Abstract
In animal models of inflammation and in farm animals, dietary inclusion of spray-dried porcine plasma (SDP) reduces mucosal inflammation. Here, we study whether these effects could be mediated by changes in the intestinal microbiota and if these changes are similar to those induced by oral antibiotics. Weaned 21-day-old C57BL/6 mice were divided into 3 groups: the CTL group, fed the control diet; the COL group, administered low doses of neomycin and colistin; and the SDP group, supplemented with 8% SDP. After 14 days, analysis of the fecal microbiome showed that the microbiota profiles induced by SDP and the antibiotics were very different, thus, SDP has prebiotic rather than antibiotic effects. At the phylum level, SDP stimulated the presence of Firmicutes, considerably increasing the lactobacilli population. It also enhanced the growth of species involved in regulatory T-lymphocyte homeostasis and restoration of the mucosal barrier, as well as species negatively correlated with expression of pro-inflammatory cytokines. At the mucosal level, expression of toll-like receptors Tlr2, Tlr4 and Tlr9, and mucous-related genes Muc2 and Tff3 with regulatory and barrier stability functions, were increased. SDP also increased expression of Il-10 and Tgf-β, as well as markers of macrophages and dendritic cells eventually promoting an immune-tolerant environment.
Similar content being viewed by others
Introduction
Dietary plasma supplements obtained from porcine and bovine sources enhance growth in several animal species1,2,3,4. Such supplements are commonly used in animal husbandry because they reduce morbidity and mortality via mechanisms that involve activation of the immune system, with a special role for gut-associated lymphoid tissue (GALT) and regulation of mucosal barrier functions5. Studies in humans provide evidence that this kind of supplement can also improve the nutritional status and gastrointestinal symptoms in patients with enteropathy6,7.
The anti-inflammatory properties of spray-dried porcine plasma (SDP) have been studied in several rodent models, specifically, the model of mild intestinal inflammation induced by systemic administration of S. aureus enterotoxin B8, the model of acute lung inflammation induced by lipopolysaccharide inhalation9,10, the model of uterine mucosal inflammation induced by transport stress11, and a colitis model using knockout mice lacking the mdr1a gene that codifies for P-glycoprotein12,13. In all cases, the mucosal responses to the challenges showed a common pattern characterized by activation of mucosal lymphocyte populations, increasing the Tact/Treg ratio, and the secretion of pro-inflammatory cytokines. However, when animals were supplemented with 2%–8% SDP, these changes in the Tact/Treg ratio were prevented, the production of pro-inflammatory cytokines reduced and the secretion of anti-inflammatory cytokines increased8,9,10,11,12,14,15. This indicates that SDP can modulate the magnitude of inflammatory responses.
Moreover, the anti-inflammatory effects of SDP are observed if it is administered before, during or after the challenge. In models of acute inflammation, SDP was given before the challenge and the results therefore suggest that the supplement modulates receptors and regulatory pathways involved in the GALT immune responses, thereby promoting a tolerogenic profile that reduces the magnitude of the response. However, SDP is also effective with protocols that start feeding once the inflammation process has begun (as in the case of the colitis model) or even when the inflammatory response is fully established, as with the model of stress-induced mucosal uterine inflammation. This means that SDP is capable of modulating GALT both before (preventive effect) and during (therapeutic effect) inflammatory syndrome.
The first step in the anti-inflammatory cascade must take place at gut inductive sites, where SDP modulates GALT resulting in the generation of the appropriate immune responses that then spreads via the lymphatic and circulatory systems to distant mucosal lymphoid tissues such as the respiratory and genito-urinary tracts, as well as the gastrointestinal tract itself, behaving as effector sites16. The mechanism by which SDP modulates GALT at the inductive sites is not fully understood. The signals initiating the regulatory mechanisms may be functional SDP components, already present in the supplement or generated by its gastrointestinal digestion. This latter possibility has been shown to be the case for milk components17. Alternatively, they may be functional immunoglobulins in SDP, binding to luminal antigens and hence reducing the activity of luminal inflammatory stimuli, as suggested by Petschow et al.3 and Pérez-Bosque et al.5. Another possibility is that SDP modulates the intestinal commensal microbiota to promote probiotic species that stimulate cell receptors at inductive sites and eventually regulate mucosal lymphoid responses at the effector sites.
Some evidence suggests that SDP supplementation can modify the composition of the intestinal microbiota. In pigs, SDP stimulates the growth of lactobacilli in the ileum and cecum18, and decreases E. coli colonies in the small intestine19. Moreover, Che et al.20 have observed, in fecal samples of weaned piglets, that SDP reduces the abundance of Proteobacteria and increases Firmicutes, tripling the population of lactobacilli. In rats, SDP increased the presence of several species of Lactobacillus in the cecum21 while ovine serum immunoglobulins enriched lactobacilli and depleted enterobacteria22. Finally, Asmuth et al.23 reported that dietary bovine serum proteins decreased the population of fecal pro-inflammatory gamma-Proteobacteria in patients with enteropathy. All these results support the hypothesis that animal plasma supplements change the composition of the microbiota towards a probiotic profile with anti-inflammatory properties.
Since SDP is effective against bacterial toxins and viruses and protects against E. coli-induced inflammation in pigs24,25, its use has been suggested as an alternative to antibiotics26. Administration of low-dose antibiotics as growth promoters was a common practice in the animal farming industry2, and it is well documented that these practices alter intestinal microbiota and affect immune homeostasis27. To determine if the effects of SDP on microbiota composition and immune responses were compatible with those attributed to antibiotics, we also included an experimental group for comparing the effects of SDP supplementation with those of an antibiotic preparation containing neomycin and colistin, a combination previously widely used in farming as a growth promoter18.
The colonic microbiota is essential for immune homeostasis and participates in microbiota–mucosal crosstalk, cell–cell regulatory interactions, and the production of regulatory metabolites with mucosal and peripheral functions28. Here, we analyzed the composition of fecal microbiota, as an indicator of colonic microbiota composition, and, specifically in the colon mucosa, the levels of regulators of mucosal homeostasis and immune markers of inflammation. We also studied the expression of receptors directly involved in the regulatory mechanisms of probiotics such as membrane toll-like receptors (TLRs) and cytosolic nucleotide-binding oligomerization domain-containing protein (NOD) receptors. They are expressed in a wide variety of cell types, including intestinal epithelial enterocytes, subepithelial myofibroblasts and immune cell subsets, such as macrophages and dendritic cells29.
