Abstract
Psychosocial stress is thought to influence gestational weight gain (GWG), but results are inconsistent. We investigated the relationship of questionnaire-based maternal stress and related constructs assessed at childbirth with maternal weight measured throughout pregnancy. Data were derived from the Ulm SPATZ Health Study, a birth cohort recruited from the general population (04/2012–05/2013, Ulm, Germany). Adjusted generalized estimating equations were performed. Regression coefficients (b) and 95% confidence intervals, each highest versus lowest tertile of stress or related constructs, are presented. In 748 women, we observed positive associations for maternal chronic stress (b = 4.36 kg (1.77; 6.95)), depressive symptoms (b = 2.50 kg (0.14; 4.86)), anxiety symptoms (b = 3.26 kg (0.62, 5.89)), and hair cortisol (b = 3.35 kg (0.86; 5.83)) with maternal weight at the first gestational month. GWG was considerably lower in mothers with higher chronic stress. Pregnancy-related anxiety was positively related to weight at first month (b = 4.16 kg (1.74; 6.58)) and overall GWG. In contrast, no association was observed between anxiety symptoms and GWG. Odds ratios for association with inadequate weight gain according to Institute of Medicine recommended cutoffs differed from the results presented obove. There is evidence of an association between stress and weight gain lying beyond the recommended cut-offs, which however needs further corroboration.
Similar content being viewed by others
Introduction
Conditions during pregnancy play a major role in shaping not only aspects of fetal development and birth outcomes but also subsequent newborn, child, and adult health outcomes1,2,3,4. Maternal psychosocial stress has been frequently studied for its potential association with dietary behavior and/or changes in body weight, however results for these associations during pregnancy have been inconsistent.
There are several common conceptualizations of ‘psychosocial stress’ (from now on referred to as stress) which include: the occurrence of stressful situations or events, individual’s perception of stress following such circumstances, and the subsequent psychological consequences (e.g., depression, anxiety symptoms)5. One proposed mechanism linking maternal stress during pregnancy to maternal or child outcomes involves steroid hormones secreted by the hypothalamic‐pituitary‐adrenal (HPA) axis following stressful events. Prolonged activation of the HPA axis may promote abdominal visceral fat accumulation as well as an increase of body weight via increased cortisol secretion (e.g.,6).
Additionally, stress and related constructs appear to alter overall energy intake through under- or overeating, depending on the nature of the stressor, its severity as well as an individual predisposition7. In a cross-sectional study in pregnant women, emotional problems and negative attitudes towards the baby were related to dietary behavior, as was the pre-pregnancy body mass index8. Apart from stress, a meta-analysis suggests that depression is linked to weight and weight gain in a bidirectional manner9.
Nevertheless, evidence for an association between stress and related constructs and weight gain during pregnancy is mixed which may be due to different measurements of stress. One systematic review10 concluded that maternal depression, but not anxiety or stress during pregnancy appears to have a direct relationship with excessive gestational weight gain (GWG). Another systematic review11 reported that only one out of the seven included studies observed a negative association between depression and excessive GWG, whereas no link between stress or anxiety and excessive GWG was reported. Furthermore, in a large study of 13,314 mothers11, depression was not meaningfully associated with inadequate or excessive GWG, yet a later study12 reported a relationship beween anxiety and GWG in multiparous women only. Notably, most of the evidence is based on self-reported pre- and post-pregnancy body weight as well as self-reported body height with higher potential for misclassification than prospective measurement of maternal weight13,14.
Given previous inconsistent results, this study aims to investigate the association of stress, anxiety, and depression symptoms with GWG based on paper documentation of routine preventive medical examination including several measurements of body weight. We hypothesized that higher stressed mothers would gain more or less weight than lower stressed mothers due to mechanisms e.g., like a change in the HPA axis or changed dietary behaviors. Thereby, hair cortisol concentrations (HCC) were measured as a potential biomarker of HPA axis activation. The assessment of the biological stress response by using HCC has gained interest, as it offers a way to assess aggregated, long-term cortisol levels with a single non-invasive sampling15,16,17. By capitalizing on the continuous incorporation of lipophilic substances into the slowly growing hair matrix, HCC are assumed to provide an easily obtainable index of hormone levels integrated over the periods of several months. HCC may thus be more representative of normal, long-term cortisol secretion than single measures in saliva, blood, or urine. We further analyzed the associations of self-reported stress or related constructs with GWG above or below cut-offs recommended by the Institute of Medicine (IOM)18 to compare the results.
