Abstract
Hearing loss becomes increasingly common with age and affects quality of life. Recently, scientists have published articles about the relationship between metabolic disease and hearing loss. Metabolic disease was previously found to be highly related to an increase in alkaline phosphatase. Thus, there may be an indirect relationship between alkaline phosphatase (ALP) and hearing loss. In this paper, we will demonstrate the relationship between ALP and hearing loss. We included 3877 National Health and Nutrition Examination Survey (NHANES) participants, who represent the noninstitutionalized civilian population in the United States from age 20 to age 69, and examined the association between ALP and frequency distributions of pure-tone air-condition (PTAC) thresholds. After adjusting for pertinent variables, the subjects who belonged to the higher quartiles of ALP tended to have worse hearing thresholds (pure tone average at high and low frequencies) than the first quartile of ALP (p < 0.001). The results showed a positive correlation between ALP and hearing loss, in both males and females (p < 0.001) and in subjects whose body mass indices (BMI) were less than 30 (p < 0.001). In conclusion, ALP may play a role in detecting hearing loss.
Similar content being viewed by others
Introduction
The United States government has paid more attention to auditory healthcare recently due to the prevalence of adult hearing loss between ages 20 and 69. Approximately 37.5 million of American adults aged 18 and over have suffered hearing loss in the national health interview survey in year 20121. About 28.8 million of them could benefit from wearing hearing devices2,3. However, only approximately 16 percent of adult aged 20–69 have ever used the hearing devices, based on the manufacturers’ voluntary reports of registered devices to the U.S. Food and Drug Administration, 2012. Therefore, strategy to postpone the onset of hearing loss is needed in order to improve quality of life and reduce medical expenses4. There are a myriad of intrinsic and extrinsic factors concerning hearing loss issues, such as age5,6, gene polymorphism5,6, noise and digital music exposure7, poor nutrition and lifestyle6, current ototoxic drugs used5,6, and secondhand smoke8. In addition to these factors, over 50% of patients with hearing impairment have previously unrecognized underlying diseases, such as hypertension9, diabetes mellitus (DM)10, metabolic syndrome11, osteoporosis12,13 and Paget disease14.
Osteoporosis is a metabolic disease that raises alkaline phosphatase (ALP) levels. ALP, a homodimeric enzyme of 86 kilodaltons, plays a vital role in metabolism within the liver and bone. Clinicians use bisphosphonates to reduce the level of ALP, thus preventing a further decrease in bone mineral density. In addition, bisphosphonates also lower the level of ALP in Paget disease15. Recently, researchers have noticed asymptomatic elevated ALP values in normal individuals14. In patients with chronic kidney disease, elevated serum ALP levels affect the inflammatory responses and are directly associated with erythropoietin stimulating agent-resistant anemia16. A chronically elevated ALP level in a normal person may imply presence of a disease, including hearing dysfunction.
Due to the lack of literature concerning the relationship between ALP and frequency distributions of pure-tone air-conduction (PTAC) thresholds to date, our study was designed to provide insight into this issue. Therefore, we investigated the association between ALP and hearing loss using the National Health and Nutrition Examination Survey (NHANES) data from the year 1999 to 2004.
Results
Characteristics of the study population
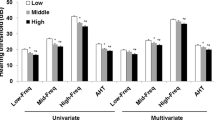
We included 3877 NHANES participants who represented noninstitutionalized civilian population. The characteristics of the study population are presented in Table 1. Our subjects had a mean age of 39.21 years old (SD = 12.4) and 46.4% of the population was male. Among subjects with higher quartiles of ALP, body mass index (BMI) and uric acid were significantly higher (P < 0.001). DM status was significantly higher in the fourth quartile of ALP (P < 0.001). Figure 1A,B showed aged between 50–80 years old have >25 dB HL (mild hearing loss) in high and low pure-tone thresholds. Figure 1C,D showed non-Hispanic white has >16 dB HL (slight hearing loss) in high pure-tone threshold. Hearing loss continues to be common in older, non-Hispanic whites according to the NHANES data.