The experimental design was based on previous studies showing that animals supplemented with SDP for 14 days, starting at weaning, and then challenged with S. aureus Enterotoxin B8 or lipopolysaccharide9, had reduced intestinal and lung inflammatory responses, respectively. Therefore, the present study tested the hypothesis that SDP exerts preventive effects by changing the microbiota composition and consequently modulating the mucosal immune mechanisms toward a tolerogenic profile.
Results
Effects of antibiotics on microbiota composition
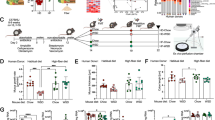
Since SDP is used as an alternative to antibiotics26, we analyzed the extent to which its effects on microbiota composition are comparable to those induced by the low doses of antibiotics. Figure 1A shows that neither SDP nor the neomycin/colistin preparation affected the Shannon (diversity) index; however, antibiotics reduced the total number of species (Fig. 1B) while SDP did not. At the phylum level (Fig. 1C), the effects on microbiota composition were very different as antibiotics enhanced the Bacteroidetes population and induced a dramatic reduction of Verrucobacteria; while these effects were not observed in the animals fed SDP (both q < 0.001). Moreover, SDP increased the Firmicutes-to-Bacteroidetes ratio, while antibiotic treatment reduced it (Fig. 1D). The effects of the antibiotic combination on families, genera, and species are shown in Supplementary Table 3 and Supplementary Fig. 1.
Microbial Shannon’s index (A), number of species (B), bacterial composition at phylum level (C) and ratio between Firmicutes and Bacteroidetes (D) in fecal microbiota. Samples were collected from mice fed a control diet (CTL) or a diet supplemented with 8% spray-dried porcine plasma (SDP) and mice fed control diet and treated with Coliphur (COL; daily dose: 25 mg/kg neomycin and 10 mg/kg colistin) for 14 days. Results are expressed as mean ± SEM (n = 9–10 mice). Statistical differences were considered significant at q < 0.05 (corrected p values).
Effects of dietary supplementation with SDP on fecal microbiota composition
In the CTL group, the dominant phyla were Bacteroidetes (33 ± 1.7%) followed by Firmicutes (25 ± 1.7%), Verrucobacteria (16 ± 1.5%), Proteobacteria (13 ± 1.5%) and Actinobacteria (10 ± 2.1%), with the remaining 3 ± 0.5% of the bacterial population dominated by the phylum Tenericutes (Fig. 1C). Dietary SDP increased the relative amount of Firmicutes up to 36.3 ± 2.0% (q < 0.001) and Bacteroidetes up to 37.1 ± 1.4% (q = 0.032), and this increased the Firmicutes-to-Bacteroidetes ratio from 0.77 to 0.98 (q = 0.026). Meanwhile, SDP decreased the population of Verrucobacteria to 11.3 ± 1.2% (q = 0.035) and Actinobacteria to only 2.6 ± 1.0% (q < 0.001). The principal coordinate analysis (PCoA) defined two different populations, as shown for the family level in Fig. 2; with the same finding repeated at other taxonomic levels.
Principal coordinate analysis (PCoA) plot of Illumina sequence data at family level, from fecal bacterial sequences from mice fed a control diet (CTL) or a diet supplemented with 8% spray-dried porcine plasma (SDP) for 14 days. The x- and y-axes are indicated by the first and second coordinates, respectively (n = 10 mice).
The effects of SDP supplementation at the family level are shown in Table 1. SDP increased Porphyriomonadaceae (q = 0.095) from the Bacteroidetes phylum, and Lactobacillaceae (q = 0.049) from the Firmicutes phylum. However, it considerably decreased the Bifidobacteriaceae population (q = 0.041) from the phylum Actinobacteria. Figure 3 shows the effects of SDP on the genera that are relevant from the functional point of view: five of them (Blautia, Lactobacillus, Pedobacter, Johnsonella and Pediococcus) were significantly stimulated, while Bifidobacterium was markedly inhibited (all q < 0.02, except Blautia and Johnsonella with q = 0.059 and q = 0.076, respectively). Figures 4 and 5 show the bacterial species whose growth was affected by SDP supplementation.
Effects of SDP on fecal microbial composition at genus level. Mice were fed a control diet (CTL) or a diet supplemented with 8% spray-dried porcine plasma (SDP) for 14 days. Results show the mean relative abundance ± SEM (n = 9–10 mice). Statistical differences were considered significant at q < 0.05 (corrected p values).
Effects of SDP on fecal microbial composition at species level. Mice were fed a control diet (CTL) or a diet supplemented with 8% spray-dried porcine plasma (SDP) for 14 days. Results are means ± SEM (n = 9–10 mice). Only significant effects on bacterial phylotypes that had >0.1% of the total sequence reads are shown. Statistical differences were considered significant at q < 0.05 (corrected p values).
Effects of SDP on fecal species of the genus Lactobacillus and Bifidobacterium. Mice were fed a control diet (CTL) or a diet supplemented with spray-dried porcine plasma (SDP) for 14 days. The figure shows all species that were modified by SDP feeding. Results are expressed as mean ± SEM (n = 9–10 mice). Statistical differences were considered significant at q < 0.05 (corrected p values).
Effects of dietary SDP on colonic mucosal receptors and immune regulators
The study of the expression of mucosal cytokines showed that SDP supplementation for 14 days stimulated the expression of the anti-inflammatory cytokines Il-10 and Tgf-β (q = 0.032 and q = 0.049, respectively); while expression of the pro-inflammatory Tnf-α was not affected (Fig. 6). Dietary SDP did not modify the abundance of the epithelial adhesion molecule E-cadherin or the junctional protein occludin (Supplementary Fig. 2) but did increase those of the goblet cell secretory products Muc2 and Tff3, which are involved in the regulation of mucosal barrier stability and permeability (q = 0.006 and q = 0.048, respectively).
Effects of SDP on Tnf-α (A), Il-10 (B), Tgfβ (C), Muc2 (D) and Tff3 (E) expression in the colon mucosa. Mice were fed a control diet (CTL) or a diet supplemented with 8% spray-dried porcine plasma (SDP) for 14 days. Results are expressed as mean ± SEM (n = 5–6 mice). Statistical differences were considered significant at q < 0.05 (corrected p values).
We analyzed some mucosal receptors with relevant roles in microbiota–mucosa crosstalk (Fig. 7A–D). SDP stimulated the expression of the membrane receptors Tlr2, Tlr4, and Tlr9 by nearly 3-fold, 2-fold, and more than 7-fold (q = 0.011, q = 0.009, and q = 0.012, respectively) but had no effect on Tlr5. Cytosolic NOD1 and NOD2 receptors, which can also respond to bacterial components, were not affected by SDP (Fig. 7E,F). Finally, we studied adaptor proteins recruited by TRLs to initiate signal transduction pathways and regulate cytokine expression. The myeloid cell-specific gene Myd88 was significantly increased in the SDP-supplemented mice, whereas TIR domain-containing adapter-inducing interferon-β (Trif) was not (Fig. 7G,H).
