Abstract
Gilles de la Tourette Syndrome (GTS) is a developmental disorder. Empirical studies and an emerging cognitive framework on GTS suggest that GTS is a disorder of abnormally strong ‘perception-action binding’. Theoretical considerations imply that the effectiveness of long-established behavioral interventions might be related to a normalization of increased binding in GTS. This has not been tested yet. We examined the effect of a standardized Comprehensive Behavior Intervention for Tics (CBIT) in N = 21 adolescent GTS patients and N = 21 healthy controls on perception-action binding in an inhibitory control paradigm. Prior to CBIT, GTS patients showed compromised performance compared to controls, specifically when inhibitory control was triggered by uni-modal visual compared to bi-modal stimuli. After CBIT intervention, GTS patient’s performance was at the same level as healthy controls. This is supported by a Bayesian data analysis. CBIT specifically affected inhibitory control in a condition where reconfigurations of perception-action bindings are necessary to perform inhibitory control. A power of 95% was evident for these effects. CBIT reduces increased ‘binding’ between perception and action in GTS and thereby increases the ability to perform response inhibition. The results are the first to provide insights as to why CBIT is effective by relating elements of this intervention to overarching cognitive theoretical frameworks on perception-action bindings.
Similar content being viewed by others
Introduction
Gilles de la Tourette Syndrome (GTS) is a developmental disorder with multi-faceted neuropsychiatric symptoms, as onset and highest prevalence in childhood or adolescence and is characterized by multiple motor and vocal tics1,2. Traditionally, GTS has been considered as a movement disorder. This is not undisputed though given that most tics can, at least partially, be controlled, are associated with premonitory sensations and might reflect motor learning and habit formation3. On the basis of these findings, it has recently been suggested that a hallmark of GTS is an abnormally strong interrelation of perceptual processes and motor actions, particularly between premonitory sensation including preceding urges and tics, and that GTS might be conceptualized as a disorder in which purposeful actions play an important role3. Indeed, several studies have reported that GTS patients make better use of multi- or bi-modal sensory stimuli for response selection4,5. Moreover, findings from procedural learning also suggest that GTS patients establish connections between stimuli and the corresponding response faster and more strongly6,7. In line with this, GTS is associated with increased habit formation tendencies8 depending on the establishment of strong stimulus-response mappings. These findings corroborate the notion that GTS might be a disorder characterized by an abnormally strong ‘binding’ between perception and action, for which a cognitive framework based on the Theory of Event Coding (TEC) detailing the link between perception and action is an attractive overarching concept for GTS3,9. The TEC framework states that whenever a stimulus presented and triggers a response, stimulus-action bindings are established. These bindings (associations) are stored in ‘event files’10. Once such bindings have been established, they affect subsequent actions, particularly when these are executed on the basis of only a slightly altered stimulus input. In such cases, previously established and still existing stimulus-response associations/bindings impede behavioral control10,11,12, because expectations about stimulus-response mapping become violated. Therefore, the context established by previous stimulus-response mappings profoundly affects a to be executed action. In a previous study by our group13, we provided evidence that a stronger contextual representation of stimulus-response bindings in GTS patients impedes performance to inhibit prepotent responses, when the same inhibitory control processes had to be exerted upon different sensory input.
The most established behavioral intervention technique aimed at reducing tics in GTS is habit reversal training (HRT)14,15,16. Data have already provided evidence for the efficacy of HRT in reducing tics in GTS17,18. HRT is based on the concept that patients learn to perceive premonitory urges before the tic and to apply antagonistic, competing muscle activity (movement or tension) to inhibit or counteract the occurrence of the tic. HRT builds the basis for the Comprehensive Behavioral Intervention for Tics (CBIT), for which studies also revealed a high efficacy to treat tics19,20. In CBIT, different psychoeducational elements, relaxation training and reward contingency plans are added to the HRT methods. According to an emerging view, tics may represent prefabricated actions stored in event files, which can be triggered by the appropriate perceptual input3. Replacing a tic by another response, as trained by the HRT component in CBIT, may therefore train to restructure or unbind tic-specific event files. It is possible that CBIT trains not only the tic-specific, but also the general ability of GTS patients to restructure event files. From a cognitive-theoretical, including the TEC perspective on GTS, the HRT component in CBIT may be effective because it restructures tic-specific event files. If this is the case, and the relevant mechanism underlying the HRT component in CBIT can be framed as a restructuring of event files, it is possible that the CBIT does also affect perception-action bindings impeding the ability to inhibit prepotent responses, when the same inhibitory control processes has to be exerted following different sensory input13. In the current study, we test the hypothesis that a standard CBIT intervention reduces such contextual modulations of stimulus-response bindings in adolescent GTS patients. To test this hypothesis we performed a follow-up behavioral study of the adolescent GTS patients investigated previously13. We examined performance in a response inhibition task (Go/Nogo task) and focused on the frequency of erroneous response executions in Nogo trials (i.e. false alarm rates). The Go/Nogo task was designed in a way that bindings between stimuli and responses facilitate the execution of erroneous responses in one condition of the task. In that particular condition the stimulus input was altered, as compared to the other two Nogo conditions and therefore caused problem in response inhibition whenever there is a strong binding between the more common combination of stimuli and responses (response inhibitions) in the other conditions of the task.
