Abstract
One of the most damaging pests in vegetable crops is the root-knot nematode (Meloidogyne incognita) worldwide. The continuous use of nematicide is costly and has unintended consequences for human and environmental health. To minimize nematicides, eco-friendly integrated nematode management is required. Trichoderma, an antagonistic fungus has been explored to control root-knot nematode. The fungal bio-control strain FbMi6 was identified as Trichoderma asperellum (accession no. MT529846.1). T. asperellum FbMi6 showed substantial nematicidal activity in the laboratory, with egg hatch suppression (96.6%) and juvenile mortality (90.3%) of M. incognita. T. asperellum FbMi6 was examined under pot and field conditions (after neem cake enrichment), both alone and in combination, and compared with controls. Application of T. asperellum FbMi6 enriched neem cake (1-ton ha-1) increased (28.3%) the okra yield and decreased (57.1%) nematode population as compared with control. T. asperellum FbMi6 enriched neem cake had higher polyphenol content (resistance enhancer) in okra compared with inoculated check.
Similar content being viewed by others
Introduction
Okra (Abelmoschus esculentus L.) is a popular vegetable crop produced for its health benefits in tropical and subtropical regions around the world. Southern root-knot nematode (Meloidogyne incognita), a phytonematode, is critical biotic stress on okra, producing substantial root damage and affecting the production value and quantity1,2. Okra production in India is being endangered by the spread of Meloidogyne spp. in all growing areas3. Root-knot nematode caused 19.6% yield losses in all vegetable crops in India3. In India, no nematicides for okra are currently suggested4. Although, efforts to develop resistant cultivars are being made, none are currently available5. As a result, biological control is suggested as a safer alternative for living biota and its environment6 to control the root-knot nematode in vegetables7. Trichoderma, a potential biocontrol agent, has successfully been utilized in vegetable crops to control root-knot nematodes8,9,10,11. Trichoderma spp. provides different plant health benefits, like promotion in plant growth, disease control, stress tolerance and development of resistance in plants. Trichoderma asperellum is an emerging and effective biocontrol agent, for its endless effectiveness in managing plant parasitic nematodes and disease complexes with other secondary pathogens12,13,14,15. Plant parasitic nematodes are inhibited by various Trichoderma species used as biocontrol agents8,16,17,18. Now, many actions of Trichoderma are documented as BCA: including competition, antibiosis, resistance, and plant tolerance against biotic and abiotic stresses and stimulation of its defenses against pathogens19. Trichoderma spp. has faster reproduction than other microorganisms/bioagents and has a strong acclimating capacity in a diversified environment20,21. Trichoderma spp. emit elicitors when they interact with plants, which promotes systemic acquired resistance and immunity against plant diseases and pests. Plant pathogens are inhibited by primary and secondary metabolites generated by Trichoderma spp. Many secondary metabolites generated by Trichoderma spp., such as flavonoids and phenols offer resistance to biotic stress22,23. Keeping in view, the present study was conducted on indigenous T. asperellum FbMi6 strain performed against M. incognita under in vitro and in vivo conditions in okra.
Results
Morphological and molecular identification of isolate FbMi6
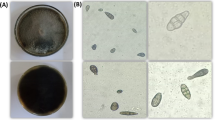
At 7 days, morphological observations of FbMi6 revealed that colonies with fluffy mycelial development and a large conidial zone at the colony's periphery, following sporulation, whitish or yellowish growth turned greenish or pale greenish features were identified as Trichoderma (Fig. 1). Morphological features of isolate FbMi-6, such as hyphae up to 7.45 µm broad, conidiophore with a maximum length of 3.2 × 197.3 µm, chlamydospores with a diameter of up to 9 × 10.4 µm, phialides range in size from 1.7–2.8 × 9.0–16.5 µm and phialospores with a diameter of 2.7–3.2 × 3.4–4.4 µm were measured (Fig. 1). Based on morphological and molecular characteristics, isolate FbMi6 was identified as T. asperellum. The sequence was submitted to GenBank (NCBI), with accession number MT529846.1. It showed 100% similarity to T. asperellum as per GenBank data. Also, according to phylogenetic analysis, FbMi6 was found most closely related to T. asperellum (Fig. 2).
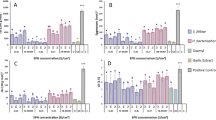
In comparison with the control, T. asperellum FbMi6 significantly reduced hatchability and having maximal juvenile mortality. T. asperellum FbMi6 inhibited egg hatching in all concentrations as compared to control. At 120 h, maximum egg hatching inhibition (96.6%) was observed at 80% concentration of T. asperellum FbMi6 as compared with control. With increasing exposure period, egg hatching inhibition gradually increased. T. asperellum FbMi6 culture filtrates cause egg deformation and hatching suppression starting at 24 h, in all the concentrations (Fig. 3).
