Abstract
This study aims to validate the current diagnostic method for the clinical detection of gastroenteritis. We analyzed 400 stool samples to detect three of the most common enteropathogens: Salmonella spp., Campylobacter spp., and Yersinia enterocolitica. All specimens were tested with a routine clinical diagnosis algorithm and with five real-time PCR assays. A total of 98 specimens (24.5%) were positive for enteropathogens. We found 24 samples positive for Salmonella enterica, 71 positive for Campylobacter spp., and 4 positive for Yersinia enterocolitica. All evaluated methods exhibited a good performance in identifying Salmonella and Yersinia enterocolitica, being the highest positive percent agreement (PPA) value of 95.8% and 100%, respectively. The clinical algorithm showed the highest PPA value identifying Salmonella, due to the enrichment in selenite broth. However, the evaluated methods showed notable differences in the identification of Campylobacter species, obtaining a wide range of PPA values: 59.2%–100%. The clinical algorithm showed the lowest PPA value since it was only able to detect Campylobacter jejuni and Campylobacter coli species. This study revealed the importance of implementing the real-time PCR technique in a clinical algorithm: it improved the accuracy of the diagnosis and provided results in a shorter time compared to routine clinical methods.
Similar content being viewed by others
Introduction
Acute gastroenteritis is a leading cause of morbidity and mortality worldwide1, particularly in at-risk populations, such as children, older individuals, and immunocompromised patients. We included three bacteria in this study: Salmonella spp., Campylobacter spp., and Yersinia enterocolitica, which are among the most common enteropathogens that cause gastrointestinal infections. Thus, their rapid, accurate identification is crucial for infection control, and in selected cases, for determining the most suitable therapy. According to the Centers for Disease Control and Prevention, during 2016, the Foodborne Active Surveillance Network identified 24,029 cases of foodborne infections, which led to 5,512 hospitalizations (22.9%) and 98 deaths (0.4%). Some of these cases were detected by culture, but others were detected with culture-independent diagnostic tests (CIDTs). Current CIDTs mainly comprise two types: antigen-based and nucleic acid-based assays. In 2016, one study showed that many infections were identified with a CIDT and without a culture confirmation; most frequently, Campylobacter (32%) and Yersinia (32%); but also Shiga toxin-producing E.coli (STEC) (24%), Shigella (23%), Vibrio (13%), and Salmonella (8%)2. That study revealed the consequences of the current change in diagnostic trends. The implementation of CIDTs in clinical laboratories has led to more exhaustive diagnoses. Most of these tests are ordered by clinicians, because CIDTs are easier to perform and they return results faster than traditional culture techniques. Moreover, samples are subjected to deep screening when multiplex panels are used; consequently, the number of reported infections has increased2,3. Routine detection of enteropathogens in clinical laboratories typically consists of a complex algorithm, based on selective culture-, biochemistry-, and immunology-based tests. Most studies have shown that culture-based methods lacked sensitivity, particularly in identifying Campylobacter, and most researchers agree that nucleic acid-based CIDTs have great potential. Consequently, CIDTs (particularly gastrointestinal panels, based on multiplex real-time PCR technology (qPCR)) are increasingly implemented in clinical laboratories4,5,6,7,8, due to their high sensitivity and their ability to screen a considerable number of enteropathogens simultaneously. This approach avoids complex algorithms and reduces the hands-on time. However, the use of stool antigen-based CIDTs remains controversial, because they have displayed highly variable sensitivities and specificities, depending on the study9,10.
This study aimed to compare five molecular CIDTs, based on qPCR technology, to culture-dependent techniques to evaluate their performance and determine the most suitable alternative for routine clinical diagnoses of enteropathogens.
