Abstract
Flowering is important for plant propagation and survival, and it is also closely related to human life. Identifying the molecular mechanisms underlying flower development is essential for plant improvement and breeding. Flower development is a complex physiological process that is regulated by multiple genes. LFY genes play important roles in the floral meristem transition and act as crucial integrators in regulating the floral gene network. Argyranthemum frutescens is an ornamental species cultivated for floral displays, yet little is known about molecular mechanisms driving its flower development. In this study, the LEAFY gene homologue, AfLFY, was identified and cloned from A. frutescens, and its role and expression patterns were characterized. Two distinct copies of AfLFY were found in the A. frutescens genome and both sequences contained a 1248 bp open reading frame that encoded 415 amino acids. The putative protein sequences have a typical LFY family domain. In addition, AfLFY was expressed at the highest levels in young leaves of the vegetative stage and in the shoot apical bud meristem of the reproductive stage. Phylogenetic analysis showed that AfLFY was most closely related to DFL from Chrysanthemum lavandulifolium. Subcellular localization studies revealed that AfLFY localized to the nucleus. Heterologous expression of AfLFY in transgenic tobacco plants shortened its period of vegetative growth, converted the lateral meristems into terminal flowers and promoted precocious flowering. In addition, transgenic plants exhibited obvious morphological changes in leaf shape. qRT-PCR analysis indicated that the expression levels genes related to flowering, FT, SOC1, and AP1 were significantly upregulated in AfLFY transgenic plants. Our findings suggested that the AfLFY gene plays a vital role in promoting flowering and leaf development in A. frutescens. These results laid a foundation for us to understand the mechanism of AfLFY in regulation flowering, and the results will be helpful in improving A. frutescens through molecular breeding.
Similar content being viewed by others
Introduction
Flowering is a vital component of the plant life cycle that influences the success of plant reproduction. The transition from vegetative to reproductive development occurs in response to various endogenous and exogenous cues, and these cues are integrated by a complex molecular network1,2,3. Many floral integrator genes are involved in this molecular network, such as FLOWERING LOCUST (FT) and SUPPRESSOR OF OVEREXPRESSION OF CONSTANS 1 (SOC1), which work with the floral meristem identity gene LEAFY (LFY), to initiate the growth of floral meristems4,5,6,7. CONSTANS (CO) mediates the floral process in the photoperiod pathway and activates LFY expression directly or indirectly through other floral integrators8,9,10.
LFY genes were initially described as floral meristem identity genes in Antirrhinum and Arabidopsis11,12, and were later shown to act as genetic switches directing the transition from inflorescence meristems to flower meristems13. Overexpression of LFY homologs stimulated flower initiation in both dicotyledonous and monocotyledonous species14,15,16,17. LFY is thought to act as a signaling gateway, integrating signals from global floral pathway processes and activating downstream ABC genes that specify unique floral meristem and organ identities18,19,20,21,22,23. For example, APETALA1 (AP1), which determines floral meristem and organ identities in Arabidopsis, is directly activated by LFY24,25,26,27.
LFY homologs have been identified among distantly related species28,29,30,31,32,33,34,35. LFY proteins from most species share conserved regions, such as a proline-rich region, a leucine zipper, an acidic region, and a basic region formed by an arginine core and lysine residues; however, in some cases the protein structures and gene expression patterns differ among species. For example, the proline-rich region is absent in gymnosperms, eucalyptus, cotton, and papaya, among others36,37,38,39,40. FLO in Antirrhinum majus was only found to be expressed in the reproductive phase. Expression of GhLFY in Gerbera hybrida is restricted to the reproductive transformation phase and to early flower development41. Although associated with flowering, low levels of LFY transcripts were also detected in vegetative tissues during the vegetative growth in some plant species33,34,35,38,41,42,43,44,45. LFY expression patterns in rice and wheat differed from those of other LFY homologs. In rice, RFL transcripts were detected in the initiation of the floral meristem much later than in developing branches and young panicle roots, and no transcripts were found in mature leaves46. In wheat, WFL transcripts were observed in all layers of the young spike except in the spikelet initiation sites, axillary meristem, and developing palea47. Identification and characterization of additional LFY homologs is needed to understand the evolution and functions of LFY and its specific motifs in flowering regulation.
LFY-like genes from Chrysanthemum are highly expressed in the flower bud33,34, which suggests that Chrysanthemum LFY genes may play important roles in the transition from vegetative to reproductive meristems; However, their functions remain unclear due to a lack of functional investigation. Argyranthemum frutescens (Asteraceae) is a popular cultivated species that is used globally as a potted plant, ground cover, or a garden ornamental for its foliage, flower color48,49 and long flowering period. However, the current research on A. frutescens has mostly focused on medicinal purposes, pathogen invasion and plant diseases50,51,52,53,54,55, and the genetic mechanisms underlying floral development in A. frutescens are not well known. Here, the LFY homolog AfLFY was identified and characterized in A. frutescens. The expression patterns of AfLFY in different tissues and organs were examined by quantitative real-time PCR (qRT-PCR). The protein subcellular localization was investigated by transient expression in onion epidermal cells. The function of AfLFY was explored by studying heterologous expression in Nicotiana tabacum L (tobacco). These studies contribute to understanding the molecular mechanisms of AfLFY in regulating floral development and will be helpful for molecular breeding of A. frutescens.