Similarly, T. asperellum FbMi6 culture filtrate showed antagonistic or nematocidal activity against M. incognita juveniles in all the concentrations (Fig. 4). T. asperellum FbMi6 showed maximum root-knot nematode juveniles’ mortality in all concentrations at 72 h exposure as compared to control. T. asperellum FbMi6 at 80% concentration had the highest (90.3%) juvenile mortality compared to control. The mortality of juveniles enhanced as exposure time increased (Fig. 4).
Effects of T. asperellum FbMi6 enriched neem cake on M. incognita and vegetative growth of okra
The application of T. asperellum FbMi6 enriched neem cake had substantial (P ≤ 0.05) suppressive effects on M. incognita population in terms of J2s 200–1 cc soil, galls and egg masses plant−1, and eggs per egg mass, as well as promote the vegetative growth of okra. The gall index and egg masses were significantly reduced with T. asperellum FbMi6 enriched neem cake (Table 1). Fewer galls on okra roots were observed with T. asperellum FbMi6 enriched neem cake at 20 g kg−1 soil. Egg masses and eggs per egg mass were also lower in all T. asperellum FbMi6 enriched neem cake treatments compared with control. In comparison to the untreated inoculated check, soil nematode population was considerably reduced in all the treatments of T. asperellum FbMi6 enriched neem cake. In contrast to the untreated control, T. asperellum FbMi6 enriched neem cake had the lowest nematode population at 20 g kg−1 soil application.
Plant biomass (shoot and root weight) differed significantly between T. asperellum FbMi6 enriched neem cake and control (untreated). When compared to the untreated inoculated control, all treatments with T. asperellum FbMi6 enriched neem cake significantly enhanced plant height (P ≤ 0.05). The results (Table 2) showed that T. asperellum FbMi6 enriched neem cake at 20 g kg−1 soil had considerably higher plant height than T. asperellum FbMi6 enriched neem cake at 15 g kg−1 soil. At increasing doses, T. asperellum FbMi6 enriched neem cake generated the maximum dry root and shoot weight.
Effect of T. asperellum FbMi6 enriched neem cake on biochemical and physiological parameters of okra infested with M. incognita
According to the results (Table 3), T. asperellum FbMi6 enriched neem cake at 20 g kg−1 soil had a considerably higher nitrogen balance index than T. asperellum FbMi6 enriched neem cake at 15 g kg−1 soil, while untreated inoculated check had the lowest nitrogen balance index. In comparison to the untreated inoculated control, all treatments of T. asperellum FbMi6 enriched neem cake had significantly higher total chlorophyll content. T. asperellum FbMi6 enriched neem cake at 20 g kg−1 soil had considerably higher anthocyanin content than the untreated inoculated control and rest of the treatments were non-significant. T. asperellum FbMi6 enriched neem cake at 20 g kg−1 soil had the highest polyphenol content, followed by T. asperellum FbMi6 enriched neem cake at 15 g kg−1 soil. When compared to the untreated inoculated control, all treatments exhibited considerably higher flavonoid content. The highest flavonoid concentration was recovered in T. asperellum FbMi6 enriched neem cake at 20 g kg−1 soil, while the lowest was recorded in the untreated inoculated control.
Effect of T. asperellum FbMi6 enriched neem cake on growth and yield of okra infecting root-knot nematode under field conditions
Colonies of T. asperellum FbMi6 culture was found to be 8 × 108 CFU g−1 after enrichment in neem cake. In a field experiment, soil application of T. asperellum FbMi6 enriched neem cake resulted in significantly greater yield and lower nematode population. The carbofuran-treated plots had less nematode reproduction than those with T. asperellum FbMi6 enriched neem cake. T.asperellum FbMi6 enriched neem cake and carbofuran inhibited root galls as comparison to control. The application of T. asperellum FbMi6 enriched neem cake and carbofuran considerably reduced the soil nematodes population (second stage juveniles). Nonetheless, results (Table 4) demonstrated that T. asperellum FbMi6 enriched neem cake at 1-ton ha−1 boosted okra output/yield substantially as compared with control. Okra yield in T. asperellum FbMi6 enriched neem cake was higher than the chemical control (carbofuran @ 1.0 kg a.i. ha−1). T. asperellum FbMi6 enriched neem cake at 1-ton ha−1 increased 28.3% okra yield above the control.