Results
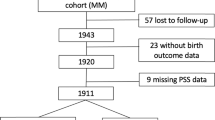
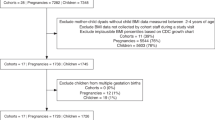
Following restriction to singleton full-term deliveries without maternal diabetes mellitus diagnosis or gestational diabetes (n = 107 excluded) or last obstetrician examination before gestational day 245 (n = 57 excluded), information was available for 748 mothers of whom the majority (70.3%) were between 26 and 35 years of age and of German nationality (85.4%) (see Table 1). A large proportion (38.4%) were classified as having experienced excessive weight gain above the IOM recommended limit for their respective pre-pregnancy body mass index (BMI). Median chronic stress (SSCS-TICS) was 50.0 (25th percentile: 43.0, 75th percentile: 56.0), median HADS-D: 2.0 (1.0, 3.0), and median HADS-A was 4.0 (2.0, 6.0). The respective values for PANX and HCC were median: 15.0 (13.0, 18.0), and 1.74 (1.23, 2.46).
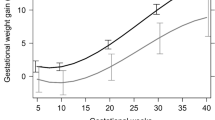
GEE model results are shown in Table 2. A positive association was observed between chronic stress (SSCS-TICS) reported at childbirth and body weight at first gestational month (regression coefficient (b) = 4.36 kg (95% CI: 1.77; 6.95)) in mothers scoring in the highest tertile (Tert3) of SSCS-TICS compared to those scoring in the lowest tertile (Tert1). Here the first gestational month is thought to reflect pre-pregnancy weight as weight gain in the first three gestational months is generally very low. However, maternal weight gain in women reporting high chronic stress was lower than in their lower stressed counterparts (see Table 2, Test for interaction month*SSCS-TICS tertiles p = 0.043), indicating that the time profiles for those who are highly stressed and those who are not differ. This holds particularly true for months three to nine (see Table 2, column 2 and 4).
Depressive symptoms retrospectively reported after delivery were positively associated with maternal body weight at first gestational month (b = 2.50 kg (0.14; 4.86), HADS-D Tert3 vs. HADS-D Tert1, see Table 3) but we did not see an association between depressive symptoms and maternal pregnancy weight gain meaning that women with high depressive symptoms did not gain more or less weight than their non-depressed counterparts. Similarly, there was a statistically significant association between HADS-A and maternal weight at the first gestational month but no significant interaction between maternal anxiety and GWG (see Supplementary Table S1). A strong positive relationship between pregnancy-related anxiety and body weight (b = 4.16 kg (1.74; 6.58) PANX Tert3 vs. Tert1, see Table 4) was shown. Furthermore, higher pregnancy anxiety was associated with higher GWG (Test for interaction month*PANX tertiles p = 0.010). Furthermore, a positive association between HCC tertiles and body weight at the first gestational month was observed. Conversely, data revealed an inverse linear relationship (p = 0.147) between HCC and GWG (see Supplementary Table S2). An additional adjustment of the association between SSCS-TICS and gestational weight (gain) by HCC leaded to a small increase of the estimate (regression coefficient (b) = 4.64 kg (95% CI: 2.01; 7.26)) for body weight at first gestational month but no relevant change in the gestational weight gain compared to the model without HCC. Substantial relations between chronic stress or related constructs and IOM GWG category (see Table 5) were only observed for PANX and HCC. High pregnancy-related anxiety was inversely associated with inadequate GWG (adjusted odds OR: 0.59 (95% CI: 0.35, 0.98), Tert3 vs. Tert1), but positively with excessive GWG (OR: 1.70 (95% CI: 1.09, 2.64), Tert3 vs. Tert1). High HCC were significantly associated with higher odds of inadequate GWG (OR: 1.76 (95% CI: 1.06, 2.93), Tert3 vs. Tert1).