Materials and Methods
Plant material
A. frutescens plants used in this study were collected from Shennongjia in Hubei Province, China, and were planted in the nursery garden of Northeastern University, China. Plant tissues for RNA extraction were collected at the relevant developmental stages and were immediately frozen in liquid nitrogen before storing at −80 °C. Total RNA was extracted using a Plant RNA kit (Omega, USA) and then was treated with DNase I (Omega, USA) to remove genomic DNA. Genomic DNA was isolated from fresh leaves using the CTAB method described by Couch and Fritz with minor modifications56.
Cloning AfLFY
First-strand cDNA was synthesized from a library of inflorescence shoot apices using a Revert Aid First Strand cDNA Synthesis kit (Thermo, USA). Full-length AfLFY cDNA was obtained by RT-PCR with primers AfLFY–F and AfLFY–R, which were designed against LFY homolog from chrysanthemum (Table 1). PCR amplifications was performed in 25 µL reaction volumes containing 1.5 μL of cDNA, 0.3 μL of LA Taq polymerase (TaKaRa, Japan), 2.5 μL of 10 × LA Buffer, 2.0 μL of dNTPs, 1.0 µL of each primer, and 16.7 μL of ddH2O. Thermocycling conditions were as follows: denaturation for 5 min at 95 °C; 35 cycles of 95 °C for 50 s, annealing at 55 °C for 30 s, and extension at 72 °C for 90 s; and final extension at 72 °C for 10 min. PCR products were assessed using 1% agarose gel electrophoresis and then were purified using a Trace agarose gel DNA recovery kit (Zhongmeitaihe, Beijing China). Purified PCR products were cloned and transformed into E. coli using a pCloneEZ-TA-Amp/HC Cloning kit (Thermo, USA). Transformed colonies were identified by PCR with gene-specific primers and restriction digestion, and six positive clones were confirmed by sequencing (Zhongmeitaihe Gene Company, Beijing, China).
The full AfLFY gene was amplified from genomic DNA using long PCR with primers LK and LB, as previously described57. PCR products were subcloned and sequenced as described above.
Sequence analysis
BLAST online searches were used to confirm that sequences from selected clones were LFY homologs. Predicted protein sequences encoded by LFY homologs were retrieved from GenBank (http://www.ncbi.nlm.nih.gov/entyez/query.fcgi and http://blast.ncbi.nlm.nih.Gov/Blast.cgi) and were used to confirm sequence identification and perform phylogenetic analysis. Amino acid sequences were aligned using Geneious 9.0 and a neighbor-joining tree was constructed in MEGA 6 using Kimura two-parameter distances and pairwise deletion of gaps.
Quantitative real-time PCR expression analysis
The temporal and spatial expression patterns of AfLFY were examined during vegetative and reproductive growth. Total RNA was extracted from roots, stems, leaves, and shoot apical meristems during vegetative development and roots, stems, leaves, inflorescence shoot apices meristems (reproductive bud), 1 mm flower buds (flower bud), 5 mm flower buds (alabastrum) and fully opened flowers during the reproductive phase. RNA (1 μg) was reverse transcribed to generate cDNA using a Revert Aid First Strand cDNA Synthesis kit (Thermo, USA). Quantitative real-time PCR was performed in 20 μL reaction volumes containing 2.0 μL of a 1:5 dilution of the cDNA, 1.0 µL of each primer, 10 μL of SYBR Green Master Mix (Applied Biosystems) and 6.0 μL of ddH2O. The qPCR conditions were as follows: denaturation at 55 °C for 2 min, 95 °C for 2 min, followed by 40 cycles at 95 °C for 15 s, 60 °C for 1 min on ABI 7500 System thermocycler (Applied Biosystems). The specificity of the amplified product was verified by a melting curve from 60 to 95 °C. Specific primers for qRT-PCR analysis of AfLFY (primers qAfLFYF2 and qAfLFYR3) were designed using Primer Express 3.0 (Table 1). Actin was used as an internal reference for normalization of relative expression levels of selected genes. The primers used were actin1 and actin234 (Table 1). Three technical replicates were performed for each sample. The 2−ΔΔCt method was used to calculate relative gene expression58.
Subcellular localization and transient expression
Subcellular localization of AfLFY was examined using transient expression in onion epidermis. The AfLFY coding sequence without the termination codon was amplified with primers harboring BamH I and Kpn I restriction sites, and it was inserted into the pBI121-EGFP vector to generate the expression vector pBI121-AfLFY-EGFP. Agrobacterium tumefaciens carrying the pBI121-AfLFY-EGFP plasmid was inoculated into 50 mL of LB liquid medium. Onion epidermis was prepared and transfected as described previously59,60,61. After incubation in darkness for 14 h, the onion epidermis was collected and washed, placed on a slide, and observed under a confocal laser microscope (Leica TSP5) with excitation at 488 nm wavelength to monitor EGFP expression.
Construction and transformation of AfLFY in tobacco
The AfLFY coding sequence was amplified with primers harboring BamH I and Kpn I restriction sites, and it was inserted into the pBI121 vector to generate the expression vector pBI121-AfLFY to examine its biological function. The pBI121-AfLFY was transformed into N. tabacum using leaf discs as described previously with the help of Agrobacterium strain EHA10562, and it was cultured in a series of MS media with antibiotics. The rooted transformants were planted in soil and grown in long day (LD) (16 h light/8 h dark) conditions.