Effects of SDP on Tlr2 (A), Tlr4 (B), Tlr5 (C), Tlr9 (D), Nod1 (E), Nod2 (F), Myd88 (G) and Trif (H) expression in the colon mucosa. Mice were fed a control diet (CTL) or a diet supplemented with 8% spray-dried porcine plasma (SDP) for 14 days. Results are expressed as mean ± SEM (n = 5–6 mice). Statistical differences were considered significant at q < 0.05 (corrected p values).
Because the lymphoid population of the colon mucosa contains different types of cells that may participate in tolerogenic responses, we analyzed some markers that are specific for macrophages (F4/80 and Cx3cr1) and dendritic cells (integrin αE). SDP stimulated both populations (all q < 0.005, Fig. 8). The expression of Foxp3, a specific marker of natural T-regulatory cells, was not stimulated by SDP under basal conditions.
Effects of SDP on Foxp3 (A), Itgαe (B), Cx3cr1 (C) and F4/80 (D) expression in the colon mucosa. Mice were fed a control diet (CTL) or a diet supplemented with 8% spray-dried porcine plasma (SDP) for 14 days. Results are expressed as mean ± SEM (n = 5–6 mice). Statistical differences were considered significant at q < 0.05 (corrected p values).
Discussion
The present study shows that feeding mice with SDP changes the microbiota composition at several taxonomic levels, enhancing probiotic families and species that regulate mucosal barrier permeability and promote mucosal tolerance. The changes in the microbiota profile induced by SDP differ markedly from those resulting from the administration of low doses of antibiotics used in farm animals to promote growth18. Administration of low-dose antibiotics alters the intestinal microbiota and affects immune homeostasis, particularly in early life, because the changes induced in the microbiota may lead to the loss of species with important functions, increasing the risk of several diseases in adulthood30. Thus, whereas antibiotics reduced the total number of bacterial species, SDP did not; at the phylum level, antibiotics reduced Verrucobacteria 16-fold, while SDP had no effect; at the family level, SDP stimulated lactobacilli and inhibited bifidobacteria and these effects were not reproduced by antibiotics. Further evidence to distinguish the effects of SDP on the microbiota from those of antibiotics is that the latter can affect the responsiveness to vaccines. For example, pigs administered cephalosporin have a reduced immune response to vaccination against the influenza A virus31 while early-life exposure of mice to antibiotics shapes their subsequent responses to vaccines32. Dietary SDP promotes a microbiota profile with probiotic properties that may even improve the response to vaccination, as shown in aged SAMP8 mice after nasal vaccination with S. aureus enterotoxin B33. These results lead us to conclude that the anti-inflammatory and growth-promoting effects of SDP rely on microbial changes unrelated to alleged “antibiotic-like” properties of the supplement.
Altogether, our results demonstrate that 14 days of SDP supplementation suffices to induce profound changes in the gut microbiota. There is a significant increase in phyla Firmicutes and Bacteroidetes both producers of short-chain fatty acids (SCFAs). Acetate, propionate and butyrate are essential for the maintenance of mucosal immunity and mucosal barrier function34, and they promote tolerance through GALT modulation35. SDP increases Parabacteroides goldsteinii, a species in the Porphyromonadaceae family that has anti-inflammatory effects in vivo, reducing Tnf-α expression in the liver36 while inducing Il-10 expression in the proximal colon of mice37. The genus Blautia (Lachnospiraceae family) is positively correlated with low inflammatory status and high cognition scores38, and these results are compatible with the effects of SDP reported on systemic inflammation39 and cognitive functions40.
SDP supplementation had notable effects on probiotic species. The intestine houses numerous lactic acid-producing bacteria that promote health by producing metabolites that can interact with the host’s own metabolism and immune system41. Most probiotics belonging to the genera Lactobacillus and Bifidobacterium modulate the GALT and prevent adhesion of and colonization by pathogens42. SDP strongly stimulated the growth of members of the Lactobacillaceae family, indicating that SDP has prebiotic effects. The abundance of Lactobacillaceae increased 3-fold and that of the genus Lactobacillus increased 5-fold. These observations confirm previous results showing that SDP enhances the frequency of detection of Lactobacillus species in the rat cecum21 as well as in pig ileum18 and pig colon20.
The effects of SDP that promote the growth of commensal species from the Lactobacillaceae family while reducing the presence of Bifidobacterium strains may not affect the number of lactic acid-producing bacteria, as the total number of microbial species in feces is not affected by SDP. This suggests that the probiotic metabolic profile of the microbiota is not modified. Interestingly, though most prebiotics stimulate growth of both lactobacilli and bifidobacteria, SDP specifically simulates the proliferation of lactobacilli at the expense of bifidobacteria. Perhaps this is due to SDP composition being markedly different from that of conventional prebiotics, as it is mostly composed of peptides and proteins. This means that while some may be digested and absorbed in the small intestine, others resist digestion and are excreted in feces43. In the rat intestine, a soy milk supplement increased Lactobacillus and decreased Bifidobacterium strains, similarly to what was observed in the present study44. The distinct effects of SDP can be attributed to the differences in bacterial substrate preferences. Bottari et al.45 demonstrated that bifidobacteria preferred peptides with 4 or 5 residues, while lactobacilli mostly consume dipeptides. Hence, the peptide composition of SDP, and the structure of the peptides resulting from digestion of its protein components, might explain the different growth of commensal lactobacilli and bifidobacteria.
The levels of L. taiwanensis and L. frumenti were increased several-fold by SDP; and L. antri, although present in only low proportion, was increased 60-fold. It is worth noting that L. antri and L. frumenti, are representative strains within the L. reuteri subgroup of lactobacilli with well-known probiotic properties46. L. taiwanensis has regulatory functions and interacts with dendritic cells of the lamina propria, as part of the bacterial-GALT crosstalk that maintains mucosal homeostasis47 and these effects are consistent with the increased expression of the dendritic cell marker integrin αE observed in the present study. Mice exposed to L. taiwanensis show increased frequency of Treg cells in both mesenteric lymph nodes and Peyer’s patches48, that then reside in the gut, where they expand and produce a tolerant environment for specific antigens49. These observations agree with our results showing that SDP supplementation consistently increases the percentage of Treg in acute models of mucosal inflammation9,50 as well as in aging39 and colitis13 mice models. SDP also stimulates the expression of Muc2, a protein that, in addition to its role in the formation of the mucous barrier, signals pathways that regulate dendritic cells of the lamina propria, thus enhancing oral tolerance51.
