Abstract
The effects of two liquid modifiers (polyacrylate compound modifier and organic polymer compound modifier) and phloem girdling (stem girdling and branch girdling) on cadmium (Cd) content, Cd transport, and photosynthetic parameters of cotton (Xinluzao 60) in Cd-contaminated soil (40 mg kg −1) were studied through barrel experiment. The results showed that the distribution ratios of Cd in stem, leaves, and bolls, leaf net photosynthetic rate (Pn), leaf stomatal conductance (Gs), leaf transpiration rate (Tr), and chlorophyll content were decreased after girdling; and the application of modifiers reduced the Cd content and the Cd transported to the shoot, while alleviating photosynthetic damage caused by girdling. In general, our results indicated that the inhibition of carbohydrate supply caused by girdling reduced the photosynthetic capacity of cotton, while the applications of the two liquid modifiers decrease the influence to cotton photosynthesis. Moreover, Cd and modifiers may be transported to the shoot through both phloem and xylem.
Similar content being viewed by others
Introduction
Cadmium (Cd) is a toxic heavy metal commonly found in agricultural soils1. It is not necessary for plants, but it is easily absorbed by plant root and enriched in different tissues and organs2. Therefore, many scholars have studied the accumulation of Cd in crops and tried to reduce the uptake of Cd by crops. For example, Sebastian et al.3 found that the application of organic acids reduced the accumulation of Cd in plant leaves, while the application of malic acid reduced the accumulation of Cd in plant roots. Shi et al.4 found that super absorbent polymer (SAP) immobilized heavy metals in soil, which had a positive effect on maize photosynthesis and growth. Moreover, to reduce the accumulation of Cd, it is necessary to understand the mechanism of Cd transport in plants5,6. Mori et al.7 indicated that the xylem unloading limited the transport of Cd from root to shoots of wild eggplant. Qin et al.8 found that phloem played a leading role in the transport of Cd from root to leaves. However, there is no conclusive study on the transport of Cd in cotton at present.
Photosynthesis is a biological process in which plants convert light energy into chemical energy that can be used in life processes and synthesize organic matter. It is the physiological basis of plant survival and one of the most basic physiological processes in plant production9. However, Cd may inhibit photosynthesis of plants10. Moradi and Ehsanzadeh11 reported that Cd inhibited chlorophyll synthesis, resulting in a decrease in the amount of light-harvesting chlorophyll a/b-binding protein. Paunov et al.12 reported that Cd interfered with the photosynthetic electron-transfer process and decreased the efficiency of energy conversion in photosystem II. Therefore, the remediation of Cd pollution has been studied through plant photosynthesis regulation. For example, Liu et al.13 found that the application of salicylic acid significantly reduced Cd absorption in most Cd-stressed plants and restored photosynthetic efficiency. An et al.14 found that the application of liquid modifiers to Cd-contaminated soil increased the gas exchange and photosynthetic pigment content of plant leaves.
Cotton (Gossypium hirsutum) is one of the main economic crops in the arid region of northern China, and drip irrigation has been widely used in cotton cultivation. Drip irrigation has obvious water-saving and yield-increasing features. In addition, it can be combined with fertilization to obviously increase the fertilizer efficiency15. Therefore, whether the application of modifiers through drip irrigation also has an obvious effect on the remediation of soil heavy metal pollution is worth exploring.
At present, most researches on the remediation of soil heavy metal pollution focus on conventional solid materials, but conventional solid materials are not suitable for drip irrigation systems; and the transport and accumulation of Cd in cotton organs have not been determined. Therefore, the study of how liquid polymer modifiers affect cotton in Cd-contaminated soils has important implications for avoiding the potential risks of heavy metals in arid regions. In this study, the effects of two liquid modifiers (polyacrylate compound modifier and organic polymer compound modifier) and phloem girdling (stem girdling and branch girdling) on Cd content, Cd transport, and photosynthetic parameters of cotton (Xinluzao 60) in Cd-contaminated soil (40 mg kg−1) were studied through barrel experiment. This study aimed to clarify the distribution and transformation of Cd in cotton, and to explore the remediable mechanism of modifiers. We hypothesized that: (1) the transport pathways of Cd and modifiers in cotton were similar; (2) the modifiers affected the transport and accumulation of Cd in cotton organs; (3) the modifiers changed the photosynthetic characteristics of cotton leaves.