Materials and Methods
Study population and clinical stool specimens
Stool samples were submitted from October to December 2015 to the Hospital Clinico Universitario Lozano Blesa (Zaragoza, Spain). This hospital had 803 beds and attended a population of 286,774 inhabitants, with 29,506 admissions annually. It included an outpatient care facility with 2,315,197 visits annually and an emergency department with 127,694 visits annually. The samples were randomly chosen from specimens received with a request for routine detection of enteropathogens (bacteria, virus, and parasites). This study was approved by the Clinical Research Ethics Committee of Aragon (25 Sept 2019; PI19/378). This evaluation does not require informed consent as it was performed with medical waste. After routine analysis, the leftover of DNA, which is considered medical waste, was analyzed with molecular methods. These non-hospital routine diagnosis methods implied no risk or burden for any individuals and the patient did not have to provide any additional sample. The study focused on the presence/absence of bacterial DNA, without attending to residual human DNA. For each patient, the following data were collected: gender, age, hospitalization status, and stool sample consistency. The non-clinicians researchers never had access to personal identifiable information or clinical record. All recorded data were robustly pseudonymized at source, so no subject-identifiable data were generated.
Study design and case definition
Several comparisons were performed simultaneously. All specimens were tested with five qPCR assays. In addition, all specimens were analyzed with the routine clinical algorithm. Discrepant samples among the qPCR assays were resolved with additional qPCR tests. Discrepancies between the qPCR assays and the routine clinical algorithm were resolved with conventional PCR and sequencing.
When an incongruence could not be resolved, the information derived from the other tests was used to resolve it. The growth in culture was considered a positive result, regardless of other results.
Consequently, taking into account the above mentioned factors, we designated the following as “cases”:
-
a)
Samples that were identified as positive in culture media.
-
b)
Discrepant samples, with positive results supported by resolution tests. We consider as resolution tests those that help us to resolve the incongruences. In this study they were the additional qPCR test and the conventional PCR and sequencing.
-
c)
Discrepant samples, without successful resolution tests, but a positive result was supported by at least three qPCR assays.
Routine clinical algorithm
Once samples arrived at the Microbiology laboratory, they were routinely plated onto various selective and differential media, including selenite broth, MacConkey (MCK) agar, Hektoen enteric (HEK) agar, cefsulodin-irgasan-novobiocin (CIN) agar, xylose lysine deoxycholate (XLD) agar, and charcoal cefoperazone deoxycholate agar (CCDA). These cultures were grown to isolate and identify the enteric pathogens. All culture media were obtained from Oxoid (Thermo Fisher Scientific, Massachusetts, US).
Stool samples were inoculated into enrichment selenite broth and incubated at 37 °C for 24 h prior to culturing in XLD agar. All media were incubated at 37 °C for 24 h, except for CCDA plates, which were incubated at 42 °C for 48–72 h under microaerophilic conditions, with a GasPak EZ Campy system (BD Diagnostics, New Jersey, US).
Salmonella is expected to grow on MCK, HEK and XLD agar, Campylobacter on CCDA plates and Yersinia enterocolitica on MCK and CIN agar.
Colonies recognized as presumptive pathogens were confirmed with matrix-assisted laser desorption/ionization-time of flight mass spectrometry (MALDI-TOF MS Biotyper 3.1; Bruker, Massachusetts, US)11 and they were further analyzed with an automated system (MicroScan®Neg Combo Type 53 Panel; Beckman Coulter, California, US) to determine the sensitivity to antibiotics. Results from the MicroScan® panels were read on the MicroScan Walk Away 96 plus platform (Siemens, Munich, Germany).
The species of Campylobacter were determined using MALDI-TOF11.
The serotype of Salmonella was determined following the Kauffmann-White scheme with the Difco Salmonella O Antiserum Group Kit (Becton Dickinson, New Jersey, US).
The serotype of Yersinia enterocolitica was determined with the Yersinia enterocolitica O:3 Antiserum Kit (BioRad, California, US).
Nucleic acid extraction
Nucleic acids were extracted from fresh stool samples with the VIASURE RNA-DNA Extraction Kit (CerTest Biotec S.L., Zaragoza, Spain). Solid (0.1 g) or liquid (200 µl) stool was added to 200 µl of phosphate-buffered saline, and extraction was performed according to the manufacturer’s instructions. Samples were eluted in 100 µl and stored at −20 °C until use in the PCR detection assay.
Real-Time PCR assays
Five different Real-Time PCR assays were evaluated in this study: VIASURE Salmonella Real-Time PCR Detection Kit, VIASURE Campylobacter Real-Time PCR Detection Kit, VIASURE Yersinia enterocolitica Real-Time PCR Detection Kit, VIASURE Salmonella, Campylobacter & Y.enterocolitica Real-Time PCR Detection Kit (CerTest Biotec S.L., Zaragoza, Spain), and RIDA®GENE Bacterial Stool Panel (R-Biopharm, Darmstadt, Germany).