Gender difference in the association of ALP and hearing thresholds
Table 2 shows the positive correlation between ALP and pure tone average at high frequencies (high-PTA) in both men and women. After additional adjustment of models 1, 2 and 3, beta coefficients for ALP and high-PTA were 0.005, 0.005, and 0.005 respectively in men and 0.003, 0.004, and 0.004, respectively, in women (P for trend <0.001). In terms of pure tone average at low frequencies (low-PTA), both men and women showed a positive correlation with ALP. The beta coefficients were 0.004, 0.004, and 0.003 (P for trend <0.001) respectively in men and 0.004, 0.004, and 0.004 (P for trend <0.001) respectively in women.
BMI and the association between ALP and hearing thresholds
Table 2 shows a positive correlation between BMI and high-PTA after additional adjustment of three models. Beta coefficients for ALP and high-PTA were 0.004, 0.004, and 0.004 in BMI<30, and in for BMI>30 (P for trend <0.001). In terms of low-PTA, only BMI<30 was related to ALP, and the beta coefficients were 0.004, 0.004, and 0.004 (P for trend <0.001).
The relationship between different quartiles of ALP and hearing thresholds
We performed a quartile-based analysis by dividing ALP into quartiles, as shown in Table 3. The subjects in the first quartile were set as our reference group. The trends showed significance in these three models regardless of high-PTA or low-PTA. Beta coefficients for Q4 versus Q1 at high-PTA were 0.039 in model 1, −0.040 in model 2 and 0.038 in model 3 (P for trend <0.001). The beta coefficients for ALP comparing Q4 to Q1 at low-PTA were 0.050 in model 1, 0.049 in model 2 and 0.045 in model 3 (P for trend <0.001).
Discussion
By evaluating representative data for the US population, we discovered the fourth quartile of ALP was highly related to high-PTA when we weighed the differences between the first, second and third quartiles’ ALP levels. This connection was found not only in obese (BMI>30) individuals but also in underweight, normal weight, and overweight (BMI<30) individuals.
Our results showed a worse hearing threshold in low-PTA was not only observed in women with BMI less than 30 but also in men. As far as we know, women who have low body mass indices are at risk for osteoporosis17. ALP has been found at higher levels in osteoporotic postmenopausal women due to high bone turnover rate12. In addition, high bone turnover states are known to raise plasma lead levels. A high turnover rates in women who had a higher bone lead level in Q2 and Q3 of ALP compared to Q1 in peri- and postmenopausal women18. Therefore, the association between high ALP and high BLL in postmenopausal women in their study corresponds to a worse hearing threshold of low-PTA in low BMI women in our study.
In recent years, scientists have recognized osteoporosis in men. Men suffer from osteoporotic fractures and have twice the 1-year fatality rate of women due to decreased lower body strength19. In addition, osteoporotic men trended to have lower free testosterone, higher levels of ALP and sex hormone-binding globulin than osteopenic and normal bone mineral density groups. This finding also supports the correlation between hearing loss and ALP in men, although the association is weak20.
Age-related hearing loss is one of the most common conditions affecting older adults. However, hearing loss is also found in younger people without evidence of hair cell loss or absence of auditory nerve synapses in their temporal bones7,21. The mechanism is related to the intense metabolic activity such as infection and inflammation, which drive the formation of free radicals and inflammatory cytokines, in both healthy and disease22,23,24,25. The elevated serum ALP is associated with C-reactive protein (CRP), which indicates ALP level may be a marker of low-grade chronic inflammation26. Chronic TNF-alpha release that enters the inner ear may cause bone conduction threshold elevation at the high frequencies27. In addition, otitis media-related inflammation also increased air conduction and bone conduction thresholds during childhood and adults stages28. While bacterial infection, eosinophilic inflammation, and chronic otitis media deteriorated bone conduction threshold29. The increases in stiffness and in mass of the ossicular chain affect bone conduction threshold shifts, which are less likely to accompany aging than are changes in air conduction thresholds30. The temporal bone houses the structures of the auditory system31. The malleus, incus, and stapes bones are the ossicles, which function to mechanically convert the vibrations into the inner ear. While ALP activity was detected in the ganglion cells and near the temporal bone31. Therefore, temporal bone and the ossicles, which contain the ALP activity in the ear, may take part in bone conduction threshold shift32.