Results
Effects of girdling and modifier application on Cd content in different organs of cotton
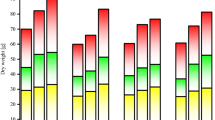
The root of cotton had the highest Cd content, followed by leaves, stem, and cotton bolls (Fig. 1). After the application of the two modifiers, the contents of Cd in root, stem, leaves, and bolls were decreased compared with CK; among them, the decreases of Cd contents in root and bolls were significant. Compared with CK, the Cd contents in root for T1 and T2 groups decreased by 12.33% (P < 0.05) and 23.79% (P < 0.05), respectively, whereas those in bolls for T1 and T2 groups decreased by 14.48% (P < 0.05) and 12.87% (P < 0.05), respectively. Compared with CK, the Cd contents in root for CK-J and CK-G groups increased by 3.76% and 6.48%, respectively, whereas the Cd contents in stem, leaves, and bolls all decreased (P < 0.05). Compared with CK-J group, the Cd contents in root, leaves, and bolls for T1-J group decreased by 5.71%, 28.84% (P < 0.05), and 8.75% (P < 0.05), respectively, and those for T2-J group decreased by 3.87%, 23.59% (P < 0.05) and 9.9% (P < 0.05), respectively. Compared with CK-G group, the Cd contents in root, leaves, and bolls for T1-G group decreased by 8.73%, 27.63% (P < 0.05), and 5.98%, respectively, and those for T2-J group decreased by 7.61%, 26.11% (P < 0.05), and 5.00%, respectively.
Effects of girdling and modifiers on cadmium transport
The distribution ratio and transport index of Cd in different organs of cotton under different treatments were shown in Table 1. Compared with CK, the distribution ratios of Cd in root and bolls for T1 group decreased by 1.55% and 3.97%, respectively, whereas those in stem and leaves increased by 7.68% (P < 0.05) and 2.45%, respectively; the distribution ratio of Cd in root for T2 group decreased by 8.91%, whereas those in stem, leaves, and bolls increased by 18.95% (P < 0.05), 14.07% (P < 0.05), and 4.14%, respectively. That is, the application of modifiers reduced the Cd content in root but increased the Cd content in stem, leaves, and bolls. After stem girdling, the distribution ratio of Cd in root for CK-J group increased by 8.43%, whereas those in stem, leaves, and bolls decreased by 31.83% (P < 0.05), 6.85%, and 3.91%, respectively, compared with CK. Compared with CK-J group, the distribution ratios of Cd in root, stem, and bolls for T1-J group increased by 5.57%, 4.18%, and 2.16%, respectively, whereas that in leaves decreased by 20.33% (P < 0.05); the distribution ratios of Cd in root and stem for T2-J group increased by 4.52% and 9.82%, respectively, whereas those in leaves and bolls decreased by 16.93% (P < 0.05) and 2.14%, respectively. After branch girdling, the distribution ratio of Cd in root for CK-G group increased by 11.80% (P < 0.05), whereas those in stem, leaves, and bolls decreased by 28.29% (P < 0.05), 12.89% (P < 0.05), and 15.27% (P < 0.05), respectively, compared with CK. Compared with CK-G group, the distribution ratios of Cd in root, stem, and bolls for T1-G group increased by 3.98%, 1.06%, and 7.11%, respectively, whereas that in leaves decreased by 17.56% (P < 0.05); the distribution ratios of Cd in root, stem, and bolls for T2-G group increased by 2.78%, 14.39% (P < 0.05), and 5.69%, respectively, whereas that in leaves decreased by 17.80% (P < 0.05).
As shown in Table 1, compared with CK, the transport indexes of Cd from root to stem for T1 and T2 groups increased by 9.38% and 30.59% (P < 0.05), respectively, whereas those from stem to leaves for T1 and T2 groups decreased by 4.86% and 4.10%, respectively; in addition, the transport indexes of Cd from stem to bolls decreased by 10.82% (P < 0.05) and 12.45% (P < 0.05), respectively. Compared with CK-J group, the transport indexes of Cd from root to stem, from stem to leaves, and from stem to bolls for T1-J group decreased by 1.32%, 23.52% (P < 0.05), and 1.93%, respectively, and those from root to stem and from stem to bolls for T2-J group decreased by 24.36% (P < 0.05) and 10.89% (P < 0.05), respectively. Compared with CK-G group, the transport indexes of Cd from root to stem and from stem to leaves for T1-G group decreased by 2.81% and 18.42% (P < 0.05), respectively, whereas that from stem to bolls increased by 5.98%; the transport index of Cd from root to stem for T2-G group increased by 11.29%, whereas those from stem to leaves and from stem to bolls decreased by 28.14% (P < 0.05) and 7.61%, respectively.