Discussion
Many countries, including the United States, Australia, India, and China, have recently added to the collection of Trichoderma sources. Trichoderma research first concentrated on soil flora and fauna, soil remediation, soil biology, pollution, and general mycology. As our understanding of Trichoderma grew, researchers concentrated on biocontrol and its applications in plant nematology and mycology. Isolate FbMi6 was identified as T. asperellum based on cultural, morphological, and molecular findings in this study. Trichoderma species has also been found in diversified environment and isolated from the upper atmosphere24, wetlands25, and rhizosphere soil26. There have been 141 or more Trichoderma species recorded worldwide27,28,29,30. T. asperellum FbMi6 showed substantial antagonistic activity on egg hatching and juvenile mortality of M. incognita in present investigation, suggesting a possible nematoxic compounds in it. Many scientists have already been conducted research on Trichoderma isolates for nematode inhibition in vitro31,32,33,34. Deformation of eggs and juveniles confirmed the presence of nematicidal compounds in culture filtrates of Trichoderma. T. asperellum FbMi6 generated nematicidal chemicals that appeared to play a key role in nematode death. Plant parasitic nematode infestations have been reduced using Trichoderma species as biocontrol agents35,36. Biocontrol effectiveness of these microbes was demonstrated by the antagonism of Trichoderma culture filtrates against M. incognita. Meyer37observed that 253 fungal isolates (Acremonium sp., Aspergillus sp., Fusarium sp., Paecilomyces sp.) had a nematicidal influence on juvenile development and egg hatch suppression.
The exceptional performance of Trichoderma enriched neem cake under pot and field circumstances is a glimmer in the nematology picture in the current study. The current findings were consistent with those of Affokpon38, who found that T. asperellum suppressed root-knot nematode and increased plant tolerance. Trichoderma asperellum biocontrol activity is attributed to the buildup of phytoalexins39. Increased plant growth of okra with Trichoderma enriched neem cake treated plants due to the reduction of root-knot nematode population. In comparison to untreated plants, treated plant roots absorb more nutrients from the soil. Several studies have shown that combining biocontrol agents with organic amendments increases plant growth and yield while also suppressing nematode populations40,41,42. However, there is a scarcity of information on the utilization of T. asperellum as a biocontrol agent against M. incognita. Present study showed that T. asperellum enriched neem cake can be used to control the root-knot nematode in vegetable crops. Nematode populations were found to be reduced in Trichoderma enriched neem cake compared to control. Plant parasitic nematodes are considerably reduced by neem and its derivatives43,44. The presence of toxic chemicals such as azadirachtin and nimbin (secondary metabolites) present in all regions of neem, which have a protective role against nematode infection and inhibited nematode proliferation45.
T. asperellum FbMi6 enriched neem cake improves plant tolerance in nematode-infected plants by boosting biochemical and physiological attributes such as polyphenols, total chlorophyll, nitrogen balance index, anthocyanin, and flavonoids as compared to control. The formation of secondary metabolites such as phenolic compounds hindered nematode reproduction46. Flavonoid inhibits nematode movement and egg hatching47, and plays an important function in plant defense48. Phenolic chemicals are produced in response to nematode stress in plants49,50. The amount of phenolic content accumulated in a plant determines its level of stress tolerance51. In comparison to the untreated inoculated control, use of bio-agents (Pseudomonas aeruginosa) in combination with neem cake produces systemic resistance in cotton52. Ammonia and higher the C/N ratio of organic inputs showed nematicidal action against plant parasitic nematodes. These substances may also affect root-knot nematode egg viability and hatching53,54. Organic additions alter the physiochemical and physical properties of the soil, which has a deleterious effect on nematode motility and host finding55. The similar technique may have worked against the root-knot nematode in okra. Trichoderma is a well-known, worldwide recognized biocontrol agent. Nonetheless, studies on Trichoderma spp. have focused on one or more parameters in a single strain. T. asperellum, the identified strain, has a quicker growth rate and a substantial nematocidal effects on M. incognita. According to our findings, pot and field studies showed that T. asperellum FbMi6 enriched neem cake considerably reduced the population of M. incognita. The remarkable effectiveness of T. asperellum FbMi6 enriched neem cake against root-knot nematode in outdoor circumstances was proved.
Materials and methods
Isolation and identification of bioagent
All methods were carried out in accordance with relevant guidelines and regulations. Wet soil samples were obtained from various places in Haryana, India. The location does not require any special permission. Trichoderma isolates were isolated on potato dextrose agar (PDA) culture plates with 50 μg mL−1 streptomycin from collected samples using the serial dilution method56 and culture was multiplied on potato dextrose broth (PDB) at 25 ± 2 °C in a BOD incubator.