The mechanism of hearing loss in regard to the increment of ALP remains an uncertainty. To date, ALP with reactive oxygen species (ROS), inflammation, and insulin resistance are possible mechanisms of hearing loss, subsequent to the disturbance of microcirculation in the cochlea. Scientist have established the role of the ROS signaling pathway in osteoblast (OB) differentiation. They demonstrated that the activation of NADPH oxidase was the key reaction to initiate ALP expression. The ROS system therefore produces oxidative stress and induces mitochondrial morphological transition33. In addition, vascular calcification raised ALP concentration asymptomatically. A statistically significant correlation between insulin resistance and serum bone ALP levels in vascular calcification, and ALP can be a predictor of cardiovascular events and mortality in patients with DM34. Moreover, ALP is capable of dephosphorylating nucleotide phosphatase and lipopolysaccharide to reduce inflammation and coagulopathy, as it shares a similar amino acid sequence with von Willebrand factor and collagen35. The positive correlation between inflammation and ALP level mostly presents in healthy elderly people with unrecognized Paget’s disease5, osteoporosis12,13,17, and presbycusis5. The sensorineural hearing loss in Paget disease includes a variety of cochlear lesions36 and inferior cochlear vein occlusion that lead to pathologic changes in the stria vascularis and spiral ligament37. Based on the findings of Nomura et al., ALP shows strong activity in spiral ligament and stria vascularis, and the microcirculatory disturbance in the stria prominence, causes the impaired function in hearing38. As previously mentioned, the increased in ALP was parallel to the hearing loss due to the evidence of ALP activity in the temporal bone and cochlea.
Our study had a few limitations. First, the cross-sectional study design with data captured from a single point in time for ALP and hearing loss was a limitation of our study design compared to hearing threshold shift in longitudinal study designs. Second, the self-reported questionnaire of inherited genetic defects and past medical history may have introduced recall bias. Third, the NHANES data only provided the current use of ototoxic medication, instead of hormonal drugs, antidepressants, and anti-inflammatory medicine.
Conclusion
We concluded that a positive relationship between bone ALP and hearing loss does exist for the general population. For an individual with a BMI<30 and hearing that has remained within the normal range, bone ALP levels seem to be a tool for detecting hearing loss. Because the causal mechanism associated ALP and hearing loss remains unclear, further research should be carried out in order to achieve a better understanding of the underlying pathophysiology of hearing loss.
Methods
Ethics statement
The NHANES data were adequately secured and approved by the National Center for Health Statistics Institutional Review Board. Informed consents for all eligible subjects were obtained before the start of a series of surveys with physical and laboratory examination. All methods were performed in accordance with the relevant guidelines and regulations of NHANES.
Study population
We included 3877 NHANES participants from ages 20 to 69 who represented the noninstitutionalized civilian population in the United States. This population were enrolled from NHANES from the period between 1999 and 2004 and had undergone a series of household interview surveys, physical examinations, and laboratory tests at a mobile examination center (MEC).
Audiometric measurements
Half of our target populations had undergone audiometric testing randomly after a series of surveys with physical and laboratory tests. Subjects who could not tolerate auditory headphones in MEC sound-isolated rooms were excluded. The test was performed by technicians who had received training from the National Institute for Occupational Safety and Health. They used an AD226 audiometer, TDH-39P headphones and EARTone 3 A earphones for both ears and recorded threshold values from −10 dB to 120 dB. According to the American Speech Language Hearing Association (ASHA), the normal range for hearing thresholds is −10 to 15 dB and the abnormal hearing thresholds (worse ear) is ≥26 dB. The frequency distributions of PTAC thresholds is 0.25–8 Hz39. We averaged the thresholds at 3,000, 4,000, 6,000, and 8,000 Hz for the high-PTA while the thresholds at 500, 1,000, and 2,000 Hz were included in the low-PTA, according to NHANES data40. In the regression model, we chose the pure-tone thresholds of the worse ear for analysis.
Covariates
We collected age, sex and race (non-Hispanic black, non-Hispanic white or other) as our demographic data. BMI was calculated as weight divided by the square of the height (kg/m2). BMI>30 was referred to as obese. The self-reported questionnaire contained questions about present medical illnesses that had ever been diagnosed by a physician, such as DM, heart disease and stroke, and the current use of ototoxic drugs.