Effect of girdling and modifiers on chlorophyll pigment in cotton leaves
For the groups without girdling, the application of modifiers increased the Chla, Chlb, and Car concentrations of cotton leaves (Fig. 2). Compared with CK, the Chla, Chlb, and Car concentrations and the Chla/b ratio for T1 group increased by 25.23% (P < 0.05), 19.28% (P < 0.05), 5.11%, and 20.67% (P < 0.05), respectively, and those for T2 group increased by 20.10% (P < 0.05), 18.54% (P < 0.05), 1.05%, and 17.17% (P < 0.05), respectively. The stem girdling and branch girdling decreased the Chla, Chlb, and Car concentrations and the Chla/b ratio of leaves, whereas the application of modifiers alleviated the damage to photosynthetic pigments. After stem girdling, the Chla, Chlb, and Car concentrations and the Chla/b ratio for CK-J group decreased by 7.37%, 5.89%, 1.58%, and 5.53%, respectively, compared with CK. Compared with CK-J group, the Chla, Chlb, and Car concentrations and the Chla/b ratio for T1-J group increased by 3.41%, 2.60%, 2.17%, and 5.89%, respectively, and those for T2-J group increased by 19.46% (P < 0.05), 16.22% (P < 0.05), 2.78%, and 20.33% (P < 0.05), respectively. After branch girdling, the Chla, Chlb, and Car concentrations and the Chla/b ratio for CK-G group decreased by 21.99% (P < 0.05), 19.05% (P < 0.05), 3.62%, and 17.87% (P < 0.05), respectively, compared with CK. Compared with CK-G group, the Chla, Chlb, and Car concentrations for T1-G group increased by 0.87%, 1.42%, and 12.11% (P < 0.05), respectively, and the Chla, Chlb, and Car concentrations and the Chla/b ratio for T2-G group increased by 12.41% (P < 0.05), 10.05% (P < 0.05), 2.13%, and 15.09% (P < 0.05), respectively.
Effect of girdling and modifiers on photosynthesis parameters of cotton leaves
For the groups without girdling, the application of modifiers increased the net photosynthesis rate (Pn) of cotton leaves (Fig. 3). Compared with CK, the Pn for T1 and T2 groups increased by 14.51% (P < 0.05) and 3.98%, respectively. The stem girdling and branch girdling decreased the leaf Pn. The Pn for CK-J and CK-G groups decreased by 4.30% and 23.02% (P < 0.05), respectively, compared with CK. The application of modifiers alleviated the decreases of Pn caused by girdling. Compared with CK-J group, the Pn for T1-J and T2-J groups increased by 1.19% and 13.39% (P < 0.05), respectively. Compared with CK-G group, the Pn for T1-G and T2-G groups increased by 12.14% (P < 0.05) and 22.34% (P < 0.05), respectively.
Modifiers application and girdling treatments significantly affected the stomatal conductance (Gs), intercellular CO2 concentration (Ci), and transpiration rate (Tr) of cotton leaves. For the groups without girdling, the application of modifiers increased the Gs and Tr but decreased the Ci (Fig. 3). After stem girdling, the Gs and Tr for CK-J group decreased by 26.86% (P < 0.05) and 20.02% (P < 0.05), respectively, whereas the Ci increased by 3.43% compared with CK; the Gs and Tr for CK-G group decreased by 34.21% (P < 0.05) and 40.96% (P < 0.05), respectively, whereas the Ci increased by 16.73% (P < 0.05) compared with CK. The application of modifiers also significantly changed the Gs, Ci and Tr of cotton leaves. Among them, compared with CK-J group, the Gs for T1-J and T2-J groups increased by 25.03% (P < 0.05) and 42.03% (P < 0.05), respectively, and the Tr for T1-J and T2-J groups increased by 1.53% and 45.44% (P < 0.05), respectively; whereas the Ci for T1-J and T2-J groups decreased by 0.37% and 8.45%, respectively. Compared with CK-G group, the Gs for T1-G and T2-G groups increased by 6.71% and 8.38%, respectively, and the Tr for T1-G and T2-G groups increased by 27.13% (P < 0.05) and 34.90% (P < 0.05), respectively; whereas the Ci for T1-G and T2-G groups decreased by 15.80% (P < 0.05) and 11.20% (P < 0.05), respectively.