The Trichoderma isolate was identified at the generic level based on morphological parameters such as growth pattern and colony colour. Genomic DNA was isolated in pure form from the culture plates. The ITS-rDNA partial gene was effectively amplified using primers ITS4 and ITS5. The ABI-BigDye® Terminatorv3.1 Cycle Sequencing Kit was used to set up the sequencing PCR. Sequence generated was manually modified for uniformity using the ABI 3100 automated DNA sequencers. Further To determine identification, the sequence data was aligned with publicly accessible sequences and compared with the GenBank database using BLSATN. The sequences were aligned in ClustalX. The partial ITS-rDNA sequence was submitted to GenBank (NCBI) to get the accession number. MegaX software was used to construct a phylogenetic tree of related Trichoderma species through the maximum likelihood method57. This culture was given the name FbMi6 and sent to the National Fungal Culture Collection (NFCCI) in Pune, Maharashtra, India for storage (http://nfcci.aripune.org/).
Maintenance of bio-agents
Trichoderma asperellum FbMi6, an isolate from the Department of Nematology at Chaudhary Charan Singh Haryana Agricultural University, Hisar, Haryana, India was kept and used in further research.
Source of seeds
The seeds of brinjal (Solanum melongena L.) cv. Hisar Shyamal okra (Abelmoschus esculentus) cv. Hisar Unnat were procured from Department of Vegetable Sciences, CCS HAU, Hisar.
Obtaining M. incognita eggs and second-stage juveniles
Meloidogyne incognita eggs and J2s were collected from a pure population kept on brinjal (Solanum melongena L.) cv. Hisar Shyamal at CCSHAU, Hisar, Haryana, India. The eggs were collected from infested brinjal roots using the sodium hypochlorite method58. The juveniles were extracted using cobb’s method59 followed by Modified Baermann Funnel Technique (MBFT)60.
In vitro assay
Culture broth 100 mL was centrifuged for 20 min at 1500 rpm containing 1 × 108 spores (CFU mL−1). Cell-free culture filtrates were recovered through 0.45 μm filters (Whatman™). Culture filtrate was checked for the presence of any fungal spores using PDA plating procedures. For Egg hatching inhibition assay, five surface sterilized egg masses were kept into tissue culture plates with four different concentrations at 20, 40, 60 and 80% of T. asperellum and distilled water and PDB were kept as control. The hatched juveniles were counted under a stereoscopic binocular microscope for alternate day upto 10 days and the percent hatching inhibition computed.
Juveniles’ mortality assay was conducted in tissue culture plates with five mL culture filtrates at four different concentrations (20, 40, 60 and 80%) with water and PDB as control. Hundred newly hatched J2 were transferred in each tissue culture plate well. Death of juveniles was examined at 24 h intervals up to 72 h under a stereo zoom binocular microscope (Gippon). The death of juveniles was confirmed after recovering in fresh water. All hatching and mortality assay were repeated twice and assay were conducted at room temperature (25 ± 2 °C).
Screen house assay
One ton of neem cake (organic carbon—10.45%, nitrogen—0.84%, phosphorous—0.69%, Potassium—0.59%) was enriched with three liters of T. asperellum FbMi6 aqueous solution. Mixed biomass (T. asperellum and neem cake) was kept under shade for 15 days with a moisture content of 25–30% and temperature of 25–28 °C for proper enrichment. T. asperellum CFU were measured in neem cake after enrichment using a serial dilution procedure (56). Before sowing, experimental pots were filled with T. asperellum FbMi6 enriched neem cake. The experiment was conducted in earthen pots (15 cm diameter) in a screen house at CCSHAU Hisar, India (29°10' N; 75°46' E).
Treatments as: T. asperellum FbMi6 enriched neem cake (5 g kg−1 soil), T. asperellum FbMi6 enriched neem cake (10 g kg−1 soil), T. asperellum FbMi6 enriched neem cake (15 g kg−1 soil), T. asperellum FbMi6 enriched neem cake (20 g kg−1 soil), Carbofuran 3G (16 mg kg−1 soil) were applied respectively. Five replications of each treatment were used in a completely randomized design (CRD). Okra cv. Hisar unnat seeds were sowed, and one plant per pot was maintained after thinning. Freshly hatched 2000 J2s were inoculated in steam sterilized soil at sowing time. Hoagland solution was applied to plants61.
Plant height, fresh shoot and root weight, dried shoot and root weight of okra, nematode galls per plant, egg masses per plant, eggs per egg mass, second stage juveniles per 200 cc soil were measured at harvesting (45 days after sowing). Galls and egg masses were counted with the help of a hand lens and soil was processed by Cobb sieving method59.