Results
For the reaction times (RTs) in the Go conditions, the ANOVA only revealed a main effect “condition” (F(1,40) = 35.94; p < 0.001; ηp2 = 0.473), showing that the reaction times were faster in the Gocompatible condition (496 ± 13 ms), than in the Gowithout condition (521 ± 11 ms). No other main or interaction effects were significant (all F <1.6; p > 0.2).
For the accuracy on Go trials, the ANOVA revealed no significant main or interaction effect (all F < 1.3; p > 0.2) and the overall rate of correct responses on Go trials was 95.71% (±2.1). For the rate of missed response on Go trials, the ANOVA only revealed a main effect “time point” (F(1,40) = 4.17; p = 0.048; ηp2 = 0.094), revealing that the rate of missed responses on Go trials was smaller at time point T2 (1.89 ± 0.61) than at T1 (2.92 ± 0.99). No other effects were significant (all F <1.05; p > 0.3).
However, the most important behavioral parameter is the rate of false alarms (FAs). For FAs, a main effect “condition” (F(2,80) = 76.89; p < 0.001; ηp2 = 0.658) showed that FAs were highest in the NoGoincompatible condition (25.59 ± 2.5), followed by the NoGowithout condition (18.19 ± 1.5) and the NoGocompatible condition (10.76 ± 1.6). All conditions differed from each other (p < 0.001). Importantly, there was an interaction “time point × condition × group” (F(2,80) = 4.90; p < 0.01; ηp2 = 0.109), indicating that the false alarm rate differed depending on whether GTS patients or controls were tested and whether testing took place before or after the treatment. The post-hoc power calculations revealed a power greater than 95% and it is shown that the effect size of this interaction is larger than that shown to be detectable in the sensitivity analysis (see methods section). No other main or interaction effect was significant (all F <1.2; p > 0.3). The interaction “time point × condition × group” is shown in Fig. 1.
A detailed analysis of this interaction using ANOVAs revealed the following for each time point separately: As can be seen in Fig. 1, there seem to be differential effects between experimental conditions and groups at time point T1, but not at T2. This is also revealed by the statistical models: For time point T1, the ANOVA revealed a main effect “condition” (F(2,40) = 55.46; p < 0.001; ηp2 = 0.581), showing that FAs were highest in the NoGowithout condition (28.11 ± 3.1), followed by the NoGoincompatible condition (20.04 ± 2.1) and the NoGocompatible condition (8.83 ± 1.4). All conditions differed from each other (p < 0.001). Importantly, and in line with the previous publication using a larger sample size of GTS and HCs13, there was an interaction “condition × group” (F(2,40) = 3.58; p = 0.032; ηp2 = 0.082). It is shown that in the NoGowithout condition (t(40) = −1.99; p = 0.015), the GTS group performed worse than the HCs group. No difference was evident in the NoGoincompatible condition and the NoGocompatible condition (all t < 1.01; p > 0.3). This is completely in line with the previous results by Petruo et al. (2018) and cross-validates these results. At time point T2, the ANOVA revealed a main effect “condition” (F(2,40) = 53.40; p < 0.001; ηp2 = 0.572), showing that the FAs were, again, highest in the NoGowithout condition (24.28 ± 3.1), followed by the NoGoincompatible condition (16.81 ± 1.8) and the NoGocompatible condition (6.66 ± 1.2). All conditions differed from each other (p < 0.001). Importantly, there was no interaction “condition × group” anymore (F(2,40) = 0.11; p = 0.950; ηp2 = 0.001). This is corroborated by a Bayesian data analysis using the method of Masson (2011), in which the probability of the null hypothesis being true given the obtained data p(H0/D) can be calculated. For the interaction effects “condition × group” at time point T2, this Bayes statistic revealed p(H0/D) = 0.97 and thus very strong evidence in favor for the null hypothesis. This suggests that after the CBIT intervention, no differential effects between GTS patients and controls were evident. Further tests showed that FAs in the NoGowithout condition decreased from time point T1 to T2 in the GTS group (t(20) = 4.25; p < 0.001). No changes in FAs between testing time points were evident in the healthy control group (t(20) = −0.15; p > 0.8). This shows that the interaction effect is driven by CBIT-induced changes in the GTS group in the NoGowithout condition. Correlation analyses showed that there was no linear relationship between the reduction of tics/symptoms between T1 and T2 in the GTS group (as examined using the YGTSS score) and the observed changes in the NoGowithout condition in GTS patients (r < 0.23; p > 0.4).