An otoscopic screening examination was used to determine the abnormality of the outer canal of the ear. In addition, tympanometry was abnormal if middle-ear peak pressure showed a value lower than −150 daPa or the compliance was lower than 0.3 ml. The Hitachi analyzer was used to determine biochemical data, such as uric acid and LDL-C. All measurements were standardized according to the guidelines that were recommended by the Centers for Disease Control and Prevention (CDC).
Statistical analysis
The study used sample weights to account for the complex sampling design and to allow approximations of the United States population, following guidelines of National Center for Health Statistics. The NHANES 1999 to 2004 sample weights adjusted for the differential probabilities of selection and nonresponse in the survey sample. The weight represented the number of individuals in the target population each sample participant was estimated to represent41,42. We used the Statistical Package for the Social Sciences version 18.0 to analyze our data. We set two-sided alpha values, with values less than 0.05 as our significant p-values. The dependent variables were the normalized hearing thresholds (high-PTA and low-PTA), which were transformed using the logarithm function. Moreover, we further divided the serum ALP into quartiles as follows: Q1 <10.6 ug/L, 10.6 ug/L ≤ Q2 <13.8 ug/L, 13.8 ug/L ≤Q3 <18.2 ug/L and Q4 ≥ 18.2 ug/L. In the multivariate regression models, we adjusted for age, gender and race for model 1; age, gender, race, BMI, LDL-C and uric acid for model 2; and age, gender, race, BMI, LDL-C, uric acid, stroke, tympanometry status, ear condition, DM status, histories of heart disease, and ototoxic status for model 3.
References
Blackwell, D. L., Lucas, J. W. & Clarke, T. C. Summary health statistics for US adults: national health interview survey, 2012. Vital and health statistics. Series 10, Data from the National Health Survey, 1–161 (2014).
Tak, S. & Calvert, G. M. Hearing difficulty attributable to employment by industry and occupation: an analysis of the National Health Interview Survey—United States, 1997 to 2003. Journal of Occupational and Environmental Medicine 50, 46–56 (2008).
Agrawal, Y., Platz, E. A. & Niparko, J. K. Prevalence of hearing loss and differences by demographic characteristics among US adults: data from the National Health and Nutrition Examination Survey, 1999–2004. Archives of internal medicine 168, 1522–1530 (2008).
Hoffman, H. J., Dobie, R. A., Losonczy, K. G., Themann, C. L. & Flamme, G. A. Declining Prevalence of Hearing Loss in US Adults Aged 20 to 69 Years. JAMA otolaryngology–head & neck surgery 143, 274–285, https://doi.org/10.1001/jamaoto.2016.3527 (2017).
Gates, G. A. & Mills, J. H. Presbycusis. The Lancet 366, 1111–1120, https://doi.org/10.1016/S0140-6736(05)67423-5 (2005).
Yamasoba, T. et al. Current concepts in age-related hearing loss: epidemiology and mechanistic pathways. Hearing research 303, 30–38, https://doi.org/10.1016/j.heares.2013.01.021 (2013).
Le Prell, C. G. et al. Digital music exposure reliably induces temporary threshold shift (TTS) in normal hearing human subjects. Ear and hearing 33, e44–e58, https://doi.org/10.1097/AUD.0b013e31825f9d89 (2012).
Lin, Y.-Y. et al. Secondhand Smoke is Associated with Hearing Threshold Shifts in Obese Adults. Scientific Reports 6, 33071, https://doi.org/10.1038/srep33071 (2016).
de Moraes Marchiori, L. L., de Almeida Rego Filho, E. & Matsuo, T. Hypertension as a factor associated with hearing loss. Brazilian journal of otorhinolaryngology 72, 533–540 (2006).
Mitchell, P. et al. Relationship of Type 2 diabetes to the prevalence, incidence and progression of age-related hearing loss. Diabetic medicine: a journal of the British Diabetic Association 26, 483–488, https://doi.org/10.1111/j.1464-5491.2009.02710.x (2009).
Sun, Y. S. et al. Components of Metabolic Syndrome as Risk Factors for Hearing Threshold Shifts. PloS one 10, e0134388, https://doi.org/10.1371/journal.pone.0134388 (2015).
Jung, D. J., Cho, H. H. & Lee, K. Y. Association of Bone Mineral Density With Hearing Impairment in Postmenopausal Women in Korea. Clinical and experimental otorhinolaryngology 9, 319–325, https://doi.org/10.21053/ceo.2015.01858 (2016).
Lee, S.-S., Han, K.-d & Joo, Y.-H. Association between low bone mineral density and hearing impairment in postmenopausal women: the Korean National Health and Nutrition Examination Survey. BMJ Open 8, e018763, https://doi.org/10.1136/bmjopen-2017-018763 (2018).
Lim, D. P. & Stephens, S. D. Clinical investigation of hearing loss in the elderly. Clinical otolaryngology and allied sciences 16, 288–293 (1991).
Nishida, Y. et al. Midterm outcome of risedronate therapy for patients with Paget’s disease of bone in the central part of Japan. Clin Rheumatol 32, 241–245, https://doi.org/10.1007/s10067-012-2109-y (2013).
Badve, S. V. et al. Association between serum alkaline phosphatase and primary resistance to erythropoiesis stimulating agents in chronic kidney disease: a secondary analysis of the HERO trial. Canadian journal of kidney health and disease 2, 33, https://doi.org/10.1186/s40697-015-0066-5 (2015).
Ravn, P. et al. Low body mass index is an important risk factor for low bone mass and increased bone loss in early postmenopausal women. Early Postmenopausal Intervention Cohort (EPIC) study group. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research 14, 1622–1627, https://doi.org/10.1359/jbmr.1999.14.9.1622 (1999).
Jackson, L. W., Cromer, B. A. & Panneerselvamm, A. Association between bone turnover, micronutrient intake, and blood lead levels in pre- and postmenopausal women, NHANES 1999–2002. Environmental health perspectives 118, 1590–1596, https://doi.org/10.1289/ehp.1002158 (2010).
Adler, R. A. Osteoporosis in men: a review. Bone research 2, 14001–14001, https://doi.org/10.1038/boneres.2014.1 (2014).
Zha, X. Y. et al. Sex hormone-binding globulin (SHBG) as an independent determinant of bone mineral density (BMD) among Chinese middle-aged and elderly men. Endocrine 47, 590–597, https://doi.org/10.1007/s12020-013-0155-0 (2014).
Makary, C. A., Shin, J., Kujawa, S. G., Liberman, M. C. & Merchant, S. N. Age-related primary cochlear neuronal degeneration in human temporal bones. Journal of the Association for Research in Otolaryngology: JARO 12, 711–717, https://doi.org/10.1007/s10162-011-0283-2 (2011).
Halliwell, B. & Gutteridge, J. M. Free radicals in biology and medicine. (Oxford University Press, USA, 2015).
Evans, P. & Halliwell, B. Free radicals and hearing: cause, consequence, and criteria. Annals of the New York Academy of Sciences 884, 19–40 (1999).
Campbell, K. Ototoxicity: understanding oxidative mechanisms. Journal of the American Academy of Audiology 14, 121 (2003).
Adams, J. C. Clinical implications of inflammatory cytokines in the cochlea: a technical note. Otology & neurotology 23, 316–322 (2002).
Kerner, A. et al. Association between elevated liver enzymes and C-reactive protein: possible hepatic contribution to systemic inflammation in the metabolic syndrome. Arteriosclerosis, thrombosis, and vascular biology 25, 193–197 (2005).
Sziklai, I., Batta, T. J. & Karosi, T. Otosclerosis: an organ-specific inflammatory disease with sensorineural hearing loss. European Archives of Oto-Rhino-Laryngology 266, 1711–1718 (2009).
Guo, J. et al. Clinical analysis of otitis media with effusion in adults and children. Lin chuang er bi yan hou tou jing wai ke za zhi= Journal of clinical otorhinolaryngology, head, and neck surgery 21, 13–15 (2007).
Iino, Y. et al. Eosinophilic inflammation in the middle ear induces deterioration of bone-conduction hearing level in patients with eosinophilic otitis media. Otology & Neurotology 31, 100–104 (2010).
Nixon, J. C., Glorig, A. & High, W. S. Changes in air and bone conduction thresholds as a function of age. The Journal of laryngology and otology 76, 288–298 (1962).
Hiraide, F. & Inouye, T. Alkaline phosphatase activity in the ganglion cells in and near the temporal bone. Archives of oto-rhino-laryngology 222, 285–294, https://doi.org/10.1007/BF01261175 (1979).
Firestein, G. S., Budd, R., Gabriel, S. E., McInnes, I. B. & O’Dell, J. R. Kelley and Firestein’s Textbook of Rheumatology E-Book. (Elsevier Health Sciences, 2016).
Arakaki, N., Yamashita, A., Niimi, S. & Yamazaki, T. Involvement of reactive oxygen species in osteoblastic differentiation of MC3T3-E1 cells accompanied by mitochondrial morphological dynamics. Biomedical research (Tokyo, Japan) 34, 161–166 (2013).
Cheung, C. L. & Cheung, B. M. Y. Bone-specific alkaline phosphatase is elevated in insulin resistance: implications for vascular calcification in diabetes. European Heart Journal 34, P5473–P5473, https://doi.org/10.1093/eurheartj/eht310.P5473 (2013).
Pike, A. F., Kramer, N. I., Blaauboer, B. J., Seinen, W. & Brands, R. A novel hypothesis for an alkaline phosphatase ‘rescue’ mechanism in the hepatic acute phase immune response. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease 1832, 2044–2056, https://doi.org/10.1016/j.bbadis.2013.07.016 (2013).
Dimitriadis, P. A., Bamiou, D. E. & Bibas, A. G. Hearing loss in Paget’s disease: a temporal bone histopathology study. Otology & neurotology: official publication of the American Otological. Society, American Neurotology Society [and] European Academy of Otology and Neurotology 33, 142–146, https://doi.org/10.1097/MAO.0b013e318241c3bd (2012).
Teufert, K. B. & Linthicum, F. Jr. Paget disease and sensorineural hearing loss associated with spiral ligament degeneration. Otology & neurotology: official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology 26, 387–391; discussion 391 (2005).
Nomura, Y. & Hiraide, F. Cochlear blood vessel. A histochemical method of its demonstration. Arch Otolaryngol 88, 231–237 (1968).
Chau, J. K., Cho, J. J. & Fritz, D. K. Evidence-based practice: management of adult sensorineural hearing loss. Otolaryngologic Clinics of North America 45, 941–958 (2012).
Spankovich, C. & Le Prell, C. G. Healthy diets, healthy hearing: National Health and Nutrition Examination Survey, 1999–2002. International Journal of Audiology 52, 369–376, https://doi.org/10.3109/14992027.2013.780133 (2013).
McDowell, M. A. et al. Hair mercury levels in US children and women of childbearing age: reference range data from NHANES 1999–2000 112, 1165 (2004).
Merikangas, K. R. et al. Prevalence and treatment of mental disorders among US children in the 2001–2004 NHANES 125, 75–81 (2010).
Author information
Authors and Affiliations
Contributions
Zhu Wei Lim contributed to the design of the study, was responsible for the management and retrieval of data, contributed to initial data analysis and interpretation, and drafted the initial manuscript. Zhu Wei Lim and Wei-Liang Chen decided upon the data collection methods. Zhu Wei Lim and Wei-Liang Chen were also responsible for the data analysis decisions. Wei-Liang Chen conceptualized and designed the study, supervised all aspects of the study, critically reviewed and revised the manuscript, and approved the final manuscript as submitted. All authors meet the ICMJE criteria for authorship.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lim, Z.W., Chen, WL. Exploring the association of Bone Alkaline Phosphatases And Hearing Loss. Sci Rep 10, 4006 (2020). https://doi.org/10.1038/s41598-020-60979-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-60979-3
This article is cited by
-
Exploring the association of organochlorine pesticides exposure and hearing impairment in United States adults
Scientific Reports (2022)
-
Adhesion-enhancing coating embedded with osteogenesis-promoting PDA/HA nanoparticles for peri-implant soft tissue sealing and osseointegration
Bio-Design and Manufacturing (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.