Blautia has also been shown to regulate the expression of tight-junction ZO-1 and maintenance of mucosal permeability52 and L. frumenti is a commensal species that, when given by oral gavage to piglets prior to weaning, decreases the relative abundance of opportunistic pathogens and improves intestinal mucosal integrity via a mechanism involving up-regulation of ZO-1, occludin, and claudin-1. We have previously shown that inflammation reduces the expression of the tight junction protein ZO-1 and adherent junction protein β-catenin in the small intestine and that SDP can prevent this decrease50,53. Because Blautia was increased 1.4-fold by SDP, we hypothesized that the effects of SDP on mucosal integrity might be mediated by this species. However, there were no changes in the levels of occludin and E-cadherin, as representative proteins of the tight junction and adherens junction, respectively, suggesting that SDP cannot affect permeability in unchallenged animals.
In the colon mucosa, dietary SDP induces Tgf-β expression, which in turn upregulates the expression of integrin αE in mucosal immune cells54. Among the cells expressing this marker are dendritic cells required for the activation of FoxP3 regulatory T cells to induce tolerogenic responses; this is important in the large intestine because this region is exposed to commensal bacteria driving inflammation55. We have also shown that SDP stimulates the expression of F4/80 and CX3CR1, which are macrophage markers in the gut with important roles in the priming of immune responses. CX3CR1+ macrophages produce immunoregulatory cytokines such as IL-10 and can also facilitate the differentiation and maintenance of Treg within the lamina propria56. They are also required for the induction of the efferent CD8+ reg-T cells required for peripheral tolerance57.
Mucosal barrier functions depend on the permeability properties of the epithelial cell lining. These in turn result from a combination of the chemical barrier composed of antimicrobial peptides (AMPs) secreted by the epithelium to control bacterial growth, and the physical barrier made of mucus, mainly secreted by goblet cells. Inflammation reduces the expression of AMPs and the secretion of mucous components such as Muc2 or the barrier stability component Tff3, both secreted by goblet cells58. Oral lactobacilli stimulate intestinal antimicrobial activity in vivo and increase Muc2 secretion59, indicating that probiotics can regulate epithelial function providing mucosal protection. SDP reinforces mucosal barrier stability and stimulates epithelial regeneration, as both Muc2 and Tff3 minimize the consequences of inflammation resulting from the challenges (and preserve these important functions). These results are also in keeping with previous observations in the mdr1−/− mouse model of colitis, showing that SDP can increase the number of goblet cells in the colon mucosa60. Our current results, showing that SDP stimulates lactobacilli proliferation, further support the view that the regulatory effects of SDP on mucosal barrier functions are mediated by changes in microbiota composition.
The microbiota interacts with epithelial and lamina propria cells through TLRs that are transmembrane receptors and cytosolic NOD receptors, with the capacity to distinguish between pathogen and commensal microbes. Our results show that TLRs rather than NOD receptors are involved in the tolerogenic responses induced by SDP. It is well known that, once stimulated, TLRs activate intracellular signal pathways mediated by MAP kinases and NF-κB that eventually trigger pro-inflammatory immune responses61. However, TLRs also modulate transduction pathways that induce anti-inflammatory responses. We hypothesized that SDP might modulate inflammation and barrier function by regulation of mucosal TLR expression in the colon mucosa. Our results show that Tlr2, Tlr4 and Tlr9 were indeed overexpressed after the 14-day SDP supplementation period, coinciding with several-fold stimulation of lactobacilli and other SCFA-producing families such as Lactobacillaceae and Porhiromonadaceae. Castillo et al.62 observed that the administration of probiotic L. casei to healthy mice for seven days increased the expression of Tlr2, Tlr4 and Tlr9 in the small intestine, which suggests that our results for TLRs may also be mediated by lactobacilli induced by SDP.
SDP stimulates Tlr2 expression and expression of both Il-10 and Tgf-β, consistent with previous observations in healthy unchallenged rodents53, and these two variables may be correlated. The capacity of Tlr2 to induce pro- or anti-inflammatory responses depends on whether it dimerizes with other receptors. Hence Tlr2-Tlr1 heterodimers induce anti-inflammatory responses mediated by Il-10 in human small intestine63 and in antigen-presenting cells from porcine Peyer’s patches incubated with lactobacilli64. Tlr2 stimulates the internalization of lactobacilli, as a pathway for the activation of Treg lymphocytes, and increases the number of mucosal tolerogenic dendritic cells, which prime Treg cells and the production of anti-inflammatory cytokines65.
Kaji et al.66 have shown that bacterial ligands for Tlr2, Tlr4, and Tlr9 may convert the cytokine production pattern from predominantly pro-inflammatory to anti-inflammatory, indicating that probiotic induction of pro- and anti-inflammatory cytokines can be modified by co-stimulation with microbial components; an effect that has also been observed in monocytes67 and in dendritic cells68. The kind of response (either pro- or anti-inflammatory) further depends on TLR compartmentalization in the cell. For example, Tlr4 expressed on the cellular membranes plays a pro-inflammatory signaling role involving MyD88 co-activation;69 while intracellular Tlr4 plays an anti-inflammatory role, inducing the expression of Il-1070. Tlr9 activation induces conventional dendritic cells to secrete anti-inflammatory Il-10, thus attenuating inflammatory responses and liver injury in mice71. In intestinal epithelial cells, Tlr9 plays an important homeostatic role, protecting mice from experimental colitis72 and these results are compatible with the anti-inflammatory effects of SDP observed in mdr1−/− mice13,73.
In summary, results from the present study and from other laboratories are consistent with the hypothesis that the mechanism by which SDP modulates GALT involves overexpression of TLR at the inductive sites and stimulation of the Myd88 pathway as first steps towards regulating inflammatory responses at the effector sites as shown by others74.
In conclusion, this study demonstrates that animal plasma supplements can modulate the composition of microbiota and provides a mechanistic explanation: it links the bacterial families and species promoted by SDP with the expression of mucosal makers and immune regulators involved in intestinal and systemic homeostasis. SDP stimulates the growth of some probiotic species and the expression of mucosal regulatory signals and anti-inflammatory pathways. Specifically, SDP increases the presence of bacterial families that enhance the intestinal barrier function and species that are well-known mediators of anti-inflammatory and tolerogenic responses.