Discussion
We observed statistically significant positive associations between maternal chronic stress and body weight at the beginning of pregnancy, but a flatter slope of GWG in higher stressed compared to lower stressed mothers. Conversely, higher pregnancy-related anxiety was associated with higher weight at first pregnancy month and higher GWG. Although the directionality of the association between stress, related constructs, and body weight is not entirely clear due to our study design, ‘emotional eating’ acting as a mediator between stress and weight gain during pregnancy could be important and might be considered by practitioners as a target to prevent health consequences of inadequate pregnancy weight gain. We do not have convincing evidence of a mediating role of HCC.
There are several limitations of our study. First, stress and the related constructs were self-reported by the mothers at only one time point just after delivery (median = 1.5 days, range = 0 to 3 days). These measurements may have been subject to recall bias or more proximal delivery-related psychological stress or anxiety. Furthermore, studies of non-pregnant women strongly support the presence of a recursive, bidirectional relationship between nutrition and stress7,19. Thus, in spite of our longitudinal study design, we cannot explicitly determine the direction of the association, since stress or related constructs may lead to higher weight gain and vice versa. Further studies should measure stress or related constructs several times throughout pregnancy allowing structural equation modelling and enlighten the complex relationship between stress, related constructs and weight (gain). This idea is also supported by the fact that recently published articles found trimester-specific associations between stress and GWG20,21. Additionally, several psychosocial stress instruments differ in their ability to measure prepartal stress22. Apart from PANX, our instruments did not account for the specific situation of pregnancy as the instruments were supposed to be used repeatedly in our cohort during long-term follow-up. Thirdly, we did not measure dietary intake and physical activity. Thus, the mechanism behind the relationship between stress and GWG is unknown. Psychological events initiate the HPA axis response resulting in an increased cortisol release, and cortisol, in turn, affects the lipid and carbohydrate metabolism showing a strong association with body weight23. Yet, by testing HCC as mediator between SSCS-TICS and weight (gain), our data revealed that HCC were not a significant promotor of weight gain in women. Whether HCC, especially in pregnancy, is therefore a marker of psychosocial stress or, more likely, a marker of metabolic conditions still needs confirmation24,25.
A primary strength in our study is the use of objectively measured weight data, documented throughout pregnancy, even though in 57 of our 748 (7.6%) mothers, we used self-reported pre-pregnancy weight as it was also done in many other studies. However, whether the method of measurement can serve as a reason for the inconsistent results reported previously is unclear. Our distribution of GWG was similar to that reported in national observational studies on GWG patterns, with only 30% of women meeting their GWG goals10. Alike our results, a strong correlation between pre-pregnancy BMI and excessive GWG26 was reported. In our models however, we took pre-pregnancy weight at first gestational month as reference and further adjusted for maternal body height. Pre-pregnancy body mass index is thought to exacerbate unhealthy dietary behavior under high-stress conditions5, and stress-induced eating in non-pregnant women with obesity is suggested27.
Our data support consistent positive associations between SSCS-TICS, HADS-D, HADS-A, PANX, and HCC and pre-pregnancy body weight, but GWG seems to be lower in mothers scoring high on SSCS-TICS. However, primarily concerning the WHO categories of GWG, one review20 reported no convincing association between chronic stress and GWG. Women with high reported stress during pregnancy tended to have low GWG in one study of racially diverse women28. Likewise, higher stress was related to inadequate GWG29 in African-American women. Conversely, other studies (see review30) found no association between chronic stress and low GWG as we did when referring to WHO categories. Given a relatively high socioeconomic status in our study population, we might underestimate the association between chronic stress and inadequate GWG as measured relative to the pre-pregnancy BMI. We did not observe an association for anxiety symptoms and GWG, which is in line with some (see, e.g.,20) but not all reports. Hartley et al.12 showed that higher anxiety was related to greater GWG for 143/256 multiparous women, but, in general, the number of studies on anxiety and weight gain is rather small31. Also, covariates including income, health behavior engaged during pregnancy, and familial or social support can modify the association between stress or related constructs and GWG32 - factors we could not account for.
Taken together, we conclude that there might be evidence of an association between stress and maternal body weight and weight gain lying beyond what is measured by the IOM categories, which however needs corroboration in other studies. Indeed, further studies should additionally analyse dietary behavior and cortisol to better understand the underling mechanisms between maternal stress, related constructs and pregnancy weight (gain).