After drying in a hot air oven, physiological and biochemical characteristics of okra were recorded. Folin–Ciocalteau assay was used to determine total phenolic content62. Oven dried 0.5 g samples were extracted in 10 ml ethanol (80%) and centrifuged at 10,000 rpm for 20 min. The supernatant was collected and evaporated until completely dry. Reagent FC (1 N) 100 µl and 20 µl of each extract were placed in test tubes and left for 8 min before adding 300 µl of sodium carbonate. For 30 min at 40 °C, the contents were allowed to incubate in the dark. At 765 nm, the absorbance was measured. On a dry weight basis, the phenolic content of the sample was reported in mg GAE/100 g of sample. Dualex sensor (Dualex® Scientific sensor) was used to calculate total chlorophyll, NBI, anthocyanin, and flavonoid.
Evaluation under field trial
At 15 days before sowing, field research plots (5 × 3 m2) were mixed with T. asperellum FbMi6 enriched neem cake (CFU 8 × 108 ml−1). The experiment was carried out on okra (cv. Hisar unnat) at the CCS HAU in Hisar, Haryana, India. The initial population 235 J2 200–1 cc soil was assessed.
Neem cake 1 ton ha−1, T. asperellum FbMi 6 enriched neem cake 1 ton ha−1, chemical check (33 kg Furadan 3G ha−1), and inoculated control were used in a Randomized Block Design (RBD) with five replications. Observations on root gall index and final nematode population were recorded at 90 days after seeding. Cobb's methods were used to assess the soil population59. Okra yield and root gall index63 were also measured.
Statistical analysis
SPSS version 10.0 was used to analyze the data statistically. The means were separated using DMRT. Differences in treatment means were considered significant at P ≤ 0.05. Values with the different letter in a column are significantly different at P ≤ 0.05. SD was calculated from excel spreadsheet Ver. 16.
Ethics approval and consent
In conducting this study, experiments on live vertebrates and/or higher invertebrates were not required any special permission for approval and consent. The variety of okra Hisar Unnat was developed by CCSHAU, Hisar therefore, it not required any permission. All images used in the manuscript are original.
Data availability
All data generated or analyzed during this study are included in this published article (and its Supplementary Information files). The sequence data obtained in this study are openly available in GenBank of NCBI at https://www.ncbi.nlm.nih.gov/ under the Accession No. MT529837.1.
References
Sasser, J. N. & Carter, C. C. An Advanced Treatise on Meloidogyne, Vols I and II (North Carolina State University Graphics, 1985).
Gugino, B. K., Abawi, G. S. & Ludwig, J. W. Damage and management of Meloidogyne hapla using oxamyl on carrot in New York. J. Nematol. 38, 483–490 (2006).
Kumar, V., Khan, M. R. & Walia, R. K. Crop loss estimations due to plant-parasitic nematodes in major crops in India. Natl. Acad. Sci. Lett. 43(5), 409–412. https://doi.org/10.1007/s40009-020-00895-2 (2020).
Patil, J. A., Kumar, A., Yadav, S. & Verma, K. K. Nematicidal effect of cruciferous bio-fumigants against the root-knot nematode, Meloidogyne incognita infesting okra. J. Nematol. 52, 1–7. https://doi.org/10.21307/jofnem-2020-080 (2020).
Fourie, H., Mc Donald, A. H. & De Waele, D. Host and yield responses of soybean genotypes resistant or susceptible to Meloidogyne incognita in vivo. Int. J. Pest Manag. 59, 111–121. https://doi.org/10.1080/09670874.2013.772261 (2013).
Rao, M. S. et al. A frontier area of research on liquid biopesticides: The way forward for sustainable agriculture in India. Curr. Sci. 108, 1590–1592 (2015).
Fourie, H., De Waele, D., McDonald, A. H., Mienie, C. M. M. & De Beer, A. Nematode pests threatening soybean production in South Africa, with reference to Meloidogyne: A review. S. Afr. J. Sci. 111, 1–9. https://doi.org/10.17159/SAJS.2015/20140212 (2015).
Poveda, J., Abril-Urias, P. & Escobar, C. Biological control of plant-parasitic nematodes by filamentous fungi inducers of resistance: Trichoderma, mycorrhizal and endophytic fungi. Front. Microbiol. https://doi.org/10.3389/fmicb.2020.00992 (2020).
Forghani, F. & Hajihassani, A. Recent advances in the development of environmentally benign treatments to control root-knot nematodes. Front. Plant Sci. https://doi.org/10.3389/fpls.2020.01125 (2020).
Harman, G. E. Trichoderma—Not just for biocontrol anymore. Phytoparasitica 39, 103–108. https://doi.org/10.1007/s12600-011-0151-y (2011).