The targets of the three monoplex and one multiplex VIASURE assays are the invA gene (Salmonella), 16S rRNA gene (Campylobacter), and ail gene (Yersinia enterocolitica), whereas RIDA®GENE Bacterial Stool Panel allows the simultaneous detection of ttr gene (Salmonella), 16S rRNA gene (Campylobacter), and ail gene (Yersinia enterocolitica). All Real-Time PCR assays were performed following the manufacturer’s protocol. Positive and negative controls were included in each run. All runs were performed on the AriaMx thermocycler (Agilent Technologies, California, US).
Real-Time PCR resolution test
The Mericon Campylobacter spp kit (Qiagen, Hilden, Germany) was used for resolving Campylobacter discrepant samples. This Real-Time PCR assay was performed according to the manufacturer’s protocol.
Conventional PCR
Conventional PCR was performed with the VIASURE ESSENTIALS DNA Master Mix kit (CerTest Biotec S.L., Zaragoza, Spain), according to the manufacturer’s instructions. The kit contained all necessary PCR reagents lyophilized in the wells, except for the primers, which were customizable, depending on the target. The primers used for detecting Salmonella (hilA gene) and Campylobacter (16S rRNA gene) were described previously12,13; we used 1 µM of each primer. We modified Salmonella primers by deleting the first nucleotides; the following forward (5′-ATTTGCGCCATGCTGAGGTAG-3′) and reverse (5′- CCGCCGGCGAGATTGTG-3′) primers were used.
PCR products were separated on a 1.0% agarose gel by electrophoresis.
Sequencing
The same conventional PCR primers were used for sequencing. Sequencing was performed by the General Research Support Service at the Health Research Institute of Aragon (IIS Aragon) and the University of Zaragoza. Sequencing was performed with the capillary sequencer, 3500 XL (Applied Biosystems, California, US). Sequences were analyzed with Chromas software, and they were identified with the basic local alignment search tool (BLAST; http://www.ncbi.nlm.nih.gov/BLAST/).
Statistical analysis
We compared the tests under study in terms of the positive percent agreement (PPA) and negative percent agreement (NPA), instead of sensitivity and specificity. This approach conforms to FDA recommendations14, which suggest that sensitivity and specificity should only be used when the comparator method is a reference standard. In this study, we did not use the culture as the reference method. Instead, we used the “case” definition for the comparator method. Samples were designated “cases” when they were positive in the culture media, in the discrepant sample analysis, or at least in three qPCR assays.
The quantification cycle (Cq) value determines the initial copy number of the target. Samples with low target concentrations display high Cq values. A Cq value greater than 40 was considered negative.
All statistical analyses were performed with SPSS software (version 22; IBM Corp., Madrid, Spain), except for the 95% confidence intervals (CI), which were calculated with the modified Wald method, with GraphPad QuickCalcs. The Chi-square test was performed to determine correlations between enteropathogen infections and patient groups. A P-value <0.05 was considered statistically significant.
Results
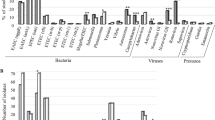
A total of 400 stool samples, acquired from 385 patients, were included in this study. Samples were grouped by stool consistency, and they were also classified according to the patient’s age group, gender, and hospitalization status. These data are shown in Table 1.
Among 400 stool samples, 98 (24.5%) met the case definition. Of these, 69 were found positive in culture media, 25 were discrepant samples that were resolved, and 5 were discrepant samples that were found positive at least in three qPCR assays. Although there were 98 cases, there were 99 positive results, because one sample corresponded to a co-infection by Campylobacter and Yersinia enterocolitica. A more detailed analysis is given in Table 2.
