. Spatial organization and coordination of slow waves in the mouse anorectum. J. Physiol 592, 3813–3829 (2014).
Kubota, M., Suita, S. & Szurszewski, J. H. Membrane properties and the neuro-effector transmission of smooth muscle cells in the canine internal anal sphincter. J. Smooth. Muscle Res 34, 173–184 (1998).
Ward, S. M. & Sanders, K. M. Upstroke component of electrical slow waves in canine colonic smooth muscle due to nifedipine-resistant calcium current. J. Physiol (Lond) 455, 321–337 (1992).
McDonnell, B., Hamilton, R., Fong, M., Ward, S. M. & Keef, K. D. Functional evidence for purinergic inhibitory neuromuscular transmission in the mouse internal anal sphincter. Am. J. Physiol Gastrointest. Liver Physiol 294, G1041–G1051 (2008).
Zheng, H., Park, K. S., Koh, S. D. & Sanders, K. M. Expression and function of a T-type Ca2+ conductance in interstitial cells of Cajal of the murine small intestine. Am. J. Physiol Cell Physiol 306, C705–C713 (2014).
Chul Kim, Y., Don Koh, S. & Sanders, K. M. Voltage-dependent inward currents of interstitial cells of Cajal from murine colon and small intestine. The Journal of Physiology 541, 797–810 (2004).
Hwang, S. J. et al. Expression of anoctamin 1/TMEM16A by interstitial cells of Cajal is fundamental for slow wave activity in gastrointestinal muscles. J. Physiol 587, 4887–4904 (2009).
Kito, Y., Mitsui, R., Ward, S. M. & Sanders, K. M. Characterization of slow waves generated by myenteric interstitial cells of Cajal of the rabbit small intestine. Am. J. Physiol Gastrointest. Liver Physiol 308, G378–G388 (2015).
Gomez-Pinilla, P. J. et al. Ano1 is a selective marker of interstitial cells of Cajal in the human and mouse gastrointestinal tract. Am. J. Physiol Gastrointest. Liver Physiol 296, G1370–G1381 (2009).
Lee, M. Y. et al. Transcriptome of interstitial cells of Cajal reveals unique and selective gene signatures. PLoS. One 12, e0176031 (2017).
Hwang, S. J., Basma, N., Sanders, K. M. & Ward, S. M. Effects of new-generation inhibitors of the calcium-activated chloride channel anoctamin 1 on slow waves in the gastrointestinal tract. Br. J. Pharmacol 173, 1339–1349 (2016).
Sanders, K. M., Zhu, M. H., Britton, F., Koh, S. D. & Ward, S. M. Anoctamins and gastrointestinal smooth muscle excitability. Exp. Physiol 97, 200–206 (2012).
Singh, R. D. et al. Ano1, a Ca2+-activated Cl- channel, coordinates contractility in mouse intestine by Ca2+ transient coordination between interstitial cells of Cajal. J. Physiol 592, 4051–4068 (2014).
Zhu, M. H. et al. A Ca(2+)-activated Cl(−) conductance in interstitial cells of Cajal linked to slow wave currents and pacemaker activity. J. Physiol 587, 4905–4918 (2009).
Malysz, J. et al. Conditional genetic deletion of Ano1 in interstitial cells of Cajal impairs Ca(2+) transients and slow waves in adult mouse small intestine. Am. J. Physiol Gastrointest. Liver Physiol 312, G228–G245 (2017).
Drumm, B. T. et al. Clustering of Ca2+ transients in interstitial cells of Cajal defines slow wave duration. The Journal of General Physiology 149, 703–725 (2017).
Drumm, B. T., Hennig, G. W., Baker, S. A. & Sanders, K. M. Applications of Spatio-temporal Mapping and Particle Analysis Techniques to Quantify Intracellular Ca2+ Signaling In Situ. J Vis Exp, 143(e58989), 1–13, https://doi.org/10.3791/58989 (2019).
Drumm, B. T., Hwang, S. J., Baker, S. A., Ward, S. M. & Sanders, K. M. Ca(2+) signalling behaviours of intramuscular interstitial cells of Cajal in the murine colon. J Physiol 597, 3587–3617 (2019).