Mukhtar, T. Management of root-knot nematode, Meloidogyne incognita in tomato with two Trichoderma species. Pak. J. Zool. 50. https://doi.org/10.17582/journal.pjz/2018.50.4.sc15 (2018).
Viterbo, A. D. A. & Chet, I. TasHyd1 a new hydrophobin gene from the biocontrol agent Trichoderma asperellum, is involved in plant root colonization. Mol. Plant Pathol. 7, 249–258. https://doi.org/10.1111/j.1364-3703.2006.00335.x (2006).
Idowu, O. O., Olawole, O. I., Idumu, O. O. & Salami, A. O. Bio-control effect of Trichoderma asperellum (Samuels) Lieck f. and Glomus intraradices Schenk on okra seedlings infected with Pythium aphanidermatum (Edson) Fitzp and Erwinia carotovora (Jones). J. Exp. Agric. Int. https://doi.org/10.9734/AJEA/2016/21348 (2016).
Qi, W. & Zhao, L. Study of the siderophore-producing Trichoderma asperellum Q1 on cucumber growth promotion under salt stress. J. Basic Microbiol. 53, 355–364. https://doi.org/10.1002/jobm.201200031 (2013).
Yoshioka, Y., Ichikawa, H., Naznin, H. A., Kogure, A. & Hyakumachi, M. Systemic resistance induced in Arabidopsis thaliana by Trichoderma asperellum SKT-1, a microbial pesticide of seed borne diseases of rice. Pest Manag. Sci. 68, 60–66. https://doi.org/10.1002/ps.2220 (2012).
Patil, J. A., Yadav, & Kumar, A. Management of root-knot nematode, Meloidogyne incognita and soil borne fungus, Fusarium oxysporum in cucumber using three bioagents under polyhouse conditions. Saudi J. Biol. Sci. 28, 7006–7011. https://doi.org/10.1016/j.sjbs.2021.07.081 (2021).
Sayed, M., Abdel-Rahman, T., Ragab, A. & Abdellatif, A. Biocontrol of root-knot nematode Meloidogyne incognita by chitinolytic Trichoderma spp.. Egypt. J. Agron. 18(1), 30–47. https://doi.org/10.21608/ejaj.2019.52842 (2019).
Patil, J., Sharma, M. K., Bharvaga, S. & Srivastava, A. S. Management of reniform nematode, Rotylenchulus reniformis on cowpea by using bio-agents and plant extracts. Indian J. Nematol. 242, 167–171 (2013).
Harman, G. E. Overview of mechanisms and uses of Trichoderma spp.. Phytopathology 96, 190–194 (2006).
Fan, H. et al. Isolation and effect of Trichoderma citrinoviride Snef 1910 for the biological control of root-knot nematode, Meloidogyne incognita. BMC Microbiol. 20(1), 1–1. https://doi.org/10.1186/s12866-020-01984-4 (2020).
Al-Hazmi, A. S. & Tariq, J. M. Effects of different inoculum densities of Trichoderma harzianum and Trichoderma viride against Meloidogyne javanica on tomato. Saudi J. Biol. Sci. 23, 288–292 (2016).
El-Sharkawy, H. H. A., Rashad, Y. M. & Ibrahim, S. A. Biocontrol of stem rust disease of wheat using arbuscular mycorrhizal fungi and Trichoderma spp.. Physiol. Mol. Plant Pathol. 103, 84–91. https://doi.org/10.1016/j.pmpp.2018.05.002 (2018).
Wang, Q. et al. The involvement of jasmonic acid, ethylene, and salicylic acid in the signaling pathway of Clonostachys rosea induced resistance to gray mold disease in tomato. Phytopathology 109, 1102–1114. https://doi.org/10.1094/PHYTO-01-19-0025-R (2019).
Yang, Y. et al. Isolation and identification of Trichoderma asperellum, the novel causal agent of green mold disease in sweet potato. Plant Dis. 10, 7. https://doi.org/10.1094/PDIS-07-20-1484-RE (2021).
Kumar, K., Amaresan, N., Bhagat, S., Madhuri, K. & Srivastava, R. C. Isolation and characterization of Trichoderma spp. for antagonistic activity against root rot and foliar pathogens. Indian J. Microbiol. 52(2), 137–144. https://doi.org/10.1007/s12088-011-0205-3 (2012).
Tondje, P. R., Daniel, P., Roberts, M. C., Bon, P. & Hebbar,. Isolation and identification of mycoparasitic isolates of Trichoderma asperellum with potential for suppression of black pod disease of cacao in Cameroon. Biol. Control. 43, 202–212. https://doi.org/10.1016/j.biocontrol.2007.08.004 (2007).