Methods
Study design and study population
Data were obtained from the Ulm SPATZ Health Study, a birth cohort study recruited from the general population in Ulm, Germany. Mothers who gave birth to a child at Ulm University Medical Center from 04/2012 to 05/2013 and their families were asked to participate (participation rate 49% of eligible mothers, n = 970 with 934 singleton births). Details of the baseline examination are described elsewhere33. Exclusion criteria were outpatient delivery, maternal age < 18 years, transfer of the newborn or the mother to intensive care immediately after delivery, insufficient knowledge of the German language, and/or stillbirth. For this analysis, the study population was restricted to singleton full-term (gestational age ≥ 37 weeks) newborns. The ethics board of Ulm University approved the study No. 311/11). All participants gave written informed consent. The study was carried out in accordance with the Declaration of Helsinki.
Data collection
Maternal demographic and lifestyle data including age at delivery (≤25, 26–35, ≥36 years, nationality (German, other), education (<12 years or ≥12 years), parity (first birth or >1 birth), and maternal occupational status before childbirth (leadership, professional, intermediate position, skilled manual or non-manual, unskilled or semi-skilled, self-employed), were collected using a self-administered questionnaire during the hospital stay following delivery. Clinical data related to the mother’s pregnancy, including gestational body weight measurements and gestational diabetes diagnosis (yes or no), were obtained from paper documentation of routine preventive medical examinations known in Germany as “Mutterpass” (expectant mother’s record of prenatal and natal care), which obstetricians are required to issue to their patients when pregnancy is clinically established and which are generally updated at each clinical visit during pregnancy (preventive medical examinations are suggested in Germany after establishment of pregnancy once a month and after 32nd week of pregnancy every 14 days). Clinical data related to the child’s delivery, including sex and birthweight, were obtained from electronic hospital records, so was the birth mode (vaginal or cesarean).
Gestational weight gain (GWG)
Pre-pregnancy body weight and height were estimated based on self-reported data recorded by the obstetrician in the Mutterpass typically at the first appointment during which pregnancy was established. GWG was calculated using obstetrician-documented weight measured nearest to the end of each gestational month (n = 906). The start of pregnancy was calculated as 280 days before the obstetrician reported full-term delivery date (“expected birth date”) estimated based on fetal ultrasound measurements at approximately 12 weeks gestation. GWG was additionally categorized into inadequate, adequate, or excessive based on total weight gain (last measured weight before delivery minus first measured weight) and according to the 2009 IOM guidelines18 (for body mass index (BMI) < 18.5 kg/m2: inadequate: <12.73, adequate: ≥12.73–18.18, excessive: >18.18; for BMI 18.5–24.9 kg/m2 <11.36, ≥11.36–15.90, >15.90, for BMI 25.0–29.9 kg/m2: <6.82, ≥6.82–11.36, >11.36, and for BMI ≥ 30 kg/m2: <5.00, ≥5.00–9.09, >9.09). Data were excluded (n = 57) if the last obstetrician examination had taken place at gestational day 245 or earlier. The weight at the first examination was self-reported body weight for calculation of GWG according to the IOM criteria if the first examination was after the 90th day of pregnancy (n = 52).
Questionnaire-based stress measurement
Chronic stress during pregnancy was measured using the screening scale of the Trier Inventory of Chronic Stress, (SSCS-TICS)34, which assesses chronic concerns, employment-related and social burden, excessive demands, and lack of social recognition experienced during the three prior months. Additionally, the German version of HADS (Hospital Anxiety and Depression Scale), referring to symptoms of anxiety and depression was used35. The revised version of the Pregnancy Related Anxiety Questionnaire (PANX)36, translated into German, measuring the anxiety of giving birth, the possibility of having a disabled child, and due to self-consciousness related to bodily appearance37 experienced throughout pregnancy was additionally used, however, the time span is not explictly specified. For each scale (or subscale) of the aformentioned questionnaires, missing values (TICS: n = 13, HADS D: n = 7, HADS-A: n = 5) were replaced by the mean of the remaining items of the same (sub-) scale.