Identification and phenotype analysis of transgenic tobacco plants
Fresh leaves were used to extract genomic DNA and total RNA from wild-type and transgenic tobacco plants to perform genome PCR, RT-PCR and qRT-PCR to verify transformation of plants. The PCR reactions were performed with the AfLFY gene-specific reverse primers. To examine the expression levels of CO, FT, AP1, SOC1 in the transgenic tobacco lines, transgenic plants were sampled for qRT-PCR analysis 0-, 7-, 14-, 21-, and 28 days after planting in soil. The actin gene of tobacco was used as an internal control. Specific primers were designed for CO, FT, AP1 and SOC1 according the tobacco sequence (Table 1). Time from rooted transgenic tobacco plantlets into an artificial soil to the first flower visible was regarded as flowering time. The date, height and number of leaves for each transgenic line were recorded when the first flower was visible. A minimum of three independent samples were conducted for each analysis. SPSS software was used to perform the statistical analyses.
Results
Cloning and sequence analysis of AfLFY
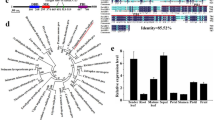
The full-length cDNA of the LFY-like gene was successfully amplified and cloned using primers designed against LFY homologous sequences. Two haplotypes were obtained among eight positive sequences. There was more than 98% identity between these two sequences. The 2 haplotypes had the same length and only had some differences in nucleotide composition. Both sequences were 1,248 bp in length and encoded 415 amino acids. (GenBank accession number: MK990596, MK990597, named AfLFYa and AfLFYb). The AfLFY protein contained the typical LFY domain: a leucine zipper, an alkaline region rich in arginine and lysine, and a central acidic region, which were also found in FLO/LFY proteins from other seed-bearing plant species (Fig. 1A). Two distinct copies of AfLFY (3024 bp and 3,123 bp) were also found in the A. frutescens genome using genomic PCR, both of which contained three exons and two introns (MG973291–MG973296, Fig. 1B). Comparison of the predicted AfLFY protein sequences with those of other FLO/LFY-like proteins showed that sequence identity between AfLFY and other LFY homologues ranged from 57% to 93%. Among them, AfLFY shared 72% identity with FLO from Antirrhinum majus, 58% with LFY from Arabidopsis, and 93% with DFL from C. lavandulifolium (Fig. 2A). These results confirmed that the sequences isolated from A. frutescens were LFY homologs. Predicted LFY amino acid sequences were used for phylogenetic analysis to determine the evolutionary relationship between AfLFY and other LFY-like proteins (Fig. 2B). Two main clades were apparent, representing monocotyledonous and dicotyledonous species. Sequences from the same taxa were clustered together. The sequences from Asteraceae species were clustered into the dicotyledonous group and were then further clustered into a subclade with a 99% bootstrap support value that was consistent with biological evolution. AfLFY was most closely related to DFL from C. lavandulifolium.
Structure of AfLFY in A. frutescens. (A) Nucleotide sequence of the open reading frame of AfLFY and the resultant amino acids. The N-terminal conserved region is indicated by a thin solid line; leucine zipper motif is indicated by a dotted line; the basic region is indicated by a dashed line; the central acidic region is indicated by a double solid line; and the C- terminal conserved region is indicated by a thick solid line. (B) Structure of the full-length AfLFYa and AfLFYb gene. Exons are indicated by black boxes and introns are represented by thin lines. Numbers indicate the size of each fragment. Start (ATG) and stop (TAG) codons are shown.
Comparison of AfLFY with LFY homologs. (A) Amino acid sequence alignment of AfLFY and plant LFY proteins, the conserved region and typical motif of LFY were indicated same as in Fig. 1. (B) Phylogenetic analysis of plant LFY amino acid sequences. PhapLFY, Phalaenopsis aphrodite (KP893636), CsLFY, Cynara scolymus (XP_024970576.1), DFL, Chrysanthemum lavandulifolium (AAT51708.1), FLO, Antirrhinum majus (AAA62574.1), MiLFY, Mangifera indica (ADX97320.1), LFY, Arabidopsis thaliana (AAM27931.1), RFL, Oryza sativa (BAA21547.1), TeLFY, Tagetes erecta (AEG88962.1), LsLFY, Lactuca sativa (XP_023744034.1), HaLFY, Helianthus annuus (XP_021984216.1), NF1, Nicotiana tabacum 1 (AAC48985.1), NF2, Nicotiana tabacum 2 (AAC48986.1), and ZmLFY, Zea mays (ABC69153.1).
Expression of AfLFY in A. frutescens
The transcription of AfLFY in different tissues of A. frutescens at the vegetative stage and reproductive stage was investigated using qRT-PCR. AfLFYa and AfLFYb showed identical expression patterns. For the discussion below, we only present results obtained from AfLFYa. AfLFY expression was observed in all tested tissues, namely, roots, leaves, stems, shoot apical meristems, and flower buds (Supplementary Fig. S1). During vegetative growth, AfLFY was most highest expressed in young leaves followed by stems and roots, with weak expression in vegetative shoot apices (Fig. 3A). During reproductive development, the highest levels of AfLFY expression were detected in inflorescence shoot apices meristem. AfLFY expression decreased gradually during flower development, with minimal expression observed in fully open flowers (Fig. 3B).