Effect of modifiers on microstructure of cotton leaf tissue
The observation of the paraffin sections of leaves in CK, T1, and T2 groups showed that leaves are composed of epidermis, palisade tissue, and sponge tissue (Fig. 4). For the control group (CK), leaf tissues were irregularly arranged; some cells contracted, shortened, and even curved, and the gap between cells increased. Compared with T1 and T2 groups, the number of sponge tissue cells for CK was decreased, the arrangement was loose, the intercellular spaces were increased, and the cells were decreased. For T1 and T2 groups, the palisade tissue cells of cotton leaves were long columnar; the cells were arranged neatly and tightly, and there were more chloroplasts compared with CK. The rounded sponge tissue cells were arranged closely.
Redundancy analysis of cotton physiological characteristics
The effects of modifiers on Cd accumulation and leaf photosynthetic parameters for different groups were analyzed by redundancy analysis (RDA). As shown in Fig. 5, PTIroot-stem, DRstem, DRleaves, and DRbolls were clearly distributed on the left side of the RDA1 axis. PTIroot-stem and DRstem were closely related to the Tr and Gs, whereas DRleaves was closely related to the Pn. STIstem-leaves, STIstem-bolls, and DRroot were clearly distributed on the right side of the RDA1 axis. The Ci was closely related to DRroot. and PTIroot-stem and DRstem were closely related to T1 and T2 groups. After stem girdling, T1-J group was closely related to the Chla/b and Ci, and T2-J group was closely related to the Chla, Chlb, Car, Tr, and Gs. After branch girdling, T2-G group was closely related to the Ci.
Discussion
Most heavy metals such as Pb, Cr, and Cu are absorbed and stored in crop root, while Cd, Ni, and Zn, etc. are easily transported to the upper part of crops16,17,18. At present, many plants have been studied in terms of the Cd distribution and accumulation8,18, and the ability of xylem to transport Cd into shoots has been considered as a major determinant of Cd accumulation in the shoots of many plants7. In addition, the absorption of Cd by peanut and wheat also showed that Cd was mobile in phloem tissue19. According to the previous results, we studied the distribution and transport of Cd in cotton organs by girdling, and found that the mobile of Cd in different organs of cotton was obvious. For the control group (CK), the root had the highest Cd accumulation, followed by leaves, stem, and bolls (Fig. 1). From the distribution ratio, we found that stem girdling significantly reduced the accumulation of Cd in stem, and branch girdling not only significantly reduced the accumulation of Cd in stem, but also reduced that in leaves and bolls (Table 2). It indicated that Cd was of high mobility in both xylem and phloem, and stem may play an important role in transport of Cd for the two pathways. This is same as the research result by Reid et al.19: Cd was transported through xylem and phloem.
The accumulation and transport of Cd in crops have been regulated through the application of exogenous materials in previous studies. For example, Huang et al.20 reported that the application of calcium can reduced the Cd accumulation in soybean and wheat roots. Guiwei et al.21 found that the application of polyacrylate polymers reduced the bioavailable Cd, which could be employed to enhance productivity of crops in Cd-contaminated soils. In this study, the modifiers used are mainly polymer materials. The carboxyl and amide groups of polyacrylamides can form coordination bonds with metal ions on the surface of soil particles to form complexes22. From transport indexes, we found that the polyacrylate compound modifier significantly reduced the transport of Cd from stem to bolls, and the organic polymer compound modifier significantly reduced the transport of Cd from root to stem and from stem to bolls (Table 2), indicating that polyacrylate compound modifier mainly reduced the mobility of Cd in cotton, and organic polymer compound modifier mainly reduced the absorption of Cd by cotton. Moreover, it was found that the application of polyacrylate compound modifier reduced the transport of Cd from stem to leaves after stem girdling and branch girdling, and the application of organic polymer compound modifier reduced the transport of Cd from stem to leaves after stem girdling and the transport of Cd from stem to bolls after branch girdling (Table 2), indicating that modifiers could be transported to leaves and bolls after phloem girdling. According to RDA analysis, without girdling, the application of polyacrylate compound modifier could significantly change the distribution and transport of Cd in cotton organs compared with CK, whereas the application of organic polymer compound modifier could significantly change the distribution and transport of Cd in cotton organs compared with CK-J and CK-G groups after girdling.