We found 24 cases positive for Salmonella enterica (11 S. Typhimurium, 6 S. Enteritidis, 1 S. Mbandaka, 1 S. Braenderup, 1 S. Paratyphi A, 3 S. serogroup G, and 1 Salmonella spp.); 71 cases positive for Campylobacter (39 C. jejuni, 7 C. concisus, 6 C. coli, 4 C. upsaliensis, 2 C. hyointestinalis, 1 C. gracilis, 1 C. helveticus, 1 C. curvus, 1 C. rectus, and 9 Campylobacter spp); and 4 cases positive for Yersinia enterocolitica O:3. The information of those Campylobacter species that were determined by 16 S rRNA sequencing is collected in Table 3.
We could detect most Salmonella positive samples with all methods. The routine clinical algorithm values were, as follows: PPA = 95.8%, CI = 78.1–99.9 and NPA = 100%, CI = 98.8–100. The VIASURE and R-Biopharm assays provided the same level of results. All gave a PPA = 87.5%, CI = 68.2–96.5 and a NPA = 100%, CI = 98.8–100.
Nine different species of Campylobacter were found. All of these were detected with the VIASURE monoplex assay (PPA = 100%, CI = 93.9–100; NPA = 100%, CI = 98.6–100); the next most accurate assays, in descending order were: the VIASURE multiplex (PPA = 95.8%, CI = 87.8–99.0; NPA = 100%, CI = 98.6–100); the R-Biopharm (PPA = 81.7%, CI = 71.0–89.1; NPA = 100%, CI = 98.6–100), and finally, the routine clinical algorithm (PPA = 59.2%, CI = 47.5–69.8; NPA = 100%, CI = 98.6–100).
The four Yersinia enterocolitica O:3 positive samples could be detected with all methods studied (PPA = 100%, CI = 45.4–100; NPA = 100%, CI = 98.8–100).
No false positive results were found in this evaluation; consequently, all NPA values were 100%. Moreover, no inhibition occurred in any qPCR reaction; therefore, it was not necessary to re-test any sample.
Samples were grouped according to the Cq value in three categories: Cq <25 (high copy samples), Cq = 25–35 (medium copy samples) and Cq >35 (low copy samples, near the detection limit). They were also classified into two groups: positive by culture and negative by culture. The minimum and maximum Cq values were calculated for each group. These data are shown in Table 4. We excluded Yersinia enterocolitica samples that were negative by culture as none of them were positive by qPCR assays.
We also evaluated whether some factors (age, hospitalization status, and stool sample consistency) might be positively associated with infections by these three enteropathogens. We performed a Chi-square test, but we did not find any statistically significant link between enteropathogen infections and the age group (P = 0.199), hospitalization status (P = 0.556), or stool sample consistency (P = 0.073). Hence, we can only report trends. When analyzed according to age, most patients affected seem to be the youngest; infection rates were 32.2% in pre-school children; 30.6% in infants; 23.1% in school-age children and adolescents; and 20.9% in adults. When analyzed according to hospitalization status, infection rates were higher among patients that required hospitalization (28.0%), compared to those attended in outpatient clinics (25.1%) and in the emergency department (18.2%). Finally, when analyzed according to sample consistency, infections were found most frequently in stools with mucous (43.8%), liquid (38.2%), and crumbly (35.7%) consistencies and in stools with traces of blood (28.6%).
Discussion
This study aimed to validate the current routine clinical diagnosis method for identifying the three main bacterial enteropathogens: Salmonella, Campylobacter, and Yersinia enterocolitica. We compared the standard culture-based techniques to three types of qPCR assays (VIASURE monoplex, VIASURE multiplex, and R-Biopharm).
In recent years, a large number of studies have been conducted to validate novel gastrointestinal molecular panels as BD Max enteric bacterial panel4, ProGastro SSCS assay7, Allplex GI-Bacteria assay8 or FilmArray GI Panel5,15. These previous studies emphasized the importance of not considering the culture technique as the reference method, due to its lack of sensitivity. Consequently, we created a reference method based on different techniques. According to this reference, samples were classified as “cases” or “non-cases” (see Materials and Methods section).