Subcellular localization of AfLFY
Subcellular localization of AfLFY was examined using an EGFP-tagged fusion protein. As there was high identity between the two AfLFY sequences, the expression vector pBI121-AfLFYa-EGFP was constructed and introduced into onion epidermal cells using Agrobacterium-mediated transformation. EGFP expression was examined using fluorescence microscopy after 12–14 hours of incubation in the dark. The pBI121-AfLFYa fusion protein localized only to the nucleus; in contrast, the EGFP control was localized to the nucleus, cytoplasm, and cell membrane (Fig. 4).
Phenotypic analysis of ectopic expression of the AfLFY gene in N. tobacum
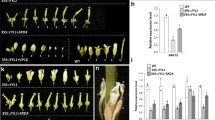
To explore the function of AfLFY in flower development, an overexpression construct with AfLFYa under the control of the CaMV 35 S promoter (35 S::AfLFY) was introduced into wild-type tobacco. Approximately 51 independent transgenic tobacco lines were obtained after rooting on MS medium containing kanamycin and rifampicin, and they were verified by genomic PCR, RT-PCR and qRT-PCR (Fig. 5; Supplementary Fig. S2). The resistant plantlets and the wild-type plants were then transferred into pots and grown in an illuminated incubator. Compared with the wild-type (Fig. 6A), all of the transgenic lines led to early flowering and showed obvious changes in flowering time (Table 2; Fig. 6B–H). The earliest flowering observed in transgenic plants overexpressing the AfLFY gene occurred 33 days earlier than that of the wild-type plants (Table 2). In addition, ectopic expression of AfLFY in transgenic tobacco produced more branches from the axillary and converted all lateral meristems into terminal flowers (Fig. 6B,H). Furthermore, we observed solitary flowers from unrooted shoots cultured on agar-solidified medium (Fig. 6K). Overexpression of AfLFY in tobacco also resulted in obvious changes in morphology, e.g., the tobacco leaf shape changed from circular to ovalar (Table 2; Fig. 6I,G), there was a shorter vegetative phase with fewer leaves, and they were shorter (Table 2; Fig. 6M,N).
Transgenic plantlet identification. (A) Genomic PCR; (B) RT-PCR; (C) AfLFY expression in the four transgenic lines and the wild-type as identified by qRT-PCR. Uncropped gels shown in Supplementary Fig. S2.
Phenotypic analysis of tobacco overexpressing AfLFY and wild-type tobacco. (A) The wild type plant (B) transgenic line, the arrow indicates the branches produced from the lower leaf axils. (C–G) Other transgenic lines. (H) The flowers were generated from the leaf axils. (I)Wild-type tobacco leaf. (J)The transgenic line. (K) Plantlets flowered on MS medium. (L) Days to the first flower opening. (M) Plant height at the time of first flower opening. (N) The number of mature leaves formed at the time of first flower opening.
To determine the relationships of AfLFY with other flowering related genes, we analyzed FT, SOC1, AP1 and CO transcript levels in different developmental stages of transgenic plants. AP1, FT, and SOC1 were activated by the ectopic expression of AfLFY and were expressed highest at day 7 after planting in soil (Fig. 7A,C,D). However, the expression level of CO showed only a subtle increase compared to that of wild-type plants (Fig. 7B).
Discussion
This study identified an LFY homolog in A. frutescens. Comparisons of predicted protein sequences showed that AfLFY belonged to the FLO/LFY superfamily and contained two highly conserved regions at the N- and C-termini. AfLFY contained three exons and two introns, which was consistent with other FLO/LFY homologs58,63,64 and revealed the evolutionary conservation of the LFY gene structure and LFY function in plants.
The majority of seed-bearing plants examined to date contain one copy of LFY in their genomes65. However, multicopy genes have been found in some polyploids (e. g., eucalyptus, Monterey pine, and chrysanthemum)30,36,63,66,67, suggesting that LFY gene has experienced ancient transient duplications events, and that duplicated paralogues were promptly lost in most land plants thus maintaining LFY as a single copy65. In our study, two haplotypes were identified in six clones from A. frutescens; they shared more than 94% identity and were in the same clade, which indicated that gene flow might occur in A. frutescens. The lengths of the three exons were consistent between the two sequences (469 bp, 383 bp, and 396 bp), but intron lengths differed (Fig. 1B). The lengths of intron 1 and intron 2 in A. frutescens were longer than the length of those introns in Arabidopsis LFY (470 bp, 910 bp)12, suggesting a rich variety of LFY introns among species.
Transcription analysis showed that AfLFY was abundantly expressed in inflorescence shoot apical meristems during reproductive development, suggesting a role for AfLFY in the transition from vegetative to reproductive development as with LFY homologs from other species such as Arabidopsis, chrysanthemums and orchids16,34,68. However, AfLFY expression was minimal in vegetative shoots and fully opened flowers (Fig. 3A,B). AfLFY expression levels were also highly expressed in the leaves during vegetative growth, but they decreased as the plant became mature, suggesting an important role for AfLFY in leaf development. A similar role for LFY during leaf development was proposed as a result of mutation analysis in legumes and tomato plants42,43,69. These results showed that the expression pattern of AfLFY diversified in A. frutescens and varied among different tissues and developmental growth phases.