Ward, S. M. et al. Interstitial cells of Cajal mediate cholinergic neurotransmission from enteric motor neurons. J. Neurosci 20, 1393–1403 (2000).
Beckett, E. A., Horiguchi, K., Khoyi, M., Sanders, K. M. & Ward, S. M. Loss of enteric motor neurotransmission in the gastric fundus of Sl/Sl(d) mice. J. Physiol 543, 871–887 (2002).
Suzuki, H., Ward, S. M., Bayguinov, Y. R., Edwards, F. R. & Hirst, G. D. Involvement of intramuscular interstitial cells in nitrergic inhibition in the mouse gastric antrum. J. Physiol 546, 751–763 (2003).
Ward, S. M., McLaren, G. J. & Sanders, K. M. Interstitial cells of Cajal in the deep muscular plexus mediate enteric motor neurotransmission in the mouse small intestine. J. Physiol 573, 147–159 (2006).
Bhetwal, B. P. et al. Ca2+ sensitization pathways accessed by cholinergic neurotransmission in the murine gastric fundus. J. Physiol 591, 2971–2986 (2013).
Klein, S. et al. Interstitial cells of Cajal integrate excitatory and inhibitory neurotransmission with intestinal slow-wave activity. Nat. Commun 4, 1630 (2013).
Baker, S. A. et al. Spontaneous Ca(2+) transients in interstitial cells of Cajal located within the deep muscular plexus of the murine small intestine. J. Physiol 594, 3317–3338 (2016).
Baker, S. A., Drumm, B. T., Cobine, C. A., Keef, K. D. & Sanders, K. M. Inhibitory Neural Regulation of the Ca (2+) Transients in Intramuscular Interstitial Cells of Cajal in the Small Intestine. Front Physiol 9, 328 (2018).
Cobine, C. A. et al. Nitrergic neuromuscular transmission in the mouse internal anal sphincter is accomplished by multiple pathways and post-junctional effector cells. Am. J. Physiol Gastrointest. Liver Physiol 307, G1057–G1072 (2014).
Horiguchi, K. & Komuro, T. Ultrastructural observations of fibroblast-like cells forming gap junctions in the W/W(nu) mouse small intestine. J. Auton. Nerv. Syst 80, 142–147 (2000).
Ishikawa, K., Komuro, T., Hirota, S. & Kitamura, Y. Ultrastructural identification of the c-kit-expressing interstitial cells in the rat stomach: a comparison of control and Ws/Ws mutant rats. Cell Tissue Res 289, 137–143 (1997).
Cobine, C. A. et al. Rhythmic calcium transients in smooth muscle cells of the mouse internal anal sphincter. Neurogastroenterol Motil 32, e13746 (2020).
Keef, K., Kaminski, R., McDowall, R. & Duffy, A. & Cobine, C. Peptidergic inhibitory neuromuscular transmission in the mouse internal anal sphincter. Neurogastroenterol. Motil 23, 49 (2011)..
Kurahashi, M. et al. A functional role for the ‘fibroblast-like cells’ in gastrointestinal smooth muscles. J. Physiol 589, 697–710 (2011).
Cobine, C. A., McKechnie, M., Brookfield, R. J., Hannigan, K. I. & Keef, K. D. Comparison of inhibitory neuromuscular transmission in the Cynomolgus monkey IAS and rectum: special emphasis on differences in purinergic transmission. J. Physiol 596, 5319–5341 (2018).
Iino, S. & Nojyo, Y. Immunohistochemical demonstration of c-Kit-negative fibroblast-like cells in murine gastrointestinal musculature. Arch. Histol. Cytol 72, 107–115 (2009).
Kurahashi, M., Nakano, Y., Hennig, G. W., Ward, S. M. & Sanders, K. M. Platelet-derived growth factor receptor alpha-positive cells in the tunica muscularis of human colon. J. Cell Mol. Med 16, 1397–1404 (2012).
Keef, K. D. et al. Functional role of vasoactive intestinal polypeptide in inhibitory motor innervation in the mouse internal anal sphincter. J. Physiol 591, 1489–1506 (2013).
Zhu, M. H., Sung, T. S., O’Driscoll, K., Koh, S. D. & Sanders, K. M. Intracellular Ca(2+) release from endoplasmic reticulum regulates slow wave currents and pacemaker activity of interstitial cells of Cajal. Am. J. Physiol Cell Physiol 308, C608–C620 (2015).
Lees-Green, R., Gibbons, S. J., Farrugia, G., Sneyd, J. & Cheng, L. K. Computational modeling of anoctamin 1 calcium-activated chloride channels as pacemaker channels in interstitial cells of Cajal. Am. J. Physiol Gastrointest. Liver Physiol 306, G711–G727 (2014).
Sanders, K. M., Kito, Y., Hwang, S. J. & Ward, S. M. Regulation of Gastrointestinal Smooth Muscle Function by Interstitial Cells. Physiology. (Bethesda.) 31, 316–326 (2016).
Sung, T. S. et al. The cells and conductance mediating cholinergic neurotransmission in the murine proximal stomach. J Physiol 596, 1549–1574 (2018).
Drumm, B. T., Rembetski, B. E., Baker, S. A. & Sanders, K. M. Tonic inhibition of murine proximal colon is due to nitrergic suppression of Ca(2+) signaling in interstitial cells of Cajal. Sci Rep 9, 4402 (2019).
Jonas-Obichere, M. et al. Comparison of the effects of nitric oxide donors and calcium channel blockers on the intrinsic myogenic tone of sheep isolated internal anal sphincter. Br. J. Surg 92, 1263–1269 (2005).
Opazo, A. et al. Specific and complementary roles for nitric oxide and ATP in the inhibitory motor pathways to rat internal anal sphincter. Neurogastroenterol. Motil 23, e11–e25 (2011).
Cook, T. A., Brading, A. F. & Mortensen, N. J. Differences in contractile properties of anorectal smooth muscle and the effects of calcium channel blockade. Br. J. Surg 86, 70–75 (1999).
McKechnie, M., Harvey, N., Cobine, C. & Keef, K. Comparison of inhibitory motor innervation in the primate and mouse internal anal sphincter. Gastroenterology 134, A686 (2008).
Caputo, C., Edman, K. A., Lou, F. & Sun, Y. B. Variation in myoplasmic Ca2+ concentration during contraction and relaxation studied by the indicator fluo-3 in frog muscle fibres. J. Physiol 478(Pt 1), 137–148 (1994).
Martini, F. In Anatomy and Physiology Ch. 9, 209–240 (Pearson Education, Inc, 2005).
Sergeant, G. P., Thornbury, K. D., McHale, N. G. & Hollywood, M. A. Interstitial cells of Cajal in the urethra. J. Cell Mol. Med 10, 280–291 (2006).
Drumm, B. T. et al. Ca2+ signalling in mouse urethral smooth muscle in situ: role of Ca2+ stores and Ca2+ influx mechanisms. The Journal of Physiology 596, 1433–1466 (2018).
Acknowledgements
We would like to extend our sincere appreciation to Nancy Horowitz for breeding, maintenance and tamoxifen injections of mice, Lauren O’Kane for genotyping mice and Yulia Bayguinov for assistance with immunohistochemistry. This work was funded by an NIH RO1 grant (DK078736) to K.D.K. and C.A.C.
Author information
Authors and Affiliations
Contributions
K.I.H., A.P.B., H.J.L.F. and C.A.C. performed the research, K.D.K. and C.A.C. designed the research study, K.I.H., S.A.B., K.D.K. and C.A.C. analysed the data, K.I.H., K.M.S., K.D.K. and C.A.C. created the figures, K.I.H., K.D.K. and C.A.C. wrote the paper and B.T.D., S.A.B., S.M.W. and K.M.S. edited the paper and contributed to discussion. All authors read and approved the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hannigan, K.I., Bossey, A.P., Foulkes, H.J.L. et al. A novel intramuscular Interstitial Cell of Cajal is a candidate for generating pacemaker activity in the mouse internal anal sphincter. Sci Rep 10, 10378 (2020). https://doi.org/10.1038/s41598-020-67142-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-67142-y
This article is cited by
-
TMEM16A in smooth muscle cells acts as a pacemaker channel in the internal anal sphincter
Communications Biology (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.