All methods showed acceptable PPA values (87.5%–95.8%) for identifying Salmonella spp. The highest PPA value was obtained with the routine clinical algorithm. This could be explained by the fact that stool samples were inoculated into a selenite enrichment broth, as reported in previous studies16. This broth contributed to bacterial replication, which enhanced the culture method. The culture method was able to detect samples that showed Cq values extremely near the detection limit (Cq > 35).The maximum Cq values obtained in Salmonella identification were 39.7, 35.8, and 39.6 with VIASURE monoplex, VIASURE multiplex, and R-Biopharm assays, respectively (Table 4). This observation revealed the high sensitivity that could be achieved with the culture method, due to the enrichment in selenite broth. We performed qPCR assays to identify Salmonella in two selenite broths whose stool sample results were negative with qPCR. As we suspected, the Salmonella DNA could be detected in the selenite broth with qPCR assays but could not be detected in the stool sample. The benefits of the Real-Time PCR in combination with the enrichment in selenite broth have been recently reported. Boer et al.17 found that the number of Salmonella positive samples increased by 2.2% when qPCR was performed after enrichment in selenite broth. Tang et al.18 have also validated the high sensitivity and specificity of a national standard protocol based on Real-Time PCR combined with guided culture.
Relative to Campylobacter, the qPCR assays exhibited notable differences in their abilities to detect many Campylobacter species. The highlight was the high capacities of the VIASURE monoplex and multiplex assays (PPA = 100% and PPA = 95.8%, respectively) in diagnosing the majority of Campylobacter species that are considered pathogenic in humans19.
The R-Biopharm test displayed less diagnostic capacity (PPA = 81.7%). It seems to be due to a failure in the recognition of less common Campylobacter species (other than C.jejuni and C.coli) rather than in the sensitivity of the test, since most of the samples were in high concentration (Cq < 25). The R-Biopharm assay could not detect any positive sample for C. upsaliensis, C. hyointestinalis, C. helveticus, or C. rectus (Table 2).
Only 3/13 samples that R-Biopharm missed were near the detection limit (Cq > 35) (1 C. concisus, 1 C. rectus and 1 Campylobacter spp.); 3/13 showed a Cq = 25–35 (1 C. concisus, 1 C. helveticus and 1 C. jejuni) and 7/13 samples showed a Cq < 25 (4 C. upsaliensis, 2 C. hyointestinalis and 1 C. coli) (Tables 2, 4).
The routine clinical algorithm was only capable of detecting C. jejuni and C. coli species (PPA = 59.2%) (Table 2). This finding was consistent with the literature, where different studies20,21,22 indicated that some antimicrobial agents incorporated into the commonly used selective media (like cefoperazone in CCDA media) could inhibit the growth of several species, including C. hyointestinalis, C. upsaliensis, and C. fetus. Moreover, not all Campylobacter species are thermophilic; consequently, the incubation at 42 °C was not suitable for all species. Additionally, some species, like C. concisus, C. rectus, C. curvus, C. gracilis, and C. showae require a special atmosphere enriched with hydrogen.
The culture technique failed to detect 3 C. jejuni and 3 C. coli samples (Table 2). None of them were low copy samples since 4/6 showed values of Cq < 25 and 2/6 showed a Cq value = 25–35. This observation revealed that sometimes even the growth of C. jejuni and C. coli in selective media could be complicated.
For the above reasons, the qPCR technique appears to be a reliable solution for Campylobacter identification since it could ensure the identification of most Campylobacter species without excessive complications in the diagnostic algorithm.
Regarding Yersinia enterocolitica, all the methods studied could detect all samples positive for Yersinia enterocolitica O:3 (PPA = 100%).
An important aspect of this study was that no false positive result was found in any qPCR assay. This high specificity (100%) could represent a key difference between molecular-based and antigen-based CIDTs. Couturier10 stated that the problem with stool antigen-based CIDTs was that they exhibited highly variable sensitivities and specificities. That problem has not occurred with molecular-based CIDTs, which, to date, have shown excellent sensitivity and specificity compared to cultures. Our findings correlate with those previous results; therefore, molecular CIDTs could be considered a reliable diagnostic tool, although more studies are needed. Additionally, some molecular CIDTs, like VIASURE assays, are very easy to use. All the components are provided lyophilized inside the wells, which implies that the user is not required to mix any reagents. Also, the lyophilized format facilitates the storage and shipping conditions as lyophilized reagents are stable at room temperature.
We did not encounter inhibition in any of the qPCR assays, although we tested a vast range of stool consistencies. This finding suggested that the qPCR assays used in this study are robust tests with a stable matrix formulation, which neutralized PCR inhibitor effects.