Kubicek, C. P., Komon-Zelazowska, M. & Druzhinina, I. S. Fungal genus Hypocera/Trichoderma: From barcodes to biodiversity. J. Zhejiang Univ. Sci. B. https://doi.org/10.1631/jzus.B0860015 (2008).
Hoyos-Carvajal, L., Orduz, S. & Bissett, J. Genetic and metabolic biodiversity of Trichoderma from Colombia and adjacent neotropic regions. Fungal Genet. Biol. 46, 615–631. https://doi.org/10.1016/j.fgb.2009.04.006 (2009).
Sadfi-Zouaoui, N. et al. Biodiversity of Trichoderma strains in Tunisia. Can. J. Microbiol. 55, 154–162. https://doi.org/10.1139/W08-101 (2009).
Błaszczyk, L. et al. Species diversity of Trichoderma in Poland. J. Appl. Genet. 52, 233–243. https://doi.org/10.1007/s13353-011-0039-z (2011).
Regina, M. D. G. C., DeSouza, I. S. & Belarmino, L. C. Nematicidal activity of Bacillus spp. strains on juveniles of Meloidogyne javanica. Nematol. Bras. 22, 12–21 (1998).
Hanna, A. I., Riad, F. W. & Tawfik, A. E. Efficacy of antagonistic rhizobacteria on the control of root-knot nematode, Meloidogyne incognita in tomato plants. Egypt. J. Agric. Res. 77, 1467–1476 (1999).
Feyisa, B., Lencho, A., Selvaraj, T. & Getaneh, G. Evaluation of some botanicals and Trichoderma harzianum against root-knot nematode (Meloidogyne incognita (Kofoid and White) Chit wood) in tomato. J. Entomol. Nematol. 8, 11–18. https://doi.org/10.5897/JEN2015.0145 (2016).
Hemeda N.F & Deeb M.A. Evaluation of biological control potential for different Trichoderma strains against root-knot nematode Meloidogyne javanica. J. Adv. Lab. Res. Biol. 10, 16–22. https://e-journal.sospublication.co.in (2019).
Papavizas, G. C. Trichoderma and Gliocladium: Biology, ecology, and potential for biocontrol. Annu. Rev. Phytopathol. 23, 23–54 (1985).
Sivan, A. & Chet, I. Biological control of Fusarium spp. in cotton, wheat and muskmelon by Trichoderma harzianum. J. Phytopathol. 116(1), 39–47. https://doi.org/10.1111/j.1439-0434.1986.tb00892.x (1986).
Meyer, S. et al. Activity of fungal culture filtrates against soybean cyst nematode and root-knot nematode egg hatch and juvenile motility. Nematology 6, 23–32. https://doi.org/10.1163/156854104323072883 (2004).
Affokpon, A. et al. Biocontrol potential of native Trichoderma isolates against root-knot nematodes in West African vegetable production systems. Soil Biol. Biochem. 43, 600–608. https://doi.org/10.1016/j.soilbio.2010.11.029 (2011).
Yedidia, I. et al. Concomitant induction of systemic resistance to Pseudomonas syringae pv. lachrymans in cucumber by Trichoderma asperellum (T-203) and accumulation of phytoalexins. Appl. Environ. Microbiol. 69, 7343–7353. https://doi.org/10.1128/AEM.69.12.7343-7353.2003 (2003).
Saravanan, P., Ilavarasan, N., Karthikeyan, A. & Padmanaban, B. Management of root knot nematode Meloidogyne incognita in banana (Cv. Robusta) through biocontrol agents and neem cake. Indian J. Agric. Res. 55, 231–234 (2021).
Choudhary, A. & Ashraf, S. Utilizing the combined antifungal potential of Trichoderma spp. and organic amendments against dry root rot of mung bean. Egypt. J. Biol. Pest Control. 29, 1–8. https://doi.org/10.1186/s41938-019-0187-8 (2019).
Singh, S. Integrated approach for the management of the root-knot nematode, Meloidogyne incognita, on eggplant under field conditions. Nematology 15, 747–757. https://doi.org/10.1163/15685411-00002715 (2013).
Ascher, K. S. Nonconventional insecticidal effects of pesticides available from the neem tree, Azadirachta indica. Arch. Insect Biochem. Physiol. 22, 433–449. https://doi.org/10.1002/arch.940220311 (1993).
Yadav, S., Kanwar, R. S. & Patil, J. A. Organic amendments with and without a synthetic nematicide for the management of Heterodera avenae in wheat. Nematology https://doi.org/10.1163/15685411-bja10083 (2021).
Ghimeray, A. K., Jin, C. W., Ghimire, B. K. & Cho, D. H. Antioxidant activity and quantitative estimation of azadirachtin and nimbin in Azadirachta indica A. Juss grown in foothills of Nepal. Afr. J. Biotechnol. 8, 3084–3091 (2009).