Hair cortisol concentrations (HCC)
Hair strands were taken scalp-near from a posterior vertex position. Cortisol concentrations were determined from the 3 cm hair segment most proximal to the scalp. Based on an average hair growth rate of 1 cm/month38, this hair segment represents hair grown over the three months before hair sampling. Wash and steroid extraction procedures of the study followed the laboratory protocol described in detail elsewhere (see39).
Statistical analysis
Firstly, the main characteristics of the study population were described. Then generalized estimating equation (GEE) models were used to incorporate intra-individual correlations of maternal weight throughout time. Thereby, a compound symmetry covariance structure was selected based on the QIC goodness of fit statistics. For predicting gestational weight gain (in kg), the main effect of gestational month and stress or symptoms of mood disorders in tertiles was tested, as well as a stress*gestational month interaction term. Based on reports from the literature40, covariates were carefully tested. In the first step, models were adjusted for maternal education, maternal age, maternal height, parity, and smoking during or before pregnancy, maternal occupational status before childbirth, maternal ethnicity, and birthweight. In a second step, parity, maternal occupational status, and ethnicity were excluded from the model, as these variables were not significantly associated (p < 0.1) with the outcome in the adjusted models. So was smoking, a possible marker of maladaptive coping mechanisms. We preferred the model without children’s birthweight, as QIC statistics were smaller after exclusion. In the GEE models, we did not adjust for pre-pregnancy BMI, since weight at the first month was the reference category, which largely corresponds to the pre-pregnancy weight. In an additional analysis, we tested if HCC works as a mediator of the association between SSCS-TICS and weight (gain) according to Baron and Kenny 198641.
For each gestational month, one examination was taken into account. If mothers had more than one examination, the last one in the respective period was chosen. Due to the longitudinal nature of the study, missing data was observed within each gestational month. Of the 906 mothers with gravidogram, no mother had an examination before the end of month 0; 485 (53.1%) had examinations during the first gestational month, 826 mothers (90.5%) in the second, 849 mothers (93.0%) in the third, 847 mothers (92.8%) in the fourth, 847 mothers (92.8%) in the fifth, 873 mothers (95.6%) in the sixth, 896 mothers (98.1%) in the seventh, 860 mothers (94.2%) in the eighth, and 471 mothers (51.6%) in the ninth gestational month. We confirmed that missing weight data were completely at random by conducting bivariate logistic regression analyses specifying missing weight as dependent and the other covariates, including stress and related constructs as independent variables. Thereby, gestational months statistically significant predicted missing values in gestational weight, revealing a lower odds of missing values with higher gestational months.
Furthermore, separate adjusted logistic regression models were used to estimate the association between stress or related constructs and the odds of each low and excessive GWG compared separately to normal GWG with 95% confidence intervals (CI). The logistic regression models were additionally adjusted for initial maternal BMI (categoricaly measured). All statistical analyses were performed with SAS® 9.4 (The SAS Institute, Cary, NC, USA).
Data availability
The datasets generated during and/or analysed during the current study are not publicly available due to ethical restrictions regarding data protection issues and the study specific consent text but are available from the corresponding author on reasonable request.
References
Entringer, S. Impact of stress and stress physiology during pregnancy on child metabolic function and obesity risk. Curr. Opin. Clin. Nutr. Metab. Care 16, 320–327 (2013).
Gentile, S. Untreated depression during pregnancy: Short- and long-term effects in offspring. A systematic review. Neurosci. 342, 154–166 (2017).
Isgut, M., Smith, A. K., Reimann, E. S., Kucuk, O. & Ryan, J. The impact of psychological distress during pregnancy on the developing fetus: biological mechanisms and the potential benefits of mindfulness interventions. J. Perinat. Med. 45, 999–1011 (2017).
Kingston, D. & Tough, S. Prenatal and postnatal maternal mental health and school-age child development: A systematic review. Matern. Child Health J. 18, 1728–1741 (2014).
Lindsay, K. L., Buss, C., Wadhwa, P. D. & Entringer, S. The interplay between maternal nutrition and stress during pregnancy: Issues and considerations. Ann. Nutr. Metab. 70, 191–200 (2017).
Rutters, F. et al. The Hypothalamic-Pituitary-Adrenal Axis, Obesity, and Chronic Stress Exposure: Foods and HPA Axis. Curr. Obes. Rep. 1, 199–207 (2012).