The main limitation of this study was that the number of positive samples depended on the prevalence of each pathogen in the study area. Consequently, we recommend that a multicenter study should be performed, and that the sample collection time should be extended.
We conclude that the three types of qPCR assays (VIASURE monoplex, VIASURE multiplex and R-Biopharm) tested could improve the routine clinical diagnosis algorithm, because they had a noticeable impact on the Campylobacter diagnosis. Additionally, they exhibited good performance in Salmonella and Yersinia enterocolitica identification too, and they substantially reduced the turnaround time (from days to hours). The ease of using molecular methods and the time they save will allow laboratories to increase the number of samples processed. Moreover, saving time is critical for avoiding the spread of infections and for providing effective treatment plans.
References
Troeger, C. et al. Estimates of global, regional, and national morbidity, mortality, and aetiologies of diarrhoeal diseases: a systematic analysis for the Global Burden of Disease Study 2015. The Lancet Infectious Diseases 17, 909–948, https://doi.org/10.1016/s1473-3099(17)30276-1 (2017).
Marder, E. P. et al. Incidence and Trends of Infections with Pathogens Transmitted Commonly Through Food and the Effect of Increasing Use of Culture-Independent Diagnostic Tests on Surveillance — Foodborne Diseases Active Surveillance Network, 10 U.S. Sites, 2013–2016. MMWR. Morbidity and Mortality Weekly Report 66, 397–403, https://doi.org/10.15585/mmwr.mm6615a1 (2017).
Cronquist, A. B. et al. Impacts of culture-independent diagnostic practices on public health surveillance for bacterial enteric pathogens. Clin Infect Dis 54(Suppl 5), S432–439, https://doi.org/10.1093/cid/cis267 (2012).
Harrington, S. M. et al. Multicenter evaluation of the BD max enteric bacterial panel PCR assay for rapid detection of Salmonella spp., Shigella spp., Campylobacter spp. (C. jejuni and C. coli), and Shiga toxin 1 and 2 genes. J Clin Microbiol 53, 1639–1647, https://doi.org/10.1128/JCM.03480-14 (2015).
Buss, S. N. et al. Multicenter evaluation of the BioFire FilmArray gastrointestinal panel for etiologic diagnosis of infectious gastroenteritis. J Clin Microbiol 53, 915–925, https://doi.org/10.1128/JCM.02674-14 (2015).
Navidad, J. F., Griswold, D. J., Gradus, M. S. & Bhattacharyya, S. Evaluation of Luminex xTAG gastrointestinal pathogen analyte-specific reagents for high-throughput, simultaneous detection of bacteria, viruses, and parasites of clinical and public health importance. J Clin Microbiol 51, 3018–3024, https://doi.org/10.1128/JCM.00896-13 (2013).
Buchan, B. W. et al. Clinical evaluation of a real-time PCR assay for identification of Salmonella, Shigella, Campylobacter (Campylobacter jejuni and C. coli), and shiga toxin-producing Escherichia coli isolates in stool specimens. J Clin Microbiol 51, 4001–4007, https://doi.org/10.1128/JCM.02056-13 (2013).
Martin, A., Perez-Ayala, A., Chaves, F., Lora, D. & Orellana, M. A. Evaluation of the multiplex PCR Allplex-GI assay in the detection of bacterial pathogens in diarrheic stool samples. J Microbiol Methods 144, 33–36, https://doi.org/10.1016/j.mimet.2017.10.016 (2018).
Fitzgerald, C. et al. Multicenter Evaluation of Clinical Diagnostic Methods for Detection and Isolation of Campylobacter spp. from Stool. J Clin Microbiol 54, 1209–1215, https://doi.org/10.1128/JCM.01925-15 (2016).
Couturier, M. R. Revisiting the Roles of Culture and Culture-Independent Detection Tests for Campylobacter. J Clin Microbiol 54, 1186–1188, https://doi.org/10.1128/JCM.03221-15 (2016).
Singhal, N., Kumar, M., Kanaujia, P. K. & Virdi, J. S. MALDI-TOF mass spectrometry: an emerging technology for microbial identification and diagnosis. Front Microbiol 6, 791, https://doi.org/10.3389/fmicb.2015.00791 (2015).