Many studies have found that phloem girdling reduces the net photosynthetic rate (Pn) of plant leaves23. Sala et al.24 showed that phloem girdling influenced the rate of photosynthetic assimilation, and Poirier-Pocovi et al.25 showed that phloem girdling reduced the photosynthesis rate of leaves. Similar findings were also shown in this study. After girdling, the Pn, Gs, and Tr all decreased, and only Ci increased, indicating that the decrease of photosynthetic rate was mainly caused by non-stomatal factors26. This is consistent with the conclusion of Zhou et al.27. Without girdling, the applications of the two modifiers significantly decreased the inhibition from Cd on Pn of cotton leaves. For groups with girdling, only T2-J group significantly decreased the inhibition on Pn, but there was significant difference in the decreases of Pn among the groups treated with modifiers. The results indicated that the modifiers could alleviate the photosynthetic damage caused by girdling. After stem girdling, the Pn for the groups treated with organic polymer compound modifier were higher than that of CK (Fig. 3), which indicated that the application of organic polymer compound modifier after stem girdling had a positive effect on Pn. This might be due to the application of organic polymer compound modifier decreased intercellular CO2 concentration, but increased stomatal conductance and transpiration rate (Fig. 3).
Consistent with the research results of Tang et al.28, the pigment chlorophyll of cotton leaves was significantly decreased by girdling, which helps to determine the compensatory photosynthesis by modifiers (Fig. 2). The decreases of Chla, Chlb, and Car concentrations for the groups treated with branch girdling was greater than those for the groups treated with stem girdling, and the changes of photosynthetic rates also showed the same phenomenon. It indicated that the damage caused by branch girdling was more serious. This may be due to the accumulation of carbohydrates in the upper part above the cut after girdling. Photosynthates could not be transported to the root, thus photosynthesis was inhibited. At this time, the plant might avoid absorbing too much light by reducing the content of photosynthetic pigments. This reaction is the feedback regulation according to the source-sink relation25. The application of modifiers improved the photosynthesis of leaves because the chlorophyll content was significantly increased, and the effect of the application of organic polymer compound modifier after stem girdling was more obvious. Moreover, Chla and Chlb play different roles in photosynthesis29. The ratios of Chla/b in cotton leaves decreased significantly after girdling, but they were increased after the application of modifiers, indicating that after girdling, the application of modifiers increased the number of molecules involved in photochemical reaction, which led to the improvement of photosynthesis29. According to the RDA analysis, the polyacrylate compound modifier has a greater effect on the protection of pigment chlorophyll of cotton without girdling than the organic polymer compound modifier, whereas the organic polymer compound modifier has a greater effect on that of cotton with girdling. In addition, Liu et al.30 found that Cd distributed in leaf epidermis, spongy tissue, and palisade tissue of crops in Cd-contaminated soils. By observing the microstructure of leaf tissues, we found that Cd did not damage the epidermis cells but the palisade tissue cells in mesophyll and sponge tissue cells, which would affect chloroplasts. Because Cd was absorbed by cotton root and transported from root to shoots, there was no direct contact with epidermis cells. By applying the modifiers, the densities of palisade tissue and sponge tissue were adjusted, which promoted the development of mesophyll cells and chloroplasts and increased the chlorophyll content. Thus, the photosynthesis of cotton leaves was improved.
Conclusions
This study proved that Cd is of high mobility in both xylem and phloem through girdling. The transport pathways of polyacrylate compound modifier and organic polymer compound modifier in cotton were the same as that of Cd. Modifies could still be transported to the shoots after phloem girdling, but the effect of modifiers on alleviating Cd stress was not greater than that without girdling. Moreover, on the one hand, the application of the two modifiers could stabilize Cd in cotton root and reduce the absorption of Cd; on the other hand, it could alleviate the inhibition of photosynthesis caused by phloem girdling.
Materials and Methods
Test site condition
Test soil (Calcaric Fluvisol) was obtained from the Test Station of Agricultural College, Shihezi University in Shihezi City, Xinjiang Province, China (86°03′E, 45°19′N). The soil pH was 7.76, soil cation-exchange capacity (CEC) was 16.25 coml kg−1, total nitrogen content was 0.89 g kg−1, organic matter content was 13.25 g kg−1, alkali-hydrolyzable nitrogen content was 60 mg kg −1, available phosphorus content was 20 mg kg −1, and available potassium content was 250 mg kg−131.