Significant changes in the expression patterns of LFY homologs were observed in different plant. The LFY gene in Arabidopsis was weakly expressed in the leaf primordia and strongly expressed in the floral meristem and floral organ primordia, but not in the inflorescence meristem12. A pattern for LFY genes similar to that in Arabidopsis was found in Antirrhinum, except that the transcripts of FLO were not detected in the stamen primordia11. In cucumbers, CsLFY was detected in SAM, FM and floral organ primordia70. In orchids, PhapLFY was strongly expressed in developing inflorescences and leaves during vegetative stage16. In hickory, CcLFY was strongly expressed in flower buds and leaves, weakly expressed in the stem and showed no expression in the roots71. The transcripts of the LFY gene in Gerbera were absent from vegetative tissues and were shown to be restricted to young capitula with emerging flower primordia41. In our data, AfLFY was strongly expressed in the inflorescence shoot meristem and young leaves during the vegetative state. AfLFY was also detected in roots, stems, leaves and young flower buds. This various expression pattern in the LFY homologs suggests the existence of a functional divergence and differentiated regulatory mechanisms associated with the flowers of different plants33,65.
Transient expression of EGFP-tagged AfLFY in onion epidermal cells revealed localization to the nucleus. This suggested that AfLFY acted as a transcription factor, which is consistent with previous studies that proposed a transcriptional regulatory role for AfLFY in the developing flower29,35,39,70.
To explore the function of AfLFY, the 35 S::AfLFY construct was introduced into tobacco. All transgenic plants showed obvious phenotypes of early flowering and converted the lateral meristems into terminal flowers. These results were consistent with previous studies, showing LFY homologs have the ability to regulate floral meristem identity and promote flowering time and cell proliferation15,32,66,72,73,74,75. In addition, the number of leaves, leaf shape and height of transgenic plants changed (Fig. 6), which correlated with the strong expression of AfLFY in vegetative leaves of A. frutescens. These phenotypes were also observed in other LFY-like genes used in the generation of transgenic plants32,46,76. Thus, our results suggested that AfLFY displays a conserved function in regulating flowering and plays a key role in leaf development during vegetative growth in A. frutescens.
Previous studies showed that LFY together with AP1, FT, SOC1 merged the signals from multiple pathways to determine the identity of floral meristems and regulate the flowering time4,54,77,78,79,80. SOC1 acted downstream of FT in the shoot apex of Arabidopsis7. In our study, the expression of endogenous genes AP1, SOC1and FT were upregulated in overexpressing AfLFY transgenic tobacco compared to what was observed in wild plants, suggesting that these genes were activated by AfLFY and led to early flowering. Thus we speculate that the function of these genes are partially redundant in promoting expression of each other, and then promoting flowering. These results indicate that AfLFY plays a central role in the flowering regulatory network. No obvious changes in CO expression were observed in transgenic plants compared with wild-type plants (Supplementary Fig. S3), which suggested that LFY might work downstream CO, and does not feedback regulate CO as observed in litchi and gloxinia’ for CO is commonly known as key upstream regulator in flowering time, while LFY works rather last step in vegetative to reproductive growth transition77,81. Further studies on determine the relationship between AfLFY and other flower related genes will give us more clues to better know the floral development process.
The mechanisms underlying floral development in A. frutescens are poorly understood. In this study, two copies of an LFY homolog, AfLFY, were identified in the A. frutescens genome. Transcriptional analysis showed that AfLFY was abundantly expressed in leaves during vegetative growth and in inflorescence shoot apices during reproductive growth, suggesting an important role for AfLFY in leaf and inflorescence development. Ectopic expression showed obvious phenotypes of precocious flowering and morphological alterations. Our findings suggested that the AfLFY gene plays a vital role in promoting flowering and leaf development in A. frutescens and might be one of the most important and last step in in vegetative to reproductive growth transition. It would be theoretical basis for foundation of key regulators work upstream. Further studies to determine target genes of AfLFY and transgenic analyses in A. frutescens would be helpful in elucidating the functions of AfLFY in regulatory networks.
References
Wellmer, F. & Riechmann, J. L. Gene networks controlling the initiation of flower development. Trends in Genetics 26, 519–527 (2010).
Li, J., Gu, H. Y., Wang, Z. M., Tang, Q. L. & Song, M. Research progress of flowering gene regulatory networks in Arabidopsis thaliana. Biotechnology Bulletin 12, 1–8 (2014).
Wils, C. R. & Kaufmann, K. Gene–regulatory networks controlling inflorescence and flower development in Arabidopsis thaliana. Biochimica et Biophysica Acta 1860, 95–105 (2017).
Lee, H. et al. The AGAMOUS-LIKE 20 MADS domain protein integrates floral inductive pathways in Arabidopsis. Gene & Development 14, 2366–2376 (2000).
Borner, R. et al. A MADS domain gene involved in the transition to flowering in Arabidopsis. The Plant Journal 24, 591–599 (2000).
Moon, J. et al. The SOC1 MADS-box gene integrates vernalization and gibberellin signals for flowering in Arabidopsis. Plant Journal 35, 613–623 (2003).
Liu, C. et al. Direct interaction of AGL24 and SOC1 integrates flowering signals in Arabidopsis. Development 135, 1481–1491 (2008).
Putterill, J., Robson, F., Lee, K., Simon, R. & Coupland, G. The CONSTANS gene of Arabidopsis promotes flowering and encodes a protein showing similarities to zinc finger transcription factors. Cell 80, 847–857 (1995).
Yanovsky, M. J. & Kay, S. A. Molecular basis of seasonal time measurement in Arabidopsis. Nature 419, 308–312 (2002).
Valverde, F. et al. Photoreceptor regulation of CONSTANS protein in photoperiodic flowering. Science 303, 1003–1006 (2004).
Coen, E. S. et al. Florieaula: A homeotic gene required for flower development in antirrhnum majus. Cell 63, 1311–1322 (1990).
Weigel, D., Alvarez, J., Smyth, D. R., Yanofsky, M. F. & Meyerowitz, E. M. LEAFY controls floral meristem identity in Arabidopsis. Cell 69, 843–859 (1992).
Yanofsky, M. F. Floral meristems to floral organ: genes controlling early events in Arabidopsis flower development. Annual Review of Plant Molecular Physiology 46, 167–188 (1995).
Weigel, D. & Coupland, G. LEAFY blooms in aspen. Nature 377, 482–483 (1995).
Ahearn, K. P., Johnson, H. A., Weigel, D. & Wagner, D. R. NFL1, a Nicotiana tabacum LFY–like gene, controls meristem initiation and floral structure. Plant Cell Physiology 42, 1130–1139 (2001).
Jang, S. H. Functional Characterization of PhapLEAFY, a FLORICAULA/LEAFY ortholog in Phalaenopsis Aphrodite. Plant Cell Physiology 56, 2234–2247 (2015).
Liu, Y. X. et al. Over–expression of EjLFY–1 Leads to an early flowering habit in strawberry (Fragaria x ananassa) and its asexual progeny. Frontiers in Plant Science 8, 496 (2017).
Nilsson, O., Lee, I., Blazquez, M. A. & Weigel, D. Flowering–time genes modulate the response to LEAFY activity. Genetics 150, 403–410 (1998).
Blázquez, M. A. & Weigel, D. Integration of floral inductive signals in Arabidopsis. Nature 404, 889–892 (2000).
Lamb, R. S., Hill, T. A., Tan, Q. K. G. & Irish, V. F. Regulation of APETALA3 floral homeotic gene expression by meristem identity genes. Development 129, 2079–2086 (2002).
Moon, J., Lee, H., Kim, M. & Lee, I. Analysis of flowering pathway integrators in Arabidopsis. Plant Cell Physiology 46, 292–299 (2005).
Benlloch, R., Berbel, A., Serrano–Mislata, A., Madueñol, F. & Notes, A. Floral initiation and inflorescence architecture: a comparative view. Annals of Botany 100, 659–676 (2007).
Liu, C. & Thong, Z. Coming into bloom: the specification of floral meristems. Development 136, 3379–3391 (2009).
Mandel, M. A. & Yanofsky, M. F. A gene triggering flower formation in Arabidopsis. Nature 377, 522–524 (1995).
Goloveshkina, E. N., Shul’ga, O. A., Shchennikova, A. V., Kamionskaya, A. M. & Skryabin, K. G. Constitutive Expression of the Sunflower and Chrysanthemum Genes of the AP1/FUL Group Changes Flowering Timing in Transgenic Tobacco Plants. Doklady Biological Sciences 434, 322–324 (2010).
Ruokolainen, S. et al. Research article Characterization of SQUAMOSA -like genes in Gerbera hybrida, including one involved in reproductive transition. BMC Plant Biology 10, 128 (2010).
Winter, C. M. et al. LEAFY target genes reveal floral regulatory logic, cis motifs, and a link to biotic stimulus response. Developmental Cell 20, 430–443 (2011).
Carmona, M. J., Cubas, P. & Martinze–Zapater, J. M. VFL, the Grapevine FLORIVAULA /LEAFY ortholog, is the expressed in meristematic regions independently of their fate (Vitis vinifera). Plant Physiology 130, 68–77 (2002).
Zhang, C. S., Zhang, H. W., Zhan, Z. X. & Liang, Y. Molecular cloning, expression analysis and subcellular localization of LEAFY in carrot (Daucus carota L.). Molecular Breeding 36, 89 (2016).
Yang, T., Du, M. F., Guo, Y. H. & Liu, X. Two LEAFY homologs ILFY1 and ILFY2 control reproductive and vegetative developments in Isoetes L. Scientificreports 7, 225 (2017).
Zhang, D. et al. Functional analysis of a homologue of the FLORICAULA/LEAFY gene in litchi (Litchi chinensis Sonn.) revealing its significance in early flowering process. Genes & Genomics 40, 1259–1267 (2018).
Ahmad, S. et al. Isolation, functional characterization and evolutionary study of LFY1 gene in Prunus mume. Plant Cell, Tissue and Organ Culture 136, 523–536 (2019).
Ma, Y. P., Fang, X. H., Chen, F. & Dai, S. L. DFL, a FLORICAULA/LEAFY homologue gene from Dendranthema lavandulifolium is expressed both in the vegetative and reproductive tissues. Plant Cell Reports 27, 647–654 (2008).
Ma, Y. P. et al. CnFL, a FLORICAULA/LEAFY, homolog in Chrysanthemum nankingense, is dramatically upregulated in induced shoot apical meristems. Biochemical Systematics and Ecology 50, 114–120 (2013).