For the reaction times in erroneous NoGo trials, the ANOVA revealed no significant main or interaction effect (all F < 1.1; p > 0.3).
Importantly, the pattern of results for the false alarm rate data was not biased by comorbidities of the patients, or medication status. When including an additional between subject factor “comorbidity” (“yes or no”) as well as “medication” (“yes” or “no”) in the ANOVA, the results were not affected (all F < 0.55, p > 0.45). In further analyses we examined whether the scores at time point T1 in the M.I.N.I. KID and CY-BOCS modulated the pattern of results. To this end, we included these scores as a covariate in the statistical models. It is shown that these covariates did not have a significant effect (all F < 0.76, p > 0.33) and also the pattern of the other effects was unaffected. All these analyses show that the effects obtained are robust.
Discussion
In the current study, we tested the effects of CBIT in children and adolescents with GTS patients on perception-action binding that was previously tested in these patients in a cognitive neurophysiological study13. The current findings significantly extend these prior data by showing how a well-established and clinically relevant intervention to reduce tics in GTS affects perception-action bindings. Doing so, the current study connects a standard clinical intervention to a new and emerging concept of GTS, i.e. GTS and its symptoms may reflect an abnormally strong ‘binding’ between perception and action3. These aspects cannot be inferred on the basis of previously published data13 using the paradigm that is also applied in the current study. CBIT has been shown to reduce tics in GTS17,18, and this was also shown in the current study (cf. reduction in YGTSS scores). We hypothesized that a CBIT intervention would reduce, i.e. normalize, increased perception-action bindings in these patients, which has previously been shown to impede the ability to inhibit prepotent responses when the same inhibitory control processes had to be exerted upon different sensory input13. The obtained behavioral data support this hypothesis.
The false alarm rate data reflecting the tendency to execute a response in NoGo trials (refer Fig. 1) did not differ between GTS patients and HCs in any of the experimental conditions of the Go/Nogo task. This was supported by Bayesian analysis providing very strong evidence for the null hypothesis. GTS patients and HCs did not differ from each other in the NoGocompatible and the NoGoincompatible condition prior to the conduction of CBIT. In the NoGocompatible and the NoGoincompatible condition, concurrent auditory information was evident that was either facilitating or impeding performance to inhibit a response in these NoGo trials. In the NoGocompatible and the NoGoincompatible conditions, both groups revealed modulatory effects that are well in line with the literature21,22, i.e., worse response inhibition performance when auditory information was conflicting with behaviorally relevant visual information. This pattern was unchanged following CBIT.
In contrast, performance in the NoGowithout condition differed between groups at baseline (i.e. prior the CBIT in the GTS group). Since the NoGowithout condition occurred as often as the multi-modal NoGo conditions (i.e. frequency NoGowithout = frequency NoGoincompatible + frequency NoGocompatible), the group difference in the NoGowithout condition cannot be attributed to a simple trial frequency effect. The important characteristic of the NoGowithout condition is that responses have to be inhibited on the basis of purely visual information. In the other conditions, concurrent auditory information was evident. Thus, in the NoGowithout condition, action control has to be exerted on the basis of altered/reduced perceptual information; i.e. in an altered context. Whereas before the intervention, the false alarm rate was significantly larger in GTS patients indicating deficits in adapting performance to an altered context, there was no group difference following the CBIT anymore, i.e. false alarms were reduced by the CBIT intervention in GTS patients. Thus, CBIT normalizes or at least reduces the increased ‘binding’ between perception and action in GTS and thereby increases the ability to perform response inhibition. The results that CBIT normalized perception-action bindings in GTS patients to the level of HCs is supported by the Bayesian data analysis results. The finding that the control group did not reveal modulations in performance in the NoGowithout condition between the repeated testings clearly shows that the changes in the data pattern are driven by the CBIT intervention in the GTS group. However, the statistical analysis showed that even though there was a reduction of tics, as assessed using the YGTSS, there was no direct linear correlation between the changes in the task performance and the reduction of tics in the YGTSS. Although this might at first sight undermine the concept and interpretation that the clinical symptoms in GTS and perception-action share a common ground it is important to consider that correlations can only capture linear relationships. However, whether the relationship between tics/GTS symptoms and the degree of alteration in perception-action bindings is in fact linear, is currently unclear. Moreover, it is important to consider that the YGTSS, albeit very useful to capture the overall severity of GTS, is not an objective tic measure because it is based on patient interview or and clinical judgement and taking into account aspects other than tic frequency including symptom intensity, distribution, complexity and interference with other activities. A more objective clinical measures (like the Rush Score23) might have been better suited to directly capture tic frequency. This, however, was not available in the current study, which is a limitation. However, the obtained findings can be explained as follows:
According to the TEC framework, deficits in performance may occur when identical action programs have to be executed on the basis of altered stimulus input10. This is particularly the case when bindings between perception and action, i.e. between stimulus and response features within an event file are particularly strong10,11,24. Bi-modal stimuli establish strong stimulus-response associations25, particularly in GTS5. It has been suggested that difficulties to reconfigure an event file and to bind uni-modal stimuli to the same action (i.e. the inhibition of a response) is difficult in GTS13. Since behavioral performance in the uni-modal NoGowithout condition was improved by the CBIT intervention, the data suggest that the CBIT fosters the ability to reconfigure event files in GTS. It seems that as a consequence of the CBIT intervention GTS patients are better able to reconfigure event files and perform the same action (i.e. the inhibition of a response) on the basis of an altered sensory input. Behavioral interventions like HRT as the basis of CBIT aim at modifying the association between tics and triggering factors and tics19,20. During HRT/CBIT, patients learn to perform an action that is physically incompatible with the tic20,26; i.e. they learn to associate identical antecedent conditions or sensory information (e.g. pre-monitory urges) with different actions. From a cognitive-theoretical, or a theory of event coding perspective, the HRT/CBIT modulates factors playing an important role in perception-action bindings and patients learn to better reconfigure event files leading to tics. In the current study, high levels of cognitive control in the NoGowithout condition require to perform similar actions on the basis of altered sensory input. While this is not directly trained in HRT/CBIT, the important aspect is that in both cases (i.e. ability to associate different actions with the identical sensory information or the different sensory input to similar actions) a reconfiguration of event files is necessary. From a TEC perspective on GTS3, it seems that HRT/CBIT trains not only the tic-specific, but also the general ability of GTS patients to restructure event files. Therefore, HRT/CBIT is not only effective to reduce tics19,20, but also normalizes alterations in higher level cognitive functions. While this has also been shown previously27, the results from the current experiment directly testing effects of bindings between perception and action are the first to provide insights into the mechanisms in relation to influencial cognitive theoretical frameworks that can explain why HRT/CBIT is effective. However, the current findings cannot answer the question whether the HRT/CBIT actually reduces abnormally strong bindings between perception and action in GTS that may emerge as a consequence of structural and functional changes in fronto-striatal circuits of3,28,29, or does ‘just’ increase the patient’s ability to handle abnormally strong bindings between perception and action more efficiently. An important aspect to consider is whether the effects obtained simply reflect learning effects in the sense that the task becomes easier to accomplish and that differences in learning effects in GTS and controls may contribute to the pattern of results. Even though this cannot be fully excluded due to the limitation of the study that no GTS group was included that did not receive any intervention, it is unlikely that the effects are simply due to learning or task-familiarization effects. The reason is that learning or task-familiarization leads to more automated processes30. Importantly, response inhibition processes are compromised by more automated responding31,32,33,34,35,36. If the observed effects had come about through pure learning, this would have led to a higher degree of automation in the reaction tendency. As a consequence, the rate of false alarms would have increased. This, however, was not the case in any of the conditions in the applied paradigm. Therefore, learning effects are unlikely to explain the pattern of results.
Materials and Methods
GTS patients and healthy controls
This is a follow-up study of Petruo et al. (2018) testing patients with GTS diagnosed according to DSM-IV using an auditory-visual Go/NoGo task. For the follow-up, a subsample of N = 21 GTS out of N = 35 previously studied patients between 9 to 19 years was available (mean age 13.09 ± 2.1; mean IQ = 107.13 ± 10.5). These N = 21 patients were tested at baseline and 8 weeks later following CBIT training that was carried out in the outpatient clinical of the Department of Child and Adolescent Psychiatry (Faculty of Medicine, TU Dresden, Germany). The Yale Global Tic Severity Scale (YGTSS) was administered before and after the conduction of the CBIT protocol. Only this scale was assessed before and after the CBIT training, since the training is specifically targeted to reduce tics. CBIT reduced tics as indicated by a significant decrease in the YGTSS score between the time point before CBIT training (39.85 ± 2.81) and after CBIT training (29.41 ± 3.51) (t(20) = 4.89; p < 0.001). The mean total TS-score before CBIT intervention was 17.38 ± 2.12 and decreased to 13.04 ± 1.85 after CBIT intervention (t(20) = 4.01; p < 0.001). Before CBIT intervention clinical scales such as the, M.I.N.I. KID (Mini International Neuropsychiatric Interview for Children and Adolescents), CY-BOCS (Children’s Yale-Brown Obsessive-Compulsive Scale), were conducted, but not after the CBIT training, since CBIT training mainly target tics. Out of the N = 21 patients, N = 1 also had a diagnosis of attention deficit hyperactivity disorder and N = 5 a diagnosis of obsessive-compulsive disorder with a mean CY-BOCS Score of 8.5 (±3.11). N = 4 of the patients were on medication during both time points including treatment with Tiapride (N = 2), Aripiprazole (N = 1), or Fluoxetine (N = 1). The doses of the drugs were not changed between the testing time points T1 and T2.
A sample of N = 21 age and gender-matched healthy controls (HCs) was also tested twice. The HCs did not show psychiatric disorders as indicated by the M.I.N.I. KID and had no history of psychiatric disorders. The mean age was mean age 12.78 ± 2.3 years, the mean IQ was 105.78 ± 9.8. The time delay between the testing was the same as for the GTS group. As with the previous study, the groups did not differ regarding the distribution of males and females (chi2 < 0.024, p = 0.90) and there were no age or IQ differences (t < 0.2; p > 0.7).
Power considerations
For the available sample size for the GTS and the control group, a ‘sensitivity analysis‘ was conducted using G*Power37. This analysis shows that with the used study design an effect size of f = 0.22, which equals a partial eta squared (ηp2) of 0.05, can be detected with a power of 90% (5% type 1 error probability). Notably, the obtained effect size in the critical effect was larger (i.e. ηp2 = 0.109; see results section). This shows that the study is sufficiently powered to reveal reliable effects.
Ethical considerations
The study was approved by the ethics committees of the Universities of Dresden and Lübeck. Written informed consent was obtained from the subjects and their legal guardians. All methods and study procedures were performed in accordance with relevant guidelines and regulations.
Task
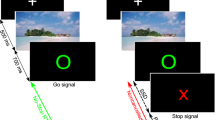
The task, an auditory-visual Go/NoGo task, was identical to a previous study in GTS patients13. It is shown in Fig. 2.
Illustration of the experimental paradigm. Upon presentation of the “PRESS” stimulus, a response had to be executed. Upon presentation of the “STOPP” stimulus, the response had to be inhibited. These conditions could either occur without or with concomitant auditory stimuli, or with an auditory stimulus. The experimental setup creates a context in which trials with bi-modal stimuli were more frequent than trials with uni-modal stimuli. Therefore, this context can interfere with performance in the Nogowithout condition.
During NoGo trials, the word stop (German: STOPP) was presented on the screen and participants had to refrain from responding. During Nogo trials, auditory sensory information was presented simultaneously to the visual NoGo stimulus. The stimuli were spoken words “DRÜCK” or “STOPP” (spoken by the neutral female computer voice of “google translate”). The length of visual and auditory stimuli was the same (400 ms). There were N = 72 NoGo trials in which an auditory NoGo stimulus (“STOPP”) was presented. Visual and auditory information were thus compatible (NoGocompatible). In another N = 72 NoGo trials, the word “DRÜCK” was the concurrent auditory stimulus, thus creating a conflict between the auditory word and the visual stimulus (NoGoincompatible), which increases demands on response inhibition. The remaining 144 NoGo trials were not accompanied by an auditory stimulus (NoGowithout). Thus, 50% of Nogo trials were uni-modal visual and the other 50% of Nogo trials were audio-visual. Since the uni-modal NoGo condition occurred as often as the multi-modal Nogo conditions differential group effects in the uni-modal Nogo condition (NoGowithout) cannot reflect a trivial frequency effect.
Go trials required the participants to press a response key, whenever the word “press” (German: “DRÜCK”) was presented. N = 336 Go trials were presented without any additional auditory stimulus and N = 336 trials with compatible auditory stimulus (i.e. the spoken word “DRÜCK” was presented). All participants received the instruction to respond only to visual stimuli and to ignore auditory stimuli. The higher ratio of Go trials than NoGo trials induces a pre-potent response tendency and increases demands on response inhibition processes. Trials were randomized and divided into six blocks with equal trial numbers. It was ensured that the frequency of the different Go and NoGo conditions was presented in each block. The inter-trial intervals varied between 1700 and 2100 ms. Each trial was terminated after the response. If no response was executed, the trial was terminated after 1000 ms. Go trials were rated as correct, if the response was given within 1000 ms after stimulus presentation. NoGo trials were treated as correct, if no response was executed within these 1000 ms. A standardized instruction was given and a practice run of 60 trials was executed to familiarize participants with the task. The rate of false alarms in each Nogo trial condition were calculated by counting the number of erroneous responses in a particular condition and dividing that number by the number of trials presented in each Nogo trial condition. For the accuracy on Go trials, the number of trials with correct responses were counted and divided by the number of presented Go trials. These parameters were used for the statistical analysis.
Comprehensive behavior intervention for tics (CBIT)
The CBIT was conducted according to previously established procedures and therapy manuals20,26. Briefly, CBIT was administered as an individualized intervention and occasionally a parent was included in sessions. CBIT includes HRT as a competing response training, but also psychoeducation about tic disorders, tic-awareness training, relaxation training, and functional analysis. Functional analysis identifies the events and situations that influence tics and develops strategies to manage these situations. Awareness training involves the detection of premonitory urges, which helps the patient to foresee a tic and intervene early. Psychoeducation included information about the neurobiological and genetic nature of tics including information about the clinical course of the disease. During competing response training, the patient is trained to develop behavior that is physically incompatible with the tic. For that, the patients learn to make use of the premonitory urges. The CBIT intervention was conducted by VP and BB who hold a master’s degree in clinical psychology and were previously trained to reliability conduct CBIT as outlined in the manual26. Supervision took place on a weekly basis by VR and all treatment sessions were video-taped. CBIT was conducted for a period of 8 weeks with weekly sessions. The first session was 90 minutes, all other sessions 60 minutes long. All patients were invited to a booster session 3 months after the end of the CBIT.
Statistical analyses
Mixed effects ANOVAs were used to analyze the behavioral data (hits, misses and FA rates, as well as corresponding RTs). The factor “group” (GTS vs. HCs) was used as between-subject factor. For the analysis of Nogo trials, the factor “condition” (NoGowithout/NoGocompatible/NoGoincompatible) was used as within-subject factor. In all analyses, the factor “time point” (before vs. after CBIT in the GTS group) served as additional within-subject factor. The analysis of Go trials comprised the factor “condition” (i.e., Gowithout and the Gocompatible). Greenhouse-Geisser correction was applied and all post-hoc tests were Bonferroni-corrected. For the descriptive statistics the mean and standard error of the mean (S.E.M.) are given. In addition to classical statistics, also Bayesian statistics were carried out to quantify the evidence for the null hypothesis in the case of non-significant results in the ANOVA. This is important because the hypotheses actually depend on the lack of differences in one task condition after the HRT. To do so, we used the method by Masson38, in which the probability of the null hypothesis being true given the obtained data p(H0/D) can be calculated on the basis of the ANOVA results.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Diagnostic and statistical manual of mental disorders: DSM-5. (American Psychiatric Publ, 2013).
Du, J.-C. et al. Tourette syndrome in children: an updated review. Pediatr. Neonatol. 51, 255–264 (2010).
Beste, C. & Münchau, A. Tics and Tourette syndrome - surplus of actions rather than disorder? Mov. Disord. 33, 238–242 (2018).
Beste, C. et al. Altered perceptual binding in Gilles de la Tourette syndrome. Cortex 83, 160–166 (2016).
Brandt, V. C., Stock, A.-K., Münchau, A. & Beste, C. Evidence for enhanced multi-component behaviour in Tourette syndrome – an EEG study. Sci. Rep. 7 (2017).
Shephard, E., Groom, M. J. & Jackson, G. M. Implicit sequence learning in young people with Tourette syndrome with and without co-occurring attention-deficit/hyperactivity disorder. J Neuropsychol, https://doi.org/10.1111/jnp.12167 (2018).
Takács, Á. et al. Is procedural memory enhanced in Tourette syndrome? Evidence from a sequence learning task. Cortex 100, 84–94 (2018).
Delorme, C. et al. Enhanced habit formation in Gilles de la Tourette syndrome. Brain 139, 605–615 (2016).
Hommel, B., Müsseler, J., Aschersleben, G. & Prinz, W. The Theory of Event Coding (TEC): a framework for perception and action planning. Behav. Brain Sci. 24, 849–878, discussion 878–937 (2001).
Hommel, B. Event files: feature binding in and across perception and action. Trends Cognit. Sci. 8, 494–500 (2004).
Colzato, L. S., Warrens, M. J. & Hommel, B. Priming and binding in and across perception and action: A correlational analysis of the internal structure of event files. Q. J. Exp. Psychol. 59, 1785–1804 (2006).
Hommel, B. Action control according to TEC (theory of event coding). Psychol. Res. 73, 512–526 (2009).
Petruo, V. et al. Altered perception-action binding modulates inhibitory control in Gilles de la Tourette syndrome. J. Child. Psychol. Psychiatry. https://doi.org/10.1111/jcpp.12938 (2018).
Bloch, M. H. Emerging treatments for Tourette’s disorder. Curr. Psychiatry Rep. 10, 323–330 (2008).
Fründt, O., Woods, D. & Ganos, C. Behavioral therapy for Tourette syndrome and chronic tic disorders. Neurol. Clin. Pract. 7, 148–156 (2017).
Ganos, C., Martino, D. & Pringsheim, T. Tics in the Pediatric Population: Pragmatic Management. Mov. Disord. Clin. Pract. 4, 160–172 (2017).
McGuire, J. F. et al. Behavior Therapy for Tic Disorders: An Evidenced-based Review and New Directions for Treatment Research. Curr. Dev. Disord. Rep. 2, 309–317 (2015).
Yates, R. et al. Habit reversal training and educational group treatments for children with tourette syndrome: A preliminary randomised controlled trial. Behav. Res. Ther. 80, 43–50 (2016).
Piacentini, J. et al. Behavior therapy for children with Tourette disorder: a randomized controlled trial. JAMA 303, 1929–1937 (2010).
Wilhelm, S. et al. Randomized trial of behavior therapy for adults with Tourette syndrome. Arch. Gen. Psychiatry 69, 795–803 (2012).
Chmielewski, W. X., Wolff, N., Mückschel, M., Roessner, V. & Beste, C. Effects of multisensory integration processes on response inhibition in adolescent autism spectrum disorder. Psychological Med. 46, 2705–2716 (2016).
Chmielewski, W. X., Mückschel, M., Dippel, G. & Beste, C. Concurrent information affects response inhibition processes via the modulation of theta oscillations in cognitive control networks. Brain Struct. Funct. 221, 3949–3961 (2016).
Goetz, C. G., Pappert, E. J., Louis, E. D., Raman, R. & Leurgans, S. Advantages of a modified scoring method for the Rush Video-Based Tic Rating Scale. Mov. Disord. 14, 502–6 (1999).
Petruo, V. A., Stock, A.-K., Münchau, A. & Beste, C. A systