Abstract
Root-knot nematode (Meloidogyne incognita) is chief plant parasitic nematode of various crops globally. Meanwhile, the negative side effects on human health and environmental concerns associated with haphazard uses of chemical nematicides. Hence, the search for a safe and effective approach is more relevant. The present study was aimed to evaluate the nematicidal potential of Snef1216 (Penicillium chrysogenum) against M. incognita at different concentrations (5%, 10%, 25%, 50%, 75% and 100%) and with the nutritious medium. The egg hatching inhibition and mortality of second stage juveniles of M. incognita were assessed after 6, 12, 24, 48 and 72 h exposure. Results revealed that egg hatching inhibition and percent mortality of M. incognita increased with increasing concentration and exposure time. The highest mortality of juveniles was recorded at 100% conc. i.e., 24.20%, 36%, 66%, 78% and 97.8% at 6, 12, 24, 48 and 72 h, respectively. The highest ovicidal activity was recorded at 100% concentration with 5.20% of eggs hatching. The outcome suggested that Snef1216 (P. chrysogenum) resulted in the lowest LC50 value was recorded as 3718.496 at 6 h exposure period followed by 10479.87, 11186.682, 14838.58 and 24001.430 at 72, 12, 48 and 24 h respectively via ovicidal assay. Whereas, in the larvicidal assay, the lowest LC50 value demonstrated at 72 h being 17.628% exposure period followed by 28.345, 50.490, 215.710 and 482.595% at 48, 24, 12 and 6 h respectively. It is concluded that Snef1216 has potential being used as a biocontrol agent against M. incognita and can serve as a source of a novel nematicidal agent of fungal origin.
Similar content being viewed by others
Introduction
Plant parasitic nematodes cause severe losses to the variety of crops by affecting their growth and yield1. They can easily damage the crops not only by feeding but also develop the association with other organisms possess a risk to agriculture globally with an estimated annual loss reached to 100–150 billion US dollars, more than half the losses only occurred by root-knot nematodes2. Root-knot nematodes (RKNs) are sedentary obligate endoparasitic in nature and are one of the major restraints in the production of economically important crops3,4.
Meloidogyne belongs to the one of the most damaging genus of root-knot nematodes owing to their polyphagous nature5,6. However, four common species of this genus, i.e. Meloidogyne arenaria, M. incognita, M. hapla and M. javanica have been reported as hazardous7. Among these species, M. incognita is the most damaging due to its extensive host range, high reproduction rate, the capability to produce complicated diseases with other pathogens and short generation time8. Plants are infested by root-knot nematodes showed the galls or knots on roots9 which disrupt the uptake of water and mineral, resulting in wilting of the plant, chlorosis, reduced tillering, excessive root branching, immature fruit drop, drying of leaf and stunted growth10,11,12. Its infection has the ability to reduce chlorophyll contents and alter numerous biochemical such as amino acids and organic acids13. At present, M. incognita can be controlled successfully by using chemical nematicides but they are hazardous for human health, other non-target organisms and environment14,15. Keeping in view the above described concerns; there is an urgent need to introduce long term integrative strategies and development of ecofriendly nematicides16,17,18.
Biological control is a safe way to control pests and pathogens. However, antagonists and nematophagous microorganisms are the best potential substitutes for chemical nematicides. Few nematophagous bacteria and fungi are commercially available to control plant parasitic nematodes19,20,21,22. Among biocontrol agents fungi have complex strategies for capturing the nematodes by sticky branches, non-constricting and constricting rings, killing by producing toxic substances, and digesting by colonizing their reproductive structures23,24,25.
Fungi belong to genera Penicillium, Fusarium, Paecilomyces, Trichoderma, Purpurocillium , Clonostachys, Chaetomium, Phyllosticta, Isaria, Arthrobotrys, Verticillium and Acremonium have been known as nematophagous or antagonistic to nematodes26,27,28. Over the recent years, the biocontrol effect against different pests and pathogens in the presence of Penicillium chrysogenum has been reported in a variety of plants and pathogens, thus providing evidence that it can be used to control nematode infection, but the available data concerning with the application of P. chrysogenum to control nematode is little so far29,30,31,32. Murali, et al.33 described the importance of P. chrysogenum that it efficiently promotes growth, induced defence-related genes and produced disease resistance against downy mildew in pearl millet. Dry mycelium of P. chrysogenum reduced the root galls to protect tomato and cucumber plants against Meloidogyne javanica and enhanced plant growth34. Siddiqui and Akhtar26 demonstrated that P. chrysogenum used alone or with the combination of Arbuscular mycorrhizal fungi AMF, Plant growth-promoting rhizobacteria PGPRS and Aspergillus niger could reduce nematode infection. Thus keeping in mind the biocontrol potential of P. chrysogenum, the current study was planned to evaluate the ovicidal and larvicidal efficacy of fungus fermentation Snef 1216 (P. chrysogenum) against M. incognita under laboratory conditions at Nematology Institute of Northern China (NINC) of Shenyang Agricultural University, P.R. China.
Results
Ovicidal assay
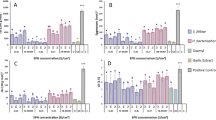
Nematicidal potential of Snef1216 was assessed on egg hatching inhibition for M. incognita (Table 1). Matured egg masses were selected and also insured eggs had the same embryonic stage. Results showed that eggs hatched in medium and distilled water were higher than those hatched by Snef1216. As mean hatching was observed in the medium as found to be statistically similar to that observed in distilled water, it showed that the medium itself did not show ovistatic or ovicidal property. The results revealed significant differences among the concentration and exposure period. The results of the tested fermentation concentrations recommended that all concentrations had antagonistic effects on egg hatching. On increasing exposure period up to 72 hours resulted in increased egg hatching. Whereas, increasing dilution of fermentation, the cumulative hatching was increased. Maximum inhibition in egg hatching was attained by 100% conc. of Snef1216. The highest percentage hatching of M. incognita was 86.8% in distilled water (Control) followed by nutritious medium and at 5% concentration of fermentation 83.2 and 33.8% respectively at 72 h exposure. The highest ovicidal activities were recorded at 100% conc. of fermentation with approximately 5.20% eggs hatched. Egg hatching inhibition of M. incognita increased with increasing concentration and exposure time. Unhatched eggs were transferred into distilled water to check their performance in the absence of Snef1216. However, it showed the strong ovicidal potential by exhibiting the egg hatching.
The data presented in (Table 2) revealed the ovicidal activity of P. chrysogenum against M. incognita. The hatching percentage was directly related to the concentration of fermentation and exposure time. Results displayed highly significant (P < 0.001) model fitness with (F = 533.28, 16594.74, 243.03, 0.72 and 1525.50) corrected model, intercept, concentration, replications and time period. The interaction/correlation between hatching versus concentration of fermentation and treatment recorded significant correlation with (P < 0.05), whereas the concentration of fermentation and exposure time also recorded highly significant (P < 0.001) positive correlation being (F = 18.67). The two-way analysis of variance provides information regarding the interaction between hatching percentage of M. incognita versus replication and exposure time recorded significant results (F = 1.30). Interaction/correlation between (concentration × replication × time) to total variance was strongly trait specific recorded significant hatching (P < 0.05).
Larvicidal assay
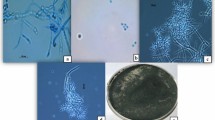
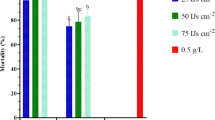
It is apparent from the perusal of the data presented in Table 3 that mean percent mortality in the fermentation of the Snef1216 (P. chrysogenum) differed significantly from that in the medium and it is concluded that medium itself had no nematostatic or nematicidal effect as the mean mortality observed in the medium was statistically similar to distilled water. Data displayed in Table 3 exposed that all concentrations of fermentation showed the nematicidal effect on M. incognita. The activity of Snef1216 was the highest, with J2s mortality 24.2%, 36%, 66%, 78% and 97.8% for 6, 12, 24, 48 and 72 h, respectively at 100% concentration. A progressive increase in the concentrations of the fermentation resulted in increased mortality of J2s. Second-stage juveniles gradually reduced their movement within 48 h and mostly were immovable after 72 h (Fig. 1). After exposure to Snef1216, immovable second-stage juveniles were transferred into distilled water but they showed no resumption of mortality.
Pictorial representation of P. chrysogenum Snef1216 and its influence upon the entire body of second-stage juveniles (J2s). Whereas; (A and B) Pure fungal colony of P. chrysogenum Snef1216, (C) Microscopic image of P. chrysogenum, (D and E): Living juveniles, (F) Natural death of juvenile, (G and H): Juveniles death due to P. chrysogenum Snef1216.
The data presented in (Table 4) revealed the larvicidal activity of P. chrysogenum against M. incognita. The percent mortality was directly related to the concentration of fermentation and exposure time. Outcomes displayed highly significant (P < 0.001) model fitness with (F = 585.09, 34637.48, 154.58, 0.819 and 3075.95) corrected model, intercept, concentration, replication and time period. The interaction/correlation between mortality versus concentration of fermentation and replication recorded a highly significant correlation with (P < 0.05), whereas the concentration of fermentation and exposure time also recorded highly significant (P < 0.001) positive correlation with (F = 11.58). The two-way analysis of variance provides information regarding the interaction between mortality (%) of M. incognita versus replication and exposure time recorded significant results (F = 0.97). Interaction/correlation between (concentration × replication × time) to total variance was strongly trait specific recorded significant mortality (P < 0.05).
Probit analysis of ovicidal and larvicidal assays
Probit analysis of data revealed the LC50 values, Chi square and fiducial limits at 95% confidence interval (Table 5). In ovicidal assay, the lowest LC50 value was demonstrated as 3718.50% at 6 h exposure period followed by 10479.87, 11186.68, 14838.58 and 24001.43 at 72, 12, 48 and 24 h respectively. Whereas, in Larvicidal assay, the lowest LC50 value was demonstrated at 72 h being 17.628 exposure period followed by 28.35, 50.49, 215.71 and 482.60 at 48, 24, 12 and 6 h respectively. In larvicidal assay, the LC50 value decreases with exposure period. Under in vitro conditions, P. chrysogenum was showed to be containing more potential in the larvicidal assay as compared to the ovicidal assay.
Model validation
Model validation performances for exposure time toward ovicidal and larvicidal assay are listed in Table 6. The exposure time revealed that R2 values of the ovicidal assay at 6, 12, 24, 48 and 72 h were 0.56, 0.79, 0.88, 0.91 and 0.83 respectively displayed better performances as compared to RMSE. However, exposure time revealed that R2 values of larvicidal were 0.75, 0.90, 0.97, 0.99 and 0.99 at 6, 12, 24, 48 and 72 h respectively displayed the best curve and best fit to the attained data.
Model validation performances for concentration of fermentation of P. chrysogenum (Snef 1216) in response to ovicidal and larvicidal assay are listed in (Table 7). The concentration of fermentation revealed that R2 values of ovicidal at 100%, 75%, 50%, 25%, 10%, 5%, medium and CK were 0.5, 0.5, 0.93, 0.99, 0.99, 0.89, 0.92 and 0.98 respectively displayed better performances as compared to RMSE. However in larvicidal assay the concentrations of fermentation revealed that R2 values were 0.98, 0.99, 0.98, 0.96, 0.91, 0.85, 0.95 and 0.87 at 100%, 75%, 50%, 25%, 10%, 5%, medium and distill water (control) respectively displayed the best curve and best fit to the attained data.
Interaction between mortality and hatching
The data presented in (Table 8) revealed the ovicidal and larvicidal activity of P. chrysogenum against M. incognita. Both mortality and hatching were directly related to the concentration of fermentation and exposure time. Outcomes displayed highly significant (P < 0.001) model fitness with (F = 888.55, 56845.43, 4022.79, 298.28 and 5053.15) corrected model, intercept, bioassay method, concentration and time. The interaction/correlation between mortality and hatching percentage versus treatment and concentration of fermentation recorded highly significant (P < 0.001) positive correlation with (F = 4831.61), whereas, treatment and exposure time also recorded highly significant (P < 0.001) positive correlation with (F = 334.92). The two-way analysis of variance provides information regarding the interaction between mortality and hatching percentage of M. incognita versus concentration and exposure time recorded highly significant results (F = 14.35). Interaction/correlation between (treatment × concentration × time) to total variance was strongly trait specific recorded significant mortality and hatching (P < 0.001 with F = 296.77).
Discussion
Meloidogyne incognita is a dominant root-knot nematode throughout the world. The complete elimination of M. incognita from the soil is difficult because of its polyphagous nature. Although different practices are employed to manage this pest however, biological control has become a good alternative to the chemical nematicides. Antagonistic microorganisms are appropriate for controlling nematodes however, still require progressive investigation12.
The present study demonstrated that Snef1216 P. chrysogenum significantly causes mortality of juveniles and lower the egg hatching of M. incognita. However, mortality and hatching are directly related to the concentration of fermentation and exposure time. As mean hatching observed in different concentrations of the medium was found to be similar to that of observed in distilled water. It showed that the medium itself did not show ovistatic or ovicidal property. By the increase in exposure time, there was a correspondingly increased in hatching but all concentrations of fermentation significantly inhibited the hatching rate as compared to control. In our results, the highest ovicidal activities were recorded at 100% concentration. Our findings agreed with Mukhtar and Pervaz35 that the medium itself has no ovicidal or ovistatic properties. Hatching inhibition was directly related to the concentration of Beauveria bassiana36. Metarhizium anisopliae was also reported as a biocontrol agent to control the hatching of eggs of Meloidogyne spp37,38. The culture filtrates of Aspergillus sp., Trichoderma harzianum, T.viride, Penicillium. sp. and Fusarium. sp. efficiently control egg hatching of M. incognita39,40. Paecilomyces lilacinus can easily parasitize females, juveniles and eggs of nematodes; therefore, it efficiently reduced their population in soil41,42,43. The conidia and hyphae of fungus can easily be penetrated in the eggs, resulting in larval death within egg44. Many fungal strains are capable to control reproduction and development of root knot nematodes in different crops25,45.
Our results also revealed that all concentrations of fermentation possess the nematicidal effect of varying degree on M. incognita. The potential of Snef1216 was the maximum, with J2 mortality up to 97.8% at 72 h at 100% concentration. However, their mortality response was concentration and time exposure dependent. Gapasin, et al.46 and Pau, et al.47 demonstrated that P. lilacinus culture filtrate had 100% nematicidal effect on J2 of Meloidogyne spp. Our results also consistent with Migunova, et al.28 that mortality percentage is directly related to the fermentation’s concentration and the exposure time. Aspergillus sp., Fusarium sp., Penicillum sp., T. harzianum and T. viride, were showed nematicidal properties on juveniles of M. incognita and exhibited more than half of juveniles mortality during 24 h incubation at 25% concentration of these fungal culture filtrate39. Hussain, et al.48 demonstrated that the effect of T. harzianum species on inactivation of J2 of Meloidogyne hapla reached 70.2% after 48 hours and 86% after 72 hours of its incubation in Clonostachys rosea strain. Regaieg, et al.49 reported that mortality of juveniles was relative to the Verticillium leptobactrum filtrate concentrations and the duration of exposure. Similarly Uddin, et al.18 reported that the culture filtrate of fungus P. chlamydosporia was effected to control M. incognita. The practice of biocontrol agents may decrease the use of harmful chemicals. However, their efficacy mainly depends upon species of nematode50,51. The outcomes of the present study clearly demonstrated that the use of Snef1216 (P. chrysogenum) for the controlling of M. incognita is effective and eco-friendly as compared to chemical nematicides.
Conclusions
Controlling of M. incognita by using the antagonistic fungus is a safe, effective and eco-friendly method as compared to injurious synthetic chemical nematicides. Snef1216 (P. chrysogenum) is apparently effective and environmentally friendly nematicides which enhanced mortality with increasing concentration of fermentation and exposure time. It is concluded that M. incognita has reasonable sensitivity toward Snef1216 (P. chrysogenum). It is reasonable to suppose that quick mortality and hatching inhibition effects connected with toxic metabolites released by the tested strain. The compounds of these metabolites and mechanisms of their actions need to be further investigations.
Materials and methods
Collection and activation of Strain Snef1216 (Penicillium chrysogenum)
Strain Snef1216 (P. chrysogenum) was obtained from China General Microbiological Culture Collection Center and stored at −80 °C in Nematology Institute of Northern China52. A small quantity of strain Snef1216 (P. chrysogenum) was put into PDA filled cavities and then placed in an incubator at 25-28 °C for 7 days to check the purity and activation. A pure single colony of strain was selected for further procedure (Fig. 1).
Fermentation
For the preparation of nutrition medium 0.003 g FeSO4.7H2O, 0.08 g MgSO4.7H2O, 0.4 g KCl, 2 g K2HPO4, 8 g NaNO3 and 50 g sucrose were added into 1 L of distilled water and then the resulting mixture was boiled for 3-5 minutes. After that, 100 ml nutrition medium was poured into the 250 ml conical flasks and sterilized in the steam autoclave machine at 121 °C for 30 min. Single pure fungus colony was cut into small pieces about 1 mm in diameters by the sterilized cutter. About five pieces were added into 100 ml of sterilized nutrition medium and placed in a shaker at 28 °C, at 150 rpm for 3 days. After that 100 ml new medium was poured into each flask and put again in a shaker for 8 days at 28 °C and150 rpm and filtered and stored at 4 °C52.
Nematode Inoculums
The population of M. incognita was cultured in tobacco plants grown in the greenhouse of Nematology Institute of Northern China (NINC), Shenyang Agricultural University (SYAU), Shenyang, Liaoning, China. Plants were carefully plucked and roots were chopped into 1-2 cm slices and then macerated in a small amount of water by using the electric blender. 0.05% sodium hypochlorite (NaOCl) was added into macerate. The mixture was manually shaken for 1-2 minutes to separate the eggs from the gelatinous matrix. Then, the suspension was poured through 200 and 500 sieve meshes respectively and washed with tap water to remove NaOCl. Eggs were further purified by centrifugation in 454gL−1 sucrose for 4 minutes at 3000 rpm. The supernatant was poured into 500 sieve mesh and rinsed several times with sterilized water. Eggs inoculums were transferred into a funnel and allowed to hatch into second stage juveniles (J2s). These J2s were then allowed to crawl through eight layers of Kim-wipe tissues into sterilized water by using the Baermann funnel method53. The J2s in the resulting suspension was adjusted at 100 J2/0.5 ml and also eggs suspension adjusted at 100 eggs/0.5 ml for subsequent experiments.
In vitro ovicidal assay
Ovicidal efficacy was evaluated by slight modification in process of Su and Mulla54. One hundred eggs of M. incognita were shifted into all of 24-well microliter plates containing different concentrations of fermentation (100%, 75%, 50%, 25%, 10% and 5%), nutritious medium and distilled water separately. The microliter plate was covered and incubated at 28 °C under humidified conditions for 72 h. The hatching of eggs was microscopically monitored after 6, 12, 24, 48 and 72 h exposure time. After 72 h, Lugol’s iodine solution (300μL) was put into all wells to stop more hatching of eggs55. To ascertain whether Snef1216 (P. chrysogenum) had ovistatic or ovicidal property the unhatched eggs were transferred to distilled water to observe hatching after exposure56,57. Five replicates were analyzed for all treatments for the accurateness of results. The percentage of hatching was calculated by the formula13.
In vitro larvicidal assay
One hundred second-stage juveniles of M. incognita carefully shifted into each well of 24-well microliter plate containing different concentrations of fermentation (100%, 75%, 50%, 25%, 10% and 5%), nutritious medium and distilled water separately. The microliter plate was incubated at 28 °C under humidified conditions for 72 h. J2’s populations were microscopically monitored after 6, 12, 24, 48 and 72 h exposure period. To ascertain whether Snef1216 (P. chrysogenum) had larvistatic or larvicidal property the immotile J2s were washed and transferred into distilled water to observe resumption of motility56,57. Juveniles were considered as dead when they remained immotile on probing with fine hair-needle and percentage mortality was calculated55. Five replicates were analyzed for all treatments for the accurateness of results.
Model validations
The association was used to construct a model for concentrations and exposure periods and the evaluation of the coefficient of determination (R2) values with root mean square error (RMSE). RMSE and R2 denote the standard deviation of the estimated errors (residuals). Prediction errors express how many values are far away from and around the regression lines. The performance of the model in the concentrations and exposure periods were assessed by RMSE. RMSE was calculated by the Equation of Debaeke, et al.58.
Data analysis
Ovicidal and larvicidal data were analyzed by two-way analysis of variance for calculating the interaction between ovicidal and larvicidal assay, concentrations and post-treatment time (exposure period) while mean between treatments were calculated for significance test by Duncan’s multiple range test at P= 0.05. All statistical processes were administered by different statistical packages such as EPA Probit analysis program (version 1.5) software, IBM-SPSS statistics (version 25.0) software and MS Excel.
References
Bogner, C. W. et al. Bioactive secondary metabolites with multiple activities from a fungal endophyte. Microbial biotechnology 10, 175–188 (2017).
Kim, T. Y. et al. Nematicidal activity of kojic acid produced by Aspergillus oryzae against Meloidogyne incognita. Journal of Microbiology and Biotechnology 26, 1383–1391 (2016).
Khan, Z. et al. A plant growth promoting rhizobacterium, Paenibacillus polymyxa strain GBR-1, suppresses root-knot nematode. Bioresource Technology 99, 3016–3023 (2008).
Singh, S. & Mathur, N. Biological control of root-knot nematode, Meloidogyne incognita infesting tomato. Biocontrol Science and Technology 20, 865–874 (2010).
Li, X. & Chen, S. Screening and identification of cucumber germplasm and rootstock resistance against the root-knot nematode (Meloidogyne incognita). Genetics and Molecular Research 16 (2017).
Xiang, N. et al. Biological control of Meloidogyne incognita by spore-forming plant growth-promoting rhizobacteria on cotton. Plant Disease 101, 774–784 (2017).
Dong, S. et al. Managing Meloidogyne incognita and Bemisia tabaci with thiacloprid in cucumber crops in China. Crop Protection 58, 1–5 (2014).
Vos, C. et al. Mycorrhiza-induced resistance against the root–knot nematode Meloidogyne incognita involves priming of defense gene responses in tomato. Soil Biology and Biochemistry 60, 45–54 (2013).
Agrios, G. N. et al. Plant pathology. Mycologia 28, 922 (2004).
Palomares-Rius, J. E., Escobar, C., Cabrera, J., Vovlas, A. & Castillo, P. Anatomical alterations in plant tissues induced by plant-parasitic nematodes. Frontiers in plant science 8, 1987 (2017).
Kingland, G. (Clemson Press, Victoria, Seychelles, 2001).
Pandey, R., Kalra, A., Gupta, M. & Sharma, P. In Proceedings of first National Interactive meet on medicinal and Aromatic Plants (ed. Mathur, S.) 188–197 (CIMAP, 2003).
Saikia, S. K., Tiwari, S. & Pandey, R. Rhizospheric biological weapons for growth enhancement and Meloidogyne incognita management in Withania somnifera cv. Poshita. Biological Control 65, 225–234 (2013).
Fuller, V. L., Lilley, C. J. & Urwin, P. E. Nematode resistance. New Phytologist 180, 27–44 (2008).
Abd-Elgawad, M. The current status of phytonematode management in Egypt with special reference to applicable nematicides. Egyptian Journal of Agronematology 6, 33–46 (2008).
Nico, A. I., Jiménez-Dı́az, R. M. & Castillo, P. Control of root-knot nematodes by composted agro-industrial wastes in potting mixtures. Crop protection 23, 581–587 (2004).
Martin, F. N. Development of alternative strategies for management of soilborne pathogens currently controlled with methyl bromide. Annual review of phytopathology 41, 325–350 (2003).
Uddin, M., Saifullah, Ahmad, M., Khan, W. & Khan, B. Evaluation of Pochonia chlamydosporia (Goddard) Isolates for Suppression of Meloidogyne incognita, Root-Knot Nematode of Tomato. Journal of Agricultural Science 11, 70–81 (2019).
Abd-Elgawad, M. Biological control agents of plant-parasitic nematodes. Egyptian Journal of Biological Pest Control 26, 423–429 (2016).
Almaghrabi, O. A., Massoud, S. I. & Abdelmoneim, T. S. Influence of inoculation with plant growth promoting rhizobacteria (PGPR) on tomato plant growth and nematode reproduction under greenhouse conditions. Saudi journal of biological sciences 20, 57–61 (2013).
Kim, T. Y. et al. Nematicidal activity of grammicin produced by Xylaria grammica KCTC 13121BP against Meloidogyne incognita. Pest management science 74, 384–391 (2018).
Wille, C. N., Gomes, C. B., Minotto, E. & Nascimento, J. S. Potential of aqueous extracts of basidiomycetes to control root-knot nematodes on lettuce. Horticultura Brasileira 37, 54–59 (2019).
Li, J. et al. Molecular mechanisms of nematode-nematophagous microbe interactions: basis for biological control of plant-parasitic nematodes. Annual review of phytopathology 53, 67–95 (2015).
Zhang, X. et al. Community composition, diversity and metabolic footprints of soil nematodes in differently-aged temperate forests. Soil Biology and Biochemistry 80, 118–126 (2015).
Karajeh, M. R. Efficacy of Saccharomyces cerevisiae on controlling the root-knot nematode (Meloidogyne javanica) infection and promoting cucumber growth and yield under laboratory and field conditions. Archives of Phytopathology and Plant Protection 46, 2492–2500 (2013).
Siddiqui, Z. A. & Akhtar, M. S. Effects of antagonistic fungi, plant growth-promoting rhizobacteria, and arbuscular mycorrhizal fungi alone and in combination on the reproduction of Meloidogyne incognita and growth of tomato. Journal of General Plant Pathology 75, 144 (2009).
Murslain, M. et al. Combined efficacy of Moringa oleifera leaves and a fungus, Trichoderma harzianum against Meloidogyne javanica on eggplant. Pakistan Journal of Zoology 46, 827–832 (2014).
Migunova, V., Sasanelli, N. & Kurakov, A. Effect of microscopic fungi on larval mortality of the root-knot nematodes Meloidogyne incognita and Meloidogyne javanica. Biological and integrated control of plant pathogens IOBC-WPRS Bulletin 133, 27–31 (2018).
Gardezi, S. R. Studies on the application of fungi and bacteria controlling insect pests in Azad Jammu and Kashmir, Pakistan. Archives of Phytopathology and Plant Protection 39, 49–67 (2006).
Yao, Q. et al. Resistance against Meloidogyne incognita in tomato induced by fermentation liquid of Penicillium chrysogenum. strain 1216. Acta Phytopathologica Sinica 44, 693–699 (2014).
Dong, H. et al. Dry mycelium of Penicillium chrysogenum protects cotton plants against wilt diseases and increases yield under field conditions. Crop protection 25, 324–330 (2006).
Sikandar, A. et al. Effects of Penicillium chrysogenum strain Snef1216 against root-knot nematodes (Meloidogyne incognita) in cucumber (Cucumis sativus L.) under greenhouse conditions. Applied Ecology and Environmental Research 17, 12451–12464 (2019).
Murali, M., Sudisha, J., Amruthesh, K., Ito, S. I. & Shetty, H. S. Rhizosphere fungus Penicillium chrysogenum promotes growth and induces defence‐related genes and downy mildew disease resistance in pearl millet. Plant Biology 15, 111–118 (2013).
Gotlieb, D., Oka, Y., Ben-Daniel, B.-H. & Cohen, Y. Dry mycelium of Penicillium chrysogenum protects cucumber and tomato plants against the root-knot nematode Meloidogyne javanica. Phytoparasitica 31, 217–225 (2003).
Mukhtar, T. & Pervaz, I. In vitro evaluation of ovicidal and larvicidal effects of culture filtrate of Verticillium chlamydosporium against Meloidogyne javanica. International Journal of Agriculture and Biology 5, 576–579 (2003).
Zhao, D. et al. Screening for nematicidal activities of Beauveria bassiana and associated fungus using culture filtrate. African Journal of Microbiology Research 7, 974–978 (2013).
Khosravi, M., Abdollahi, M. & Sadravi, M. Effect of Metarhizium anisopliae and Trichoderma harzianum on root knot nematode, Meloidogyne javanica. Biological Control of Pests and Plant Diseases 3, 67–76 (2014).
Jahanbazian, L., Abdollahi, M. & Rezaie, R. Combined effect of Metarhizium anisopliae and Pseudomonas fluorescens CHA0 on root-knot nematode, Meloidogyne incognita in tomato. Iranian Journal of Plant Pathology 51, 339–355 (2015).
Devi, G. & Bora, L. Effect of some biocontrol agents against root-knot nematode (Meloidogyne incognita race2). International Journal of Environment, Agriculture and Biotechnology 3, 1748–1755 (2018).
Xalxo, P., Karkun, D. & Poddar, A. Rhizospheric fungal associations of root knot nematode infested cucurbits: in vitro assessment of their nematicidal potential. Research Journal of Microbiology 2, 81–91 (2013).
Kiewnick, S. & Sikora, R. A. Biological control of the root-knot nematode Meloidogyne incognita by Paecilomyces lilacinus strain 251. Biological Control 38, 179–187 (2006).
Ganaie, M. & Khan, T. Biological potential of Paecilomyces lilacinus on pathogenesis of Meloidogyne javanica infecting tomato plant. European Journal of Applied Sciences 2, 80–84 (2010).
Sabet, F., Olia, M., Sharifnabi, B. & Fadaei, T. A. A. Biological control of the root-knot nematode, Meloidogyne javanica by four isolates of Paecilomyces lilacinus and an isolate of Isaria farinosa on tomato plants. Iranian Journal of Plant Pathology 49, 65–67 (2013).
Druzhinina, I. S. & Kubicek, C. P. Environmental and Microbial Relationships. (Springer, 2016).
Mokbel, A. A. & Alharbi, A. A. Suppressive effect of some microbial agents on root-knot nematode,‘Meloidogyne javanica’infected eggplant. Australian Journal of Crop Science 8, 1428–1434 (2014).
Gapasin, R. M., Vasquez, E. A. & Rendon, G. A. Bioassay-guided identification of the nematicidal secondary metabolites from Paecilomyces lilicanus for the control of root-knot nematode (Meloidogyne graminicola, Golden and Birchfield). Annals of Tropical Research 33, 22–43 (2011).
Pau, C. G. et al. Isolation of Indigenous Strains of Paecilomyces lilacinus with Antagonistic Activity against Meloidogyne incognita. International Journal of Agriculture and Biology 14, 197–203 (2012).
Hussain, M., Zouhar, M. & Ryšánek, P. Effects of nematophagous fungi on viability of eggs and juveniles of Meloidogyne incognita. The Journal of Animal and Plant Sciences 27, 252–258 (2017).
Regaieg, H., Ciancio, A., Raouani, N. H., Grasso, G. & Rosso, L. Effects of culture filtrates from the nematophagous fungus Verticillium leptobactrumon viability of the root-knot nematode Meloidogyne incognita. World Journal of Microbiology & Biotechnology 26, 2285–2289 (2010).
Kerry, B. Rhizosphere interactions and the exploitation of microbial agents for the biological control of plant-parasitic nematodes. Annual Review of Phytopathology 38, 423–441 (2000).
Huang, W.-K. et al. Testing various biocontrol agents against the root-knot nematode (Meloidogyne incognita) in cucumber plants identifies a combination of Syncephalastrum racemosum and Paecilomyces lilacinus as being most effective. Biological control 92, 31–37 (2016).
Jiang, M., Duan, Y., Chen, L. & Zhu, X. Study on the Fermentation of Penicillium Snef1216 Inducing the Resistance of Tomato to Root-knot Nematode. Journal of Henan Agricultural Sciences 4, 032 (2011).
Selim, M. E. M. Biological, chemical and molecular studies on the systemic induced resistance in tomato against Meloidogyne incognita caused by the endophytic Fusarium oxysporum, Fo162 PH.D. thesis, University of Bonn, (2010).
Su, T. & Mulla, M. Ovicidal activity of neem products (azadirachtin) against Culex tarsalis and Culex quinquefasciatus (Diptera: Culicidae). Journal of the American Mosquito Control Association 14, 204–209 (1998).
Zhao, D. et al. Isolation and identification of bacteria from rhizosphere soil and their effect on plant growth promotion and root-knot nematode disease. Biological control 119, 12–19 (2018).
Giacometti, R., d’Errico, G. & d’Errico, F. In vitro nematicidal activity of the experimental formulation Tequil against Meloidogyne incognita and Heterodera daverti. Nematropica 40, 263–268 (2010).
d’Errico, G. et al. Nematicidal efficacy of new abamectin-based products used alone and in combination with indolebutyric acid against the root-knot nematode Meloidogyne incognita. REDIA-GIORNALE DI ZOOLOGIA 100, 95–101 (2017).
Debaeke, P., Caussanel, J. P., Kiniry, J. R., Kafiz, B. & Mondragon, G. Modelling crop: weed interactions in wheat with ALMANAC. Weed Research 37, 325–341 (1997).
Acknowledgements
The financial support was provided by the National Natural Science Foundation of China (31471748), the special fund for Agro-scientific Research in the Public Interest (201503114) and China Agriculture Research System CARS-04-PS13.
Author information
Authors and Affiliations
Contributions
Y.D, L.C., A.S. and M.Z. conceived the experiment. A.S conducted the experiment. A.S., Y.W., X.Z., X.L., H.F. and Y.X analyzed the results. A.S wrote the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sikandar, A., Zhang, M., Wang, Y. et al. In vitro evaluation of Penicillium chrysogenum Snef1216 against Meloidogyne incognita (root-knot nematode). Sci Rep 10, 8342 (2020). https://doi.org/10.1038/s41598-020-65262-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-65262-z
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.