Zhang, T., Chao, Y., Kang, J., Ding, W. & Yang, Q. H. Molecular cloning and characterization of a gene regulating flowering time from Alfalfa (Medicago sativa L.). Molecular Biology Reports 40, 4597–4603 (2013).
Mouradov, A. et al. NEEDLY, a Pinus radiata ortholog of FLORICAULA/LEAFY genes, expressed in both reproductive and vegetative meristems. Proceedings of the National Academy of Sciences of USA 95, 6537–6542 (1998).
Southerton, S. G. et al. Eucalyptus has a functional equivalent of the Arabidopsis floral meristem identity gene LEAFY. Plant Molecular Biology 37, 897–910 (1998).
Dornelas, M. C. & Rodriguez, A. P. The tropical cedar tree (Cedrela fissilis Vell., Meliaceae) homolog of the Arabidopsis LEAFY gene is expressed in reproductive tissues and can complement Arabidopsis leafy mutants. Planta 223, 306–314 (2006).
Li, J. et al. Cloning and characterization of a FLO/LFY ortholog in Gossypium hirsutum L. Plant Cell Reports 32, 1675–1686 (2013).
Yu, Y. C. et al. WRKY71 accelerates flowering via the direct activation of FLOWERING LOCUS T and LEAFY in Arabidopsis thaliana. Plant Journal 85, 96–106 (2016).
Zhao, Y. et al. Evolutionary Co-Option of Floral Meristem Identity Genes for Patterning of the Flower-Like Asteraceae Inflorescence. Plant Physiology 172, 284–296 (2016).
Hofer, J. et al. UNIFOLIATA regulates leaf and flower morphogenesis in pea. Current Biology 7, 581–587 (1997).
Molinero–Rosales, N. et al. FALSIFLORA, the tomato orthologue of FLORICAULA and LFY, controls flowering time and floral meristem identity. Plant Journal 20, 685–693 (1999).
Tang, M. Y. et al. An ortholog of LEAFY in Jatropha curcas regulates flowering time and floral organ development. Scientific Reports 6, 37306 (2016).
Nian, Y. W. et al. Cloning and expression profile of LEAFY gene in Ananas comosus. Molecular. Plant Breeding 16, 2107–2115 (2018).
Kyozuka, J., Konishi, S., Nemoto, K., Izawa, T. & Shimamoto, K. Down–regulation of RFL, the FLO/LFY homolog of rice, accompanied with panicle branch initiation. Proceedings of the National Academy of Sciences of USA 95, 1979–1982 (1998).
Shitsukawa, N., Takagishi, A., Ikari, C., Takumi, S. & Murai, K. WFL, a wheat FLORICAULA/LEAFY ortholog, is associated with spikelet formation as lateral branch of the inflorescence meristem. Genes & Genetic Systems 81, 13–20 (2006).
Ye, J. Q. A new potted plant variety of international popular flower series–Argyranthemum frutescens. Garden 45 (2006).
Wen, Y. G. Study on National Flowers in the World. Beijing Forestry University (2013).
Paulsen, E. & Andersen, K. E. Contact sensitization to florists’ chrysanthemums and marguerite daisies in Denmark: a 21-year. Contact Dermatitis 82 (2019).
Marais, A., Faure, C., Deogratias, J. M. & Candresse, T. First Report of Chrysanthemum stunt viroid in Various Cultivars of Argyranthemum frutescens in France. APS Publications 95, 1196 (2019).
Garibaldi, A., Bertetti, D. & Gullino, M. L. Susceptibility of chrysanthemum and Paris daisy varieties to several isolates of Fusarium oxysporum f. sp. chrysanthemi. Commun Agric Appl Biol Sci 74, 651–657 (2019).
Garibaldi, A., Pensa, P. & Gullino, M. L. First Report of Sclerotinia sclerotiorum on Argyranthemum frutescens in Italy. Plant Disease 92, 1250 (2008).
Garibaldi, A., Bertetti, D., Frati, S., Minuto, A. & Gullino, M. L. Powdery Mildew Caused by Golovinomyces cichoracearum on Paris Daisy (Argyranthemum frutescens) in Italy. Plant Disease 92, 1153 (2008).
Koike, S. T., Fogle, D., Tjosvold, S. A. & King, A. I. Downy Mildew Caused by Peronospora radii on Marguerite Daisy (Argyranthemum frutescens) in California. Plant Disease 88, 1163 (2004).
Couch, J. A. & Fritz, P. J. Isolation of DNA from plants high in polyphenolics. Plant Molecular Biology 8, 8–12 (1990).
Ma, Y. P. et al. Origin of Chrysanthemum cultivars–Evidence from nuclear low–copy LFY gene sequences. Biochemical Systematics and Ecology 65, 129–136 (2016).
Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real–time quantitative PCR and the 2(–Delta Delta C(T)) method. Methods 25, 402–408 (2001).
Khan, M. R. I., Tabe, L. M., Heath, L. C., Spencer, D. & Higgins, T. J. V. Agrobacterium–mediated transformation of subterranean clover (Trifolium subterraneum L.). Plant Physiology 105, 81–88 (1994).
Hiei, Y. & Komari, T. Agrobacterium–mediated transformation of rice using immature embryos or calli induced from mature seed. Nature Protocols 3, 824–834 (2008).