Torres, S. J. & Nowson, C. A. Relationship between stress, eating behavior, and obesity. Nutr. 23, 887–894 (2007).
Gonçalves, S., Freitas, F., Freitas-Rosa, M. A. & Machado, B. C. Dysfunctional eating behaviour, psychological well-being and adaptation to pregnancy: A study with women in the third trimester of pregnancy. J. Health Psychol. 20, 535–542 (2015).
Mannan, M., Mamun, A., Doi, S. & Clavarino, A. Prospective Associations between Depression and Obesity for Adolescent Males and Females- A Systematic Review and Meta-Analysis of Longitudinal Studies. PLOS ONE 11, e0157240 (2016).
Hartley, E., McPhie, S., Skouteris, H., Fuller-Tyszkiewicz, M. & Hill, B. Psychosocial risk factors for excessive gestational weight gain: A systematic review. Women Birth 28, e99–e109 (2015).
Molyneaux, E., Poston, L., Khondoker, M. & Howard, L. M. Obesity, antenatal depression, diet and gestational weight gain in a population cohort study. Arch Wom. Ment. Health 19, 899–907 (2016).
Hartley, E., McPhie, S., Fuller-Tyszkiewicz, M., Hill, B. & Skouteris, H. Psychosocial factors and excessive gestational weight gain: The effect of parity in an Australian cohort. Midwifery 32, 30–37 (2016).
Sui, Z., Turnbull, D. & Dodd, J. Effect of body image on gestational weight gain in overweight and obese women. Women Birth 26, 267–272 (2013).
Flegal, K. M., Kit, B. K. & Graubard, B. I. Bias in Hazard Ratios Arising From Misclassification According to Self-Reported Weight and Height in Observational Studies of Body Mass Index and Mortality. Am. J. Epidemiol. 187, 125–134 (2018).
Meyer, J. S. & Novak, M. A. Minireview: Hair cortisol: a novel biomarker of hypothalamic-pituitary-adrenocortical activity. Endocrinol. 153, 4120–4127 (2012).
Staufenbiel, S. M., Penninx, B. W. J. H., Spijker, A. T., Elzinga, B. M. & van Rossum, E. F. C. Hair cortisol, stress exposure, and mental health in humans: A systematic review. Psychoneuroendocrinology 38, 1220–1235 (2013).
Stalder, T. & Kirschbaum, C. Analysis of cortisol in hair - State of the art and future directions. Brain, Behavior, Immun. 26, 1019–1029 (2012).
Institute of Medicine (US) and National Research Council (US) Committee to Reexamine IOM Pregnancy Weight Guidelines. Weight gain during pregnancy: Reexamining the guidelines. (National Academies Press (US), 2009).
Adam, T. C. & Epel, E. S. Stress, eating and the reward system. Physiol. Behav. 91, 449–458 (2007).
Kapadia, M. Z. et al. Psychological factors and trimester-specific gestational weight gain: A systematic review. J. Psychosom. Obstet. Gynaecol. 36, 15–22 (2015).
Matthews, J., Huberty, J., Leiferman, J. & Buman, M. Psychosocial predictors of gestational weight gain and the role of mindfulness. Midwifery 56, 86–93 (2018).
Chen, M. J., Grobman, W. A., Gollan, J. K. & Borders, A. E. B. The use of psychosocial stress scales in preterm birth research. Am. J. Obstet. Gynecol. 205, 402–434 (2011).
Chrousos, G. P. & Kino, T. Glucocorticoid action networks and complex psychiatric and/or somatic disorders. Stress 10, 213–219 (2007).
Braig, S., Stalder, T., Kirschbaum, C., Rothenbacher, D. & Genuneit, J. The association of potential stressors with hair steroids in parents with small children: The Ulm SPATZ health study. Psychoneuroendocrinology 102, 37–43 (2018).
Stalder, T. et al. Cortisol in hair and the metabolic syndrome. J. Clin. Endocrinol. Metab. 98, 2573–2580 (2013).
Fuller-Tyszkiewicz, M., Skouteris, H., Hill, B., Teede, H. & McPhie, S. Classification tree analysis of postal questionnaire data to identify risk of excessive gestational weight gain. Midwifery 32, 38–44 (2016).