Test program
Two-year continuous remediation of Cd-contaminated soil using polymer modifiers was conducted in 2017 and 2018. On April 5th, 2017, to maintain the original soil profile, soils were packed into plastic barrels (length × width × height = 30 cm × 30 cm × 80 cm), and then barrels were buried back into the field. Based on previous studies14 and pre-test results, cadmium chloride (CdCl2•2.5H2O) solution was added into the barrels and mixed fully with the plough layer. The Cd content in the plough layer reached approximately 40 mg kg−1 after three weeks. On April 26th, urea (345 kg hm−2) and compound fertilizer (17-17-17) (795 kg hm−2) was applied.
A total of nine groups were set in a randomized block design, and two treatments were employed, including: (1) the application of modifiers (non-modifier, polyacrylate compound modifier, and organic polymer compound modifier (Table 3)); and (2) phloem girdling (non-girdling, stem gridling, and branch girdling) (Table 2). Each group had three repetitions. On April 30th, two modifiers (8.48 kg hm−2) were diluted with water and applied through drip irrigation. Cotton (Xinluzao 60) was sown on May 2th. After emergence, six seedlings were retained in each barrel. The first irrigation was conducted on June 14th. The irrigation cycle was 3 days during the growth period, and the irrigation volume was 4,500 m3 hm−2. No fertilizers and modifiers were applied at later stages. Cotton was harvested on September 5, 2017. After that, the stalks were pulled out and the field was plowed.
On April 5th, 2018, the Cd content in plough layer reached 32.44 mg kg−1 in the soil without modifiers, 31.57 mg kg−1 in the soil with polyacrylate compound modifier, and 31.22 mg kg−1 in the soil with organic polymer compound modifier. In 2018, no cadmium was applied into the soils, and the amounts of modifiers applied (8.48 kg hm−2) and the other managements were the same as those in 2017.
To evaluate the transport pathway of Cd (phloem or xylem), and analyze the effects of modifiers on Cd content and regulating photosynthesis in cotton, phloem girdling was employed to inhibit the transport of Cd and modifiers through phloem in 2018. Phloem girdling was conducted at the cotton flowering boll-setting stage (the 10th day after manual topping). Girdling sites of stem girdling and branch girdling were located at the main stem of cotton (3–5 cm from the ground) and the top fruit branch, respectively. The width of the cut was 1 cm, and the depth was up to the xylem. Ten days after girdling, functional leaves from each barrel were randomly selected to determine photosynthetic parameters, and three cotton plants were randomly selected from each barrel to determine Cd content.
Experimental determination
Measurement of Cd content
Root, stem, leaves, and cotton bolls of cotton plants were separated, and then they were dried at 105 °C for 0.5 h and dried to constant at 80 °C. Samples of each organ (0.5 g) were acid-digested using sulfuric and nitric acid (1:5, v/v) at 60 °C for 24 h, and treated with HNO3/HClO4 (5/1, v/v). A Hitachi Z2000 graphite atomic absorption spectrophotometer (PinAAcle900T, PerkinElmer, USA) was used for the determination of Cd content31.
Chlorophyll pigment measurement
Fresh leaves (20 mg) from each group were cut into small pieces. Photosynthetic pigments were extracted in 80% (v/v) acetone, and centrifuged. Pellets were used to re-extract the pigments until they became colorless. The absorbance was determined at 663.2, 646.5, and 470 nm spectrophotometrically. The concentrations of chlorophyll (Chla and Chlb) and carotenoids (Car) were calculated according the method of Lichtenthaler32.
Photosynthesis parameters
Net photosynthetic rate (Pn), stomatal conductance (Gs), transpiration rate (Tr), and intracellular CO2 concentration (Ci) were determined with a portable photosynthesis system LI-6400 (LI-COR, Lincoln, USA) according to Liu et al.33.