Benedict, R. G. & Brady, L. R. Antimicrobial activity of mushroom metabolites. J. Pharm. Sci. 61, 1820–1822. https://doi.org/10.1002/jps.2600611130 (1972).
Wuyts, N., Swennen, R. & De Waele, D. Effects of plant phenylpropanoid pathway products and selected terpenoids and alkaloids on the behaviour of the plant-parasitic nematodes Radopholus similis, Pratylenchus penetrans and Meloidogyne incognita. Nematology 8, 89–101. https://doi.org/10.1163/156854106776179953 (2006).
Harborne, J. B. & Williams, C. A. Advances in flavonoid research since 1992. Phytochemistry 55(6), 481–504. https://doi.org/10.1016/S0031-9422(00)00235-1 (2000).
Briskin, D. P. Medicinal plants and phytomedicines. Linking plant biochemistry and physiology to human health. Plant Physiol. 124, 507–514. https://doi.org/10.1104/pp.124.2.507 (2000).
Dai, J. & Mumper, R. J. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules 15, 7313–7352. https://doi.org/10.3390/molecules15107313 (2010).
Khan, M. R., Fatma, M., Per, T. S., Anjum, N. A. & Khan, N. A. Salicylic acid-induced abiotic stress tolerance and underlying mechanisms in plants. Front. Plant Sci. 6, 462. https://doi.org/10.3389/fpls.2015.00462 (2015).
Rahman, A., Sultana, V., Ara, J. & Ehteshamul-Haque, S. Induction of systemic resistance in cotton by the neem cake and Pseudomonas aeruginosa under salinity stress and Macrophomina phaseolina infection. Pak. J. Bot. 48, 1681–1689 (2016).
Pandey, R., & Sikora, R.A. Influence of aqueous extracts of organic matter on the sensitivity of Heterodera schachtii Schmidt and Meloidogyne incognita (Kofoid and White) Chitwood eggs to Verticillium chlamydosporium Goddard infection. J. Plant Dis. Prot. 107, 494–497. https://www.jstor.org/stable/43215352 (2000).
Oka, Y. & Yermiyahu, U. Suppressive effects of composts against the root-knot nematode Meloidogyne javanica on tomato. Nematology 4, 891–898. https://doi.org/10.1163/156854102321122502 (2002).
Thoden, T. C., Korthals, G. W. & Termorshuizen, A. J. Organic amendments and their influences on plant-parasitic and free-living nematodes: A promising method for nematode management. Nematology 13, 133–153. https://doi.org/10.1163/138855410X541834 (2011).
Waksman, S. A. A method for counting the number of fungi in the soil. J. Bacteriol. 7, 339–341 (1922).
Tamura, K., Stecher, G. & Kumar, S. MEGA11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 38, 3022–3027. https://doi.org/10.1093/molbev/msab120 (2021).
Hussey, R. S. A comparison of methods of collecting inocula of Meloidogyne spp., including a new technique. Plant Dis. Rep. 57, 1025–1028 (1973).
Cobb, N. A. Estimating the nema population of soil, with special references to the sugarbeet and root-gall nemas, Heterodera schachtii Schmidt and Heterodera radicicola (Greef) Muller, and with a description of Tylencholaimus aequalis n. sp.. Agricult. Technol. Circ. 1, 48 (1918).
Schindler, A. F. A simple substitute for a Baermann funnel. Plant Dis. 45, 747–748 (1961).
Hoagland, D. R. & Arnon, D. I. The water-culture method for growing plants without soil. Circular (California Agricultural Experiment Station) 347, 32 (1950).
Folin, O. & Ciocalteu, V. On tyrosine and tryptophane determinations in proteins. J. Biol. Chem. 73, 627–650 (1927).
Heald, C.M., Bruton, B.D. & Davis, R.M. Influence of Glomus intradices and soil phosphorus on M. incognita infecting Cucumis melo. J. Nematol. 21, 69–73 (1989).
Acknowledgements
The authors are thankful to the Agharkar Research Institute, Pune, India, for morphological and molecular identification of fungal bioagent.
Author information
Authors and Affiliations
Contributions
J.P. and S.Y. designed the experiments and wrote the manuscript. R.S. and J.P. performed the experiments. J.P., R.S., S.Y., A.K., V.G. analyzed the results. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Saharan, R., Patil, J.A., Yadav, S. et al. The nematicidal potential of novel fungus, Trichoderma asperellum FbMi6 against Meloidogyne incognita. Sci Rep 13, 6603 (2023). https://doi.org/10.1038/s41598-023-33669-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-33669-z
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.