Risacher, T., Craze, M., Bowden, S., Pual, W. & Barsby, T. Highly efficient Agrobacterium–mediated transformation of wheat via in planta inoculation. Methods in Molecular Biology 478, 115–124 (2009).
Sparkes, I. A., Runions, J., Kearns, A. & Hawes, C. Rapid, transient expression of fluorescent fusion proteins in tobacco plants and generation of stably transformed plants. Nature Protocols 1, 2019–2025 (2006).
Frolich, M. W. & Parker, D. S. The mostly male theory of flowers evolutionary origins: from genes to fossils. Systematic Botany 25, 155–170 (2000).
Zheng, X. Y. et al. Molecular evolution of Adh and LEAFY and the phylogenetic utility of their introns in Pyrus (Rosaceae). BMC Evolutionary Biology 11, 225 (2011).
Gao, B., Chen, M. X., Li, X. S. & Zhang, J. H. Ancient duplications and grass-specific transposition influenced the evolution of LEAFY transcription. Communication. Biology 2, 237 (2019).
Dornelas, M. C., Amaral, W. A. N. & Rodriguez, A. P. M. EgLFY, the eucalyptus grandis homolog of the Arabidopsis gene LEAFY is expressed in reproductive and vegetative tissues. Braz. J. Plant Physiology 16, 105–114 (2004).
Mellerowicz, E. J., Horgan, K., Walden, A., Coker, A. & Walter, C. PRFLL– a Pinus radiata homologue of FLORICAULA and LEAFY is expressed in buds containing vegetative shoot and undifferentiated male cone primordia. Planta 206, 619–629 (1998).
Moyroud, E., Kusters, E., Monniaux, M., Koes, R. & Parcy, F. LEAFY blossoms. Trends in Plant Science 15, 346–352 (2010).
Champagne, C. E. et al. Compound leaf development and evolution in the Legumes. Plant Cell 19, 3369–3378 (2007).
Zhao, W. S. et al. CsLFY is required for shoot meristem maintenance via interaction with WUSCHEL in cucumber (Cucumis sativus). New Phytologist 218, 344–356 (2017).
Wang, Z. J., Huang, J. Q., Huang, Y. J., Chen, F. F. & Zheng, B. S. Cloning and characterization of a homologue of the floricaula/leafy gene in hickory (Carya cathayensis Sarg). Plant Molecular Biology Report 30, 794–805 (2012).
Weigel, D. & Nilsson, O. A developmental switch sufficient for flower initiation in diverse plants. Nature 377, 495–500 (1995).
Liu, C. et al. Specification of Arabidopsis floral meristem identity by repression of flowering time genes. Development 134, 1901–1910 (2007).
Shiokawa, T. et al. Isolation and functional analysis of the CjNdly gene, a homolog in Cryptomeria japonica of FLORICAULA/LEAFY genes. Tree Physiology 28, 21–28 (2008).
Ding, F. et al. Functional analysis of a homologue of the FLORICAULA/LEAFY gene in litchi (Litchi chinensis Sonn.) revealing its significance in early flowering process. Genes & Genomics 40, 1259–1267 (2018).
Flachowsky, H., Hättasch, C., Höfer, M., Peil, A. & Hanke, M. Overexpression of LEAFY in apple leads to a columnar phenotype with shorter internodes. Planta 231, 251–263 (2010).
Simpson, G. G. & Dean, C. Arabidopsis, the Rosetta stone of flowering time? Science 296, 285–289 (2002).
Parcy, F. Flowering: a time for integration. International Journal of Development Biology 49, 585–593 (2005).
Lee, J. & Lee, I. Regulation and function of SOC1, a flowering pathway integrator. Journal of Experimental Botany 61, 2247–2254 (2010).
Hepworth, S. R. et al. Antagonistic regulation of flowering-time gene SOC1 by CONSTANS and FLC via separrate promoter motifs. EMBO Journal 21, 4327–4337 (2002).
Zhang, M. Z. et al. Overexpression of the cucumber LEAFY homolog CFL and hormone treatments alter flower development in gloxinia (Sinningia speciosa). Plant Molecular Biology 67, 419–427 (2008).
Acknowledgements
This study was supported by National Natural Science Foundation of China (No. 31872710, 31470699).
Author information
Authors and Affiliations
Contributions
Hu J. performed the gene isolation, expression analysis, vector construction, plant transformation, phenotype analysis and draft of the manuscript. Jin Q. helped in the sequence analysis and data processing. Ma Y.P. participated in the design of the study and revision of the manuscript. All authors have read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The manuscript is approved by all authors for publication and no conflict of interest exits in the submission of this manuscript.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hu, J., Jin, Q. & Ma, Y. AfLFY, a LEAFY homolog in Argyranthemum frutescens, controls flowering time and leaf development. Sci Rep 10, 1616 (2020). https://doi.org/10.1038/s41598-020-58570-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-58570-x
This article is cited by
-
Genome-wide screening and characterization of long noncoding RNAs involved in flowering/bolting of Lactuca sativa
BMC Plant Biology (2023)
-
Identification of Alfalfa SPL gene family and expression analysis under biotic and abiotic stresses
Scientific Reports (2023)
-
Molecular cloning and functional analysis of a Chrysanthemum vestitum GME homolog that enhances drought tolerance in transgenic tobacco
Scientific Reports (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.