Anatomical type of cotton leaves
In the flowering and boll-setting stage, the leaves at the same position of cotton plants in each group were collected and wrapped up using wet gauze. After that, they were put in ice box and taken back to the laboratory. Leaves were washed with distilled water, and then, they were blotted with absorbent paper. A small piece (about 1 cm × 0.5 cm) was cut with a blade and put into the FAA fixing solution (formaldehyde: 5 mL, glacial acetic acid: 5 mL, and 70% alcohol: 90 m L) for making paraffin sections. Paraffin sectioning34 was conducted using hematoxylin and Canadian neutral gum. The thickness of the slice was 6 to 8 μm, and the slices were photographed with a microscope (eyepiece × objective lens: 10 × 40) (DMB: Motic Digital Microscope).
Statistical analysis
The data were processed using Excel 2016 (Microsoft, USA), and one-way analysis of variance (ANOVA) was performed using SPSS 23.0 (SPSS Inc., Chicago, IL, USA). Multiple comparisons between different groups were conducted using Duncan’s new multiple range method (significance level: α = 0.05). Charts were drawn using Origin 8.0 (Origin Lab, Massachusetts, USA).
Calculation formulas in data processing:
References
Pena, L. B., Barcia, R. A., Azpilicueta, C. E., Méndez, A. A. E. & Gallego, S. M. Oxidative post translational modifications of proteins related to cell cycle are involved in cadmium toxicity in wheat seedlings. Plant Sci. 196, 1–7 (2012).
Vitória, A. P., Rodriguez, A. P. M., Cunha, M., Lea, P. J. & Azevedo, R. A. Structural changes in radish seedlings exposed to cadmium. Biol. Plantarum 47, 561–568 (2004).
Sebastian, A. & Prasad, M. N. V. Exogenous citrate and malate alleviate cadmium stress in Oryza sativa L.: Probing role of cadmium localization and iron nutrition. Ecotoxicol. Environ. Saf. 166, 215–222 (2018).
Shi, Y. et al. Environmental materials for remediation of soils contaminated with lead and cadmium using maize (Zea mays L.) growth as a bioindicator. Environ. Sci. Pollut. Res. Int. 23, 6168–6178 (2016).
Schroeder, J. et al. Using membrane transporters to improve crops for sustainable food production. Nature 497, 60–66 (2013).
Uraguchi, S., Kamiya, T., Clemens, S. & Fujiwara, T. Characterization of OsLCT1, a cadmium transporter from indica rice (Oryza sativa). Physiol. Plant. 151, 339–347 (2014).
Mori, S., Uraguchi, S., Ishikawa, S. & Arao, T. Xylem loading process is a critical factor in determining Cd accumulation in the shoots of Solanum melongena and Solanum torvum. Environ. Exp. Bot. 67, 127–132 (2009).
Qin, Q. et al. Long-distance transport of cadmium from roots to leaves of Solanum melongena. Ecotoxicology 24, 2224–2232 (2015).
Salisbury, A. B., Gallagher, F. J., Caplan, J. S. & Grabosky, J. C. Maintenance of photosynthesis by Betula populifolia in metal contaminated soils. Sci. Total. Environ. 625, 1615–1627 (2018).
Li, S., Yang, W., Yang, T., Chen, Y. & Ni, W. Effects of cadmium stress on leaf chlorophyll fluorescence and photosynthesis of Elsholtzia argyi–a cadmium accumulating plant. Int. J. Phytoremediation 17, 85–92 (2015).
Moradi, L. & Ehsanzadeh, P. Effects of Cd on photosynthesis and growth of safflower (Carthamus tinctorius L.) genotypes. Photosynthetica 53, 506–518 (2015).
Paunov, M., Koleva, L., Vassilev, A., Vangronsveld, J. & Goltsev, V. Effects of Different Metals on Photosynthesis: Cadmium and Zinc Affect Chlorophyll Fluorescence in Durum Wheat. Int. J. Mol. Sci. 19, 787 (2018).
Liu, Z., Ding, Y., Wang, F., Ye, Y. & Zhu, C. Role of salicylic acid in resistance to cadmium stress in plants. Plant Cell Rep. 35, 719–731 (2016).
An, M. J. et al. Effects of Modifiers on the Growth, Photosynthesis, and Antioxidant Enzymes of Cotton Under Cadmium Toxicity. J. Plant. Growth Regul. 38, 1196–1205 (2019).
Zhong, F., Hou, M., He, B. & Chen, I. Assessment on the coupling effects of drip irrigation and organic fertilization based on entropy weight coefficient model. PeerJ 5, e3855 (2017).