Material and Methods
Animals and diets
Male C57BL/6 mice were purchased from Envigo (Bresso, Italy) and kept under stable temperature and humidity conditions, with a 12 h light–12 h dark cycle and free access to food and water. All protocols used in the present study were approved by the Animal Experimentation Ethics Committee of the Universitat de Barcelona (Ref. 503/14), following the guidelines for the Care and Use of Laboratory Animals of the regional government (DAAM 7939, Generalitat de Catalunya, Spain). At day 21 (weaning) 30 mice were equally distributed at random in three groups; the CTL group, fed the control diet, the SDP group, supplemented with 8% SDP, and the COL group fed the control diet and administered with a mixture of antibiotics in the drinking water, as described below.
SDP is a protein-rich ingredient obtained from industrial fractionation of blood from healthy pigs. Blood is collected with an anticoagulant (sodium citrate or sodium phosphate) and centrifuged to separate the plasma fraction from blood cells. The plasma is then concentrated through membranes and spray-dried. With this procedure, proteins and peptides preserve most of their biological activity75. Control and SDP diets were designed to provide balanced energy and nutrients. The experimental diets were prepared by APC-Europe SLU from base ingredients provided by Envigo, and their composition is detailed in Supplementary Table 1.
Antibiotic treatment
A group of mice fed with the control diet received a pharmaceutical preparation containing 100 mg/L neomycin and 40 mg/L colistin (Coliphur; Maymó, Spain; COL group). The estimated daily doses of antibiotics were 25 mg/kg for neomycin and 10 mg/kg for colistin. This antibiotic dose is similar to that being used in pigs18.
Sample collection
Feces were collected at days 33–35 of life (12–14 days on diet) in clean conditions. Samples were immediately frozen in liquid N2 and maintained at −80 °C until use. At the end of the experiment, mice were anaesthetized with xylazine/ketamine killed by exsanguination. Colon samples were obtained as previously described14. Briefly, the colon was washed with phosphate-buffered saline, and the colon mucosa was scraped and quickly frozen at −80 °C for further analysis.
Extraction and purification of total genomic DNA
DNA was extracted according to Santiago et al.76 with some modifications. The method is based on microbial disruption by bead-beating because it allows a better detection of Gram-positive bacteria and because it reduces the miscellaneous populations to very low values. Briefly, samples (70 mg feces/mice) were suspended in 0.25 mL of 4 M guanidine thiocyanate (Sigma Aldrich, St. Louis, MO, USA), 40 μL of 10% N-lauroyl sarcosine (Sigma Aldrich) and 0.5 mL of 5% N-lauroyl sarcosine (Sigma Aldrich). DNA extraction was carried out by mechanical disruption of the microbial cell wall using Zirconia/silica beads of 0.1 mm diameter (BioSpec Products, Bartlesville, OK, USA). The disruption was performed by shaking the mixture using the FastPrep®−24 (MP Biomedicals, Solon, OH, USA). Tubs were added with polyvinylpolypyrrolidone (15 mg, Sigma Aldrich) and then vortexed and centrifuged for 3 min at 12,000 g. After recovery of the supernatant, the pellet was washed with 0.5 mL of TENP (50 mM Tris [pH 8], 20 mM EDTA [pH 8], 100 mM NaCl, 1% polyvinylpolypyrrolidone) and centrifuged for 3 min at 12,000 g. Supernatants were transferred to new tubes and pellets were washed three times. Finally, nucleic acids were recovered from clear lysates by isopropanol precipitation. Pellets were resuspended and pooled in 0.225 mL of 100 mM phosphate buffer, pH 8, and 50 μL of 5 M potassium acetate (all reagents were from Sigma Aldrich). Tubes were placed on ice for 90 min and centrifuged at 16,000 g for 30 min. The supernatants were transferred to tubes containing 5 μL of RNase (4 mg/ml, Qiagen, Venlo, The Netherlands) and incubated at 37 °C for 30 min. Nucleic acids were precipitated by the addition of 50 μL of 3 M sodium acetate (Sigma Aldrich) and 1 mL of absolute ethanol (JT Baker, Deventer, The Netherlands). Tubes were then incubated for 10 min at room temperature, and nucleic acids were recovered by centrifugation at 20,000 g for 15 min. DNA pellets were finally washed with 70% ethanol, dried, and resuspended in 0.1 mL of water. The quantification of DNA was done using a NanoDrop ND-100 Spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA).
16S rDNA gene analysis
The extracted genomic DNA was processed and the variable V3 and V4 regions of the 16S rRNA gene were amplified. The primers to detect 16S rRNA gene were: 16S forward 5′-TCGTCGGCAGCGTCAGATGTGTATAAGAGACAGCCTACGGGNGGCWGCAG-3′ and reverse 5′ -GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAGGACTACHVGGGTATCTAATCC-3′. High-through sequencing was done using the Illumina MiSeq platform (Illumina, San Diego, CA, USA) at the Genomics and Bioinformatics Service, Universitat Autònoma de Barcelona (Bellaterra, Spain).
Real-time PCR analysis
Total RNA was extracted with TRIzol reagent (Life Technologies, Carlsbad, CA, USA) following the manufacturer’s instructions. RNA extraction and retrotranscription were carried out as previously described39. The mouse primers used are listed in Supplementary Table 2. Real-time PCR was performed using a cDNA template in a 20 µL reaction containing 0.2 μmol/L of each primer and SsoAdvanced Universal SYBR Green Supermix (Bio-Rad, Hercules, CA, USA) and carried out on a MiniOpticon Real-Time PCR System (Bio-Rad, Hercules, CA, USA). To confirm PCR amplification of the intended product, representative samples were analyzed by electrophoresis on a 4% agarose gel. Product fidelity was confirmed by melt-curve analysis. TaqMan gene expression assays (Applied Biosystems, Foster City, CA, USA) were used for the following genes: Toll-like receptor 2 (Tlr2, Mm00442346_m1), Toll-like receptor 4 (Tlr4, Mm00445273_m1), Toll-like receptor 9 (Tlr9, Mm00446193_m1) and Myeloid differentiation primary response 88 (Myd88, Mm00440338_m1) following the manufacturer’s instruction. Each PCR run included duplicates of reverse transcription for each sample and negative controls (reverse transcription-free samples, RNA-free sample). Quantification of the targeted gene transcripts was done using hypoxanthine phosphoribosyltransferase 1 (Hprt1, Mm00446968_m1) gene expression as reference, and was carried out with the 2−ΔΔCT method77.