Klatzkin, R. R., Gaffney, S., Cyrus, K., Bigus, E. & Brownley, K. A. Stress-induced eating in women with binge-eating disorder and obesity. Biol. Psychol. 131, 96–106 (2018).
Brawarsky, P. et al. Pre-pregnancy and pregnancy-related factors and the risk of excessive or inadequate gestational weight gain. Int. J. Gynaecol. Obstet. 91, 125–131 (2005).
Orr, S. T. et al. Psychosocial stressors and low birthweight in an urban population. Am. J. Prev. Med. 12, 459–466 (1996).
Kapadia, M. Z. et al. Psychological antecedents of excess gestational weight gain: A systematic review. BMC Pregnancy Childbirth 15, 107 (2015).
Nagl, M., Linde, K., Stepan, H. & Kersting, A. Obesity and anxiety during pregnancy and postpartum: A systematic review. J. Affect. Disord. 186, 293–305 (2015).
Hill, B. et al. A conceptual model of psychosocial risk and protective factors for excessive gestational weight gain. Midwifery 29, 110–114 (2013).
Braig, S. et al. Determinants of maternal hair cortisol concentrations at delivery reflecting the last trimester of pregnancy. Psychoneuroendocrinology 52, 289–296 (2015).
Schultz, P., Schlotz, W. & Becker, P. TICS - Trierer Inventar Zum Chronischen Stress. (Hogrefe, 2004).
Hermann-Lingen, C., Buss, U. & Snaith, R. Hospital Anxiety and Depression Scale-Deutsche Version (HADS-D). (Hans Huber, 2011).
Huizink, A. C., Mulder, E. J. H., Robles de Medina, P. G., Visser, G. H. A. & Buitelaar, J. K. Is pregnancy anxiety a distinctive syndrome? Early Hum. Dev. 79, 81–91 (2004).
Rothenberger, S. E., Moehler, E., Reck, C. & Resch, F. Prenatal stress: course and interrelation of emotional and physiological stress measures. Psychopathology 44, 60–67 (2011).
Wennig, R. Potential problems with the interpretation of hair analysis results. Forensic Sci. Int. 107, 5–12 (2000).
Kirschbaum, C., Tietze, A., Skoluda, N. & Dettenborn, L. Hair as a retrospective calendar of cortisol production-Increased cortisol incorporation into hair in the third trimester of pregnancy. Psychoneuroendocrinology 34, 32–37 (2009).
Kominiarek, M. A. et al. Stress during pregnancy and gestational weight gain. J. Perinatol. 38, 462–467 (2018).
Baron, R. & Kenny, D. Moderator-mediator variables distinction in social psychological research: Conceptual, strategic, and statistical considerations. J. Pers. Soc. Psych. 51, 1173–82 (1986).
Acknowledgements
C.A.L. is working at Roche Diagnostics Europe since 09/2018.
Author information
Authors and Affiliations
Contributions
S.B. designed and performed the statistical analysis and wrote the main manuscript text. C.A.L. assisted with the design of the statistical analysis. F.R. oversaw the baseline study. D.R. and J.G. designed and oversaw the study. J.G. assisted with the design of the statistical analysis. C.A.L., D.R., J.G. assisted with the writing of the manuscript.
Corresponding author
Ethics declarations
Competing interests
C.A.L. is working at Roche Diagnostics Europe since 09/2018, the design of the statistical analysis was done before 09/2018. The other authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Braig, S., Logan, C.A., Reister, F. et al. Psychosocial stress and longitudinally measured gestational weight gain throughout pregnancy: The Ulm SPATZ Health Study. Sci Rep 10, 1996 (2020). https://doi.org/10.1038/s41598-020-58808-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-58808-8
This article is cited by
-
Effects of maternal pre-pregnancy body mass index and gestational weight gain on antenatal mental disorders in China: a prospective study
BMC Pregnancy and Childbirth (2023)
-
Gestational Weight Gain During the COVID-19 Pandemic
Maternal and Child Health Journal (2023)
-
Gestational weight gain outside the 2009 Institute of Medicine recommendations: novel psychological and behavioural factors associated with inadequate or excess weight gain in a prospective cohort study
BMC Pregnancy and Childbirth (2021)
-
Maternal mental health and gestational weight gain in a Brazilian Cohort
Scientific Reports (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.