Gaiss, S., Amarasiriwardena, D., Alexander, D. & Wu, F. Tissue level distribution of toxic and essential elements during the germination stage of corn seeds (Zea mays, L.) using LA-ICP-MS. Environ. Pollut. 252, 657–665 (2019).
Ahmad, M. S. & Ashraf, M. Essential roles and hazardous effects of nickel in plants. Rev. Environ. Contam. Toxicol. 214, 125–167 (2011).
Yan, B. F. et al. Cadmium allocation to grains in durum wheat exposed to low Cd concentrations in hydroponics. Ecotoxicol. Environ. Saf. 184, 109592 (2019).
Reid, R. J., Dunbar, K. R. & McLaughlin, M. J. Cadmium loading into potato tubers: the roles of the periderm, xylem and phloem. Plant Cell Environ. 26, 201–206 (2010).
Huang, D. et al. Effects of calcium at toxic concentrations of cadmium in plants. Planta. 245, 863–873 (2017).
Guiwei, Q. et al. Improvement in soil and sorghum health following the application of polyacrylate polymers to a Cd-contaminated soil. J. Hazard. Mater. 173, 570–575 (2010).
Ruehrwein, R. A. & Ward, D. W. Mechanism of Clay Aggregation by Polyelectrolytes. Soil Sci. 73, 485–492 (1952).
De Schepper, V., Kathy, S., Van Labeke, M. C. & Lemeur, R. Detailed analysis of double girdling effects on stem diameter variations and sap flow in young oak trees. Environ. Exp. Bot. 68, 149–156 (2010).
Sala, A., Woodruff, D. R. & Meinzer, F. C. Carbon dynamics in trees: feast or famine? Tree Physiol. 32, 764–775 (2012).
Poirier-Pocovi, M., Lothier, J. & Buck-Sorlin, G. Modelling temporal variation of parameters used in two photosynthesis models: influence of fruit load and girdling on leaf photosynthesis in fruit-bearing branches of apple. Ann. Bot. 121, 821–832 (2018).
Bloomfield, K. J., Farquhar, G. D. & Lloyd, J. Photosynthesis–nitrogen relationships in tropical forest tree species as affected by soil phosphorus availability: a controlled environment study. Funct. Plant. Biol. 41, 820 (2014).
Zhou, X., Sun, C., Zhu, P. & Liu, F. Effects of Antimony Stress on Photosynthesis and Growth of Acorus calamus. Front Plant Sci. 9, 579 (2018).
Tang, G. L., Li, X. Y., Lin, L. S., Hu, Y. & Zeng, F. J. Inhibition of root respiration induces leaf senescence in Alhagi sparsifolia. Photosynthetica 55, 588–602 (2017).
Akrami, M. & Arzani, A. Physiological alterations due to field salinity stress in melon (Cucumis melo L.). Acta Physiol. Plant. 40, 91 (2018).
Liu, M. et al. Microstructural and physiological responses to cadmium stress under different nitrogen levels in Populus cathayana females and males. Tree Physiol. 40, 30–45 (2020).
Bao, S. D. Soil and agricultural chemistry analysis. (China Agriculture Press, Beijing, 2000).
Lichtenthaler, H. K. Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Method Enzymol. 148, 350–382 (1987).
Liu, X. et al. Linking stomatal traits and expression of slow anion channel genes HvSLAH1 and HvSLAC1 with grain yield for increasing salinity tolerance in barley. Front. Plant Sci. 5, 634 (2014).
Pereira, E. G., Oliva, M. A., Kuki, K. N. & Cambraia, J. Photosynthetic changes and oxidative stress caused by iron ore dust deposition in the tropical CAM tree Clusia hilariana. Trees (Berlin) 23, 277 (2009).
Acknowledgements
This research was supported by the National Key Research and Development Program of China (2016YFC0501406), and the International Science & Technology Cooperation Program of Shihezi University (GJHZ201802).
Author information
Authors and Affiliations
Contributions
Mengjie An analyzed the data and wrote the manuscript; Mengjie An, Hua Fan, and Xiaoli Wang did the experiment and collected the data; Changzhou Wei revised the manuscript; Kaiyong Wang designed the experiment and revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
An, M., Wei, C., Wang, K. et al. Study on the effects of polymer modifiers and phloem girdling on cotton in cadmium-contaminated soil in Xinjiang Province, China. Sci Rep 10, 6356 (2020). https://doi.org/10.1038/s41598-020-63421-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-63421-w
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.