Statistics
Hierarchical clustering and ordination of the community structures were performed using a Principal Coordinate Analysis (PCoA) plot by Illumina MiSeq analyzer (Illumina, San Diego, CA, USA). Results are presented as mean ± SEM. The statistical analysis was performed using GrapPad Prism® software v 7.01 (GraphPad Software, Inc., La Jolla, CA, USA). Grubb´s test was performed to determine outliers and the Shapiro-Wilk test was used to check the normality of data distribution. When comparing three groups (i.e. CTL, SDP and COL), the ANOVA test was used when data were normally distributed; otherwise the non-parametric Kruskal-Wallis test was carried out. When only Control and SDP conditions were compared, the Student t-test was used when data were normally distributed; otherwise, the non-parametric Mann-Whitney U-test test was used. All p-values were corrected for multiple testing using the false discovery rate (FDR) correction (Benjamini-Hochberg). Statistical differences were considered significant at q < 0.05. A q value between 0.05 and 0.1 was suggestive of a true effect78.
References
Van Dijk, A. J., Margry, R. J. C. F., Van der Lee, A. G., Hemke, G. & Beynen, A. C. Growth performance and health status in weanling piglets fed spray-dried porcine plasma under typical Northern European conditions. J. Anim. Physiol. Anim. Nutr. 86, 17–25 (2002).
Lallès, J. P., Bosi, P., Janczyk, P., Koopmans, S. J. & Torrallardona, D. Impact of bioactive substances on the gastrointestinal tract and performance of weaned piglets: a review. Animal 3, 1625–1643 (2009).
Petschow, B. W. et al. Bovine immunoglobulin protein isolates for the nutritional management of enteropathy. World J. Gastroenterol. 20, 11713–11726 (2014).
Weaver, A. C., Campbell, J. M., Crenshaw, J. D., Polo, J. & Kim, S. W. Efficacy of dietary spray dried plasma protein to mitigate the negative effects on performance of pigs fed diets with corn naturally contaminated with multiple mycotoxins. J. Anim. Sci. 92, 3878–3886 (2014).
Pérez-Bosque, A., Polo, J. & Torrallardona, D. Spray dried plasma as an alternative to antibiotics in piglet feeds, mode of action and biosafety. Porc. Health Manag. 2, 16, https://doi.org/10.1186/s40813-016-0034-1 (2016).
Kuchibhatla, R., Petschow, B. W., Odle, J. & Weaver, E. M. Nutritional impact of dietary plasma proteins in animals undergoing experimental challenge and Implications for patients with inflammatory bowel disorders: A meta-analysis. Adv. Nutr. 6, 541–551 (2015).
Petschow, B. W., Burnett, B. P., Shaw, A. L., Weaver, E. M. & Klein, G. L. Dietary requirement for serum-derived bovine immunoglobulins in the clinical management of patients with enteropathy. Dig. Dis. Sci. 60, 13–23 (2015).
Pérez-Bosque, A. & Moretó, M. A rat model of mild intestinal inflammation induced by Staphylococcus aureus enterotoxin B. Proc. Nutr. Soc. 69, 447–453 (2010).
Maijó, M. et al. Dietary plasma proteins attenuate the innate immunity response in a mouse model of acute lung injury. Br. J. Nutr. 107, 867–875 (2012).
Maijó, M. et al. Dietary plasma proteins modulate the adaptive immune response in mice with acute lung inflammation. J. Nutr. 142, 264–270 (2012).
Song, M. et al. Spray-dried plasma attenuates inflammation and improves pregnancy rate of mated female mice. J. Anim. Sci. 93, 298–305 (2015).
Moretó, M. & Pérez-Bosque, A. Dietary plasma proteins, the intestinal immune system, and the barrier functions of the intestinal mucosa. J. Anim. Sci. 87, E92–E100 (2009).
Pérez-Bosque, A. et al. Dietary intervention with serum-derived bovine immunoglobulins protects barrier function in a mouse model of colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 308, G1012–G1018 (2015).
Pérez-Bosque, A., Miró, L., Amat, C., Polo, J. & Moretó, M. The anti-inflammatory effect of spray-dried plasma is mediated by a reduction in mucosal lymphocyte activation and infiltration in a mouse model of intestinal inflammation. Nutrients 8, E657, https://doi.org/10.3390/nu8100657 (2016).
Liu, Y. et al. Spray-dried plasma attenuates inflammation and lethargic behaviors of pregnant mice caused by lipopolysaccharide. PLoS One 13, e0203427, https://doi.org/10.1371/journal.pone.0203427 (2018).
Kiyono, H., Kweon, M. N., Hiroi, T. & Takahashi, I. The mucosal immune system: from specialized immune defense to inflammation and allergy. Acta Odontol. Scand. 59, 145–53 (2001).
Chatterton, D. E., Nguyen, D. N., Bering, S. B. & Sangild, P. T. Anti-inflammatory mechanisms of bioactive milk proteins in the intestine of newborns. Int. J. Biochem. Cell Biol. 45, 1730–1747 (2013).
Torrallardona, D., Conde, M. R., Badiola, I., Polo, J. & Brufau, J. Effect of fishmeal replacement with spray-dried animal plasma and colistin on intestinal structure, intestinal microbiology, and performance of weanling pigs challenged with Escherichia coli K99. J. Anim. Sci. 81, 1220–1226 (2003).
Ferreira, A. S., Barbosa, F. F., Tokach, M. D. & Santos, M. Spray-dried plasma for pigs weaned at different ages. Recent Pat. Food Nutr. Agric. 1, 231–235 (2009).
Che, L., et al. Microbial insight into dietary protein source affects intestinal function of pigs with intrauterine growth retardation. Eur. J. Nutr. 2019, https://doi.org/10.1007/s00394-019-01910-z (2019).
Martín-Orúe, S. M., Pérez-Bosque, A., Gómez de Segura, A. & Moretó, M. Feed added spray-dried porcine plasma modifies cecal microbiota in rats. Paper presented at Gut Microbiome Meeting, Clermont-Ferran, France, Abstract book (2008).
Balan, P., Han, K. S., Lawley, B. & Mougham, P. J. Orally administered ovine serum immunoglobulins modulate the intestinal levels of Lactobacillus and enterobacteria in the growing rat. J. Anim. Sci. 91, 3724–3732 (2013).
Asmuth, D. M. et al. Oral serum-derived bovine immunoglobulin improves duodenal immune reconstitution and absorption function in patients with HIV enteropathy. AIDS 27, 2207–2217 (2013).