Pathmanathan, S. G. et al. Simple and rapid detection of Salmonella strains by direct PCR amplification of the hilA gene. J Med Microbiol 52, 773–776, https://doi.org/10.1099/jmm.0.05188-0 (2003).
Platts-Mills, J. A. et al. Detection of Campylobacter in stool and determination of significance by culture, enzyme immunoassay, and PCR in developing countries. J Clin Microbiol 52, 1074–1080, https://doi.org/10.1128/JCM.02935-13 (2014).
Administration, U. F. a. D. Guidance for industry and FDA staff. Statistical Guidance on reporting results from studies evaluating diagnostic tests. US Department of Health and Human Services, FDA, Silver Spring, MD. (2007).
Calderaro, A. et al. Contribution of the FilmArray((R)) Gastrointestinal Panel in the laboratory diagnosis of gastroenteritis in a cohort of children: a two-year prospective study. Int J Med Microbiol 308, 514–521, https://doi.org/10.1016/j.ijmm.2018.04.007 (2018).
Cunningham, S. A. et al. Three-hour molecular detection of Campylobacter, Salmonella, Yersinia, and Shigella species in feces with accuracy as high as that of culture. J Clin Microbiol 48, 2929–2933, https://doi.org/10.1128/JCM.00339-10 (2010).
Boer, M. D. et al. Selenite enrichment broth to improve the sensitivity in molecular diagnostics of Salmonella. J Microbiol Methods 157, 59–64, https://doi.org/10.1016/j.mimet.2018.12.018 (2019).
Tang, X. J. et al. Verification and large scale clinical evaluation of a national standard protocol for Salmonella spp./Shigella spp. screening using real-time PCR combined with guided culture. J Microbiol Methods 145, 14–19, https://doi.org/10.1016/j.mimet.2017.12.007 (2018).
Man, S. M. The clinical importance of emerging Campylobacter species. Nat Rev Gastroenterol Hepatol 8, 669–685, https://doi.org/10.1038/nrgastro.2011.191 (2011).
Kulkarni, S. P. et al. Detection of campylobacter species: a comparison of culture and polymerase chain reaction based methods. J Clin Pathol 55, 749–753 (2002).
Lawson, A. J., Linton, D., Stanley, J. & Owen, R. J. Polymerase chain reaction detection and speciation of Campylobacter upsaliensis and C. helveticus in human faeces and comparison with culture techniques. J Appl Microbiol 83, 375–380 (1997).
Maher, M. et al. Evaluation of culture methods and a DNA probe-based PCR assay for detection of Campylobacter species in clinical specimens of feces. J Clin Microbiol 41, 2980–2986 (2003).
Acknowledgements
We acknowledge the technical personnel for their microbiology service at the Hospital Clinico Universitario Lozano Blesa (Spain). CerTest Biotec S.L. provided the materials, kits, and laboratory instruments. This study was also supported by a fellowship from the cluster “AraBioTech”, mediated by the institution “Fundación Empresa Universidad de Zaragoza (FEUZ)”. S. Valledor and I. Valledor were awarded this fellowship in 2015 and 2016, respectively. Now, they and MC Gil-Rodríguez work at Certest Biotec S.L. This work was partially supported by the Departamento de Ciencia, Tecnología, and Universidad from the Gobierno de Aragón, Spain (Project DGA-European Social Fund (FSE)/Grupos consolidados, B13–17R. (“Investing in your future”).
Author information
Authors and Affiliations
Contributions
S. Valledor and I. Valledor collected the data and performed the analysis. S. Valledor also wrote the article with input from all authors. M.C. Gil-Rodríguez conceived and designed the analysis. C. Seral and J. Castillo performed the routine clinical methods and were involved in planning and supervised the work. All authors provided critical feedback and helped shape the research, analysis and manuscript.
Corresponding author
Ethics declarations
Competing interests
The other authors have no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Valledor, S., Valledor, I., Gil-Rodríguez, M.C. et al. Comparison of several Real-Time PCR Kits versus a Culture-dependent Algorithm to Identify Enteropathogens in Stool Samples. Sci Rep 10, 4301 (2020). https://doi.org/10.1038/s41598-020-61202-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-61202-z
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.