Bosi, P. et al. Spray-dried plasma improves growth performance and reduces inflammatory status of weaned pigs challenged with enterotoxigenic Escherichia coli K88. J. Anim. Sci. 82, 1764–1772 (2004).
Corl, B. A. et al. Effect of animal plasma proteins on intestinal damage and recovery of neonatal pigs infected with rotavirus. J. Nutr. Biochem. 18, 778–784 (2007).
Torrallardona, D. Spray dried animal plasma as an alternative to antibiotics in weanling pigs. Asian-Aust. J. Anim. Sci. 23, 131–148 (2010).
Blaser, M. J. Antibiotic use and its consequences for the normal microbiome. Science 352, 544–545 (2016).
Shanahan, F. The colonic microbiota in health and disease. Curr. Opin. Gastroenterol. 29, 49–54 (2013).
De Kivit, S., Tobin, M. C., Forsyth, C. B., Keshavarzian, A. & Landay, A. L. Regulation of intestinal immune responses through TLR activation: implications for pro- and prebiotics. Front. Immunol. 5, 60, https://doi.org/10.3389/fimmu.2014.00060 (2014).
Cox, L. M. et al. Altering the intestinal microbiota during a critical developmental window has lasting metabolic consequences. Cell 158, 705–721 (2014).
Pomorska-Mól, M., Kwit, K., Wierzchosławski, K., Dors, A. & Pejsak, Z. Effects of amoxicillin, ceftiofur, doxycycline, tiamulin and tulathromycin on pig humoral immune responses induced by erysipelas vaccination. Vet. Rec. 178, 559, https://doi.org/10.1136/vr.103533 (2016).
Lynn, M. A. et al. Early-life antibiotic-driven dysbiosis leads to dysregulated vaccine immune responses in mice. Cell Host Microbe 23, 653–660 (2018).
Miró, L., et al. Spray-dried animal plasma supplementation improves protection of vaccination in senescent mice. Paper presented at 42nd Congress of the Spanish Society of Biochemsitry and Molecular Biology, Madrid, Spain, Abstract book (2019).
Rooks, M. G. & Garrett, W. S. Gut microbiota, metabolites and host immunity. Nat. Rev. Immunol. 16, 341–352 (2016).
Lathrop, S. K. et al. Peripheral education of the immune system by colonic commensal microbiota. Nature 478, 250–254 (2011).
Neyrinck, A. M. et al. Rhubarb extract prevents hepatic inflammation induced by acute alcohol intake, an effect related to the modulation of the gut microbiota. Mol. Nutr. Food Res. 61, 1–12, https://doi.org/10.1002/mnfr.201500899 (2016).
Wu, R.-T. et al. Gut commensal Parabacteroides goldsteinii plays a predominant role in the anti-obesity effects of polysaccharides isolated from Hirsutella sinensis. Gut 68, 248–262 (2019).
Bajaj, J. S. et al. Colonic mucosal microbiome differs from stool microbiome in cirrhosis and hepatic encephalopathy and is linked to cognition and inflammation. Am. J. Physiol. Gastrointest. Liver Physiol. 303, G675–G685 (2012).
Miró, L. et al. Dietary animal plasma proteins improve the intestinal immune response in senescent mice. Nutrients 9, E1346, https://doi.org/10.3390/nu9121346 (2017).
Garcia-Just, A., et al. Dietary Spray-Dried Porcine Plasma Prevents Cognitive Decline in Senescent Mice and Reduces Neuroinflammation and Oxidative Stress. J. Nutr., nxz239, https://doi.org/10.1093/jn/nxz239 (2019).
Puebla-Barragan, S. & Reid, G. Forty-five-year evolution of probiotic therapy. Microbial Cell 6, 184–196 (2019).
Zielinska, D. & Kolohyn-Krajewska, D. Food-origin lactic acid bacteria may exhibit probiotic properties: Review. Biomed. Res. Int. 2018, 5063185, https://doi.org/10.1155/2018/5063185 (2018).
Rodríguez, C. et al. Porcine immunoglobulins survival in the intestinal tract of adult dogs and cats fed dry food kibbles containing spray-dried porcine plasma (SDPP) or porcine immunoglobulin concentrate (PIC). Anim. Feed Sci. Technol. 139, 201–211 (2007).
Bedani, R., Pauly-Silveira, N. D., Roselino, M. N., de Valdez, G. F. & Rossi, E. A. Effect of fermented soy product on the fecal microbiota of rats fed on a beef-based animal diet. J. Sci. Food Agric. 90, 233–238 (2010).
Bottari, B. et al. Characterization of the peptide fraction from digested Parmigiano Reggiano cheese and its effect on growth of lactobacilli and bifidobacteria. Int. J. Food Microbiol. 255, 32–41 (2017).
Roos, S., Engstrand, L. & Jonsson, H. Lactobacillus gastricus sp. nov., Lactobacillus antri sp. nov., Lactobacillus kalixensis sp. nov. and Lactobacillus ultunensis sp. nov., isolated from human stomach mucosa. Int. J. Syst. Evol. Microbiol. 55, 77–82 (2005).
Morikawa, M. et al. Microbiota of the small intestine is selectively engulfed by phagocytes of the lamina propria and Peyer’s patches. Plos One 11, e0163607, https://doi.org/10.1371/journal.pone.0163607 (2016).
Reynolds, L. A. et al. Commensal-pathogen interactions in the intestinal tract. Gut Microbes 5, 522–532 (2014).
Hadis, U. et al. Intestinal tolerance requires gut homing and expansion of FoxP3+ regulatory T Cells in the lamina propria. Immunity 34, 237–246 (2011).
Pérez-Bosque, A. & Moretó, M. Dietary spray-dried animal plasma alleviates mucosal inflammation in experimental models. Recent Adv. Pharmac. Sci. V, 117–132 (2015).
Shan, M. et al. Mucus enhances gut homeostasis and oral tolerance by delivering immunoregulatory signals. Science 342, 447–453 (2013).
Zhou, D. et al. Sodium butyrate attenuates high-fat diet-induced steatohepatitis in mice by improving gut microbiota and gastrointestinal barrier. World J. Gastroenterol. 23, 60–75 (2017).
Pérez-Bosque, A. et al. Oral plasma proteins attenuate gut inflammatory effects induced by S. aureus enterotoxin B challenge in rats. Livestock Sci. 133, 242–245 (2010).
Hardenberg, J. B., Braun, A. & Schön, M. P. A Yin and Yang in Epithelial Immunology: The Roles of the αE(CD103)β7 Integrin in T Cells. J. Invest. Dermatol. 138, 23–31 (2018).
Scott, C. L., Aumeunier, A. M. & Mowat, A. M. Intestinal CD103+ dendritic cells: master regulators of tolerance? Trends Immunol. 32, 412–419 (2011).
Lee, M., Lee, Y., Song, J., Lee, J. & Chang, S.-Y. Tissue-specific Role of CX3CR1 Expressing Immune Cells and Their Relationships with Human Disease. Immune network 18, e5, https://doi.org/10.4110/in.2018.18.e5 (2018).
Van den Berg, T. K. & Kraal, G. A function for the macrophage F4/80 molecule in tolerance induction. Trends Immunol. 26, 506–509 (2005).
Pelaseyed, T. et al. The mucus and mucins of the goblet cells and enterocytes provide the first defense line of the gastrointestinal tract and interact with the immune system. Immunol. Rev. 260, 8–20 (2014).
Cazorla, S. I., Maldonado-Galdeano, C., Weill, R., De Paula, J. & Perdigón, G. D. V. Oral administration of probiotics increases paneth cells and intestinal antimicrobial activity. Front. Microbiol. 9, 736, https://doi.org/10.3389/fmicb.2018.00736 (2018).
Moretó, M. et al. Dietary porcine plasma protein supplements attenuate the changes in colitis markers in the mdr1a-/- mouse model of colitis. Paper presented at Digestive Diseases Week 2009, Chicago, IL, USA. Gastroenterology 136, A–771 (2009).
Frosali, S. et al. How the intricate interaction among toll-like receptors, microbiota, and intestinal immunity can influence gastrointestinal pathology. J. Immunol. Res. 2015, 489821, https://doi.org/10.1155/2015/489821 (2015).
Castillo, N. A., Perdigón, G. & De Moreno de LeBlanc, A. Oral administration of a probiotic Lactobacillus modulates cytokine production and TLR expression improving the immune response against Salmonella enterica serovar Typhimurium infection in mice. BMC Microbiology 11, 177, https://doi.org/10.1186/1471-2180-11-177 (2011).
Hug, H., Mohajeri, M. H. & La Fata, G. Toll-like receptors: Regulators of the immune response in the human gut. Nutrients 10, 203, https://doi.org/10.3390/nu10020203 (2018).
Villena, J. et al. Immunobiotic Lactobacillus rhamnosus strains differentially modulate antiviral immune response in porcine intestinal epithelial and antigen presenting cells. BMC Microbiology 14, 126, https://doi.org/10.1186/1471-2180-14-126 (2014).
Hoang, T. K. et al. Protective effect of Lactobacillus reuteri DSM 17938 against experimental necrotizing enterocolitis is mediated by Toll-like receptor 2. Am. J. Physiol. Gastrointest. Liver Physiol. 315, G231–G240 (2018).
Kaji, R., Kiyoshima-Shibata, J., Tsujibe, S., Nanno, M. & Shida, K. Probiotic induction of interleukin-10 and interleukin-12 production by macrophages is modulated by co-stimulation with microbial components. J. Dairy Sci. 101, 2838–2841 (2018).
Barkman, C., Martner, A., Hessle, C. & Wold, A. E. Soluble bacterial constituents down-regulate secretion of IL-12 in response to intact Gram-positive bacteria. Microbes. Infect. 10, 1484–1493 (2008).
Baba, N., Samson, S., Bourdet-Sicard, R., Rubio, M. & Sarfati, M. Selected commensal-related bacteria and Toll-like receptor 3 agonist combinatorial codes synergistically induce interleukin-12 production by dendritic cells to trigger a T helper type 1 polarizing programme. Immunology 128, e523–e531 (2009).
Marongiu, L., Gornati, L., Artuso, I., Zanoni, I. & Granucci, F. Below the surface: The inner lives of TLR4 and TLR9. J. Leukoc. Biol. 106, 147–160 (2019).
Siegemund, S. & Sauer, K. Balancing pro- and anti-inflammatory TLR4 signaling. Nat. Immunol. 13, 1031–1033 (2012).
Bamboat, Z. M. et al. Conventional DCs reduce liver ischemia/reperfusion injury in mice via IL-10 secretion. J. Clin. Invest. 20, 559–569 (2010).
Lee, J., Rachmilewitz, D. & Raz, E. Homeostatic Effects of TLR9 signaling in experimental colitis. Ann. NY Acad. Sci. 1072, 351–355 (2006).
Pérez-Bosque, A. et al. Oral serum-derived bovine immunoglobulin/protein isolate has immunomodulatory effects on the colon of mice that spontaneously develop colitis. PLoS One 11, e0154823, https://doi.org/10.1371/journal.pone.0154823 (2016).
Wang, S. et al. MyD88 adaptor-dependent microbial sensing by regulatory T cells promotes mucosal tolerance and enforces commensalism. Immunity 43, 289–303 (2015).
Borg, B. S. et al. Evaluation of the chemical and biological characteristics of spray-dried plasma protein collected from various locations around the world. Am. Assoc. Swine Vet. 33, 97–100 (2002).
Santiago, A. et al. Processing faecal samples: a step forward for standards in microbial community analysis. BMC Microbiology 14, 112–121 (2014).
Schmittgen, T. D. & Livak, K. J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 3, 1101–1108 (2008).
Curran-Everett, D. & Benos, D. J. Guidelines for reporting statistics in journals published by the American Physiological Society. Physiol. Genomics 18, 249–251 (2004).
Acknowledgements
This study was funded by APC-Europe SLU (Granollers, Spain) by research contracts with the Bosch i Gimpera Foundation of the University of Barcelona. The research group was also supported by grant 2017SGR945 for Consolidated Research Groups, Generalitat de Catalunya, Spain. L. Miró was supported by a grant from the Torres Quevedo programme (reference PTQ-12-05394) of the Spanish Ministerio de Economía y Competitividad). APC-Europe SLU provided the experimental diets.
Author information
Authors and Affiliations
Contributions
M.M., A.P.B., C.A. and L.M. conceived and designed the experiments; M.M., A.P.B. conducted the research; L.M. and A.P.B. performed data analysis; C.M., J.P. contributed reagents/materials/analysis tools; M.M., C.A., A.P.B. and L.M. wrote the paper. All authors discussed the results and edited the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
J.P. and L.M. are employed by APC-Europe SLU; A.P.B., C.A., C.M. and M.M. have no conflicts of interests. The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Moretó, M., Miró, L., Amat, C. et al. Dietary supplementation with spray-dried porcine plasma has prebiotic effects on gut microbiota in mice. Sci Rep 10, 2926 (2020). https://doi.org/10.1038/s41598-020-59756-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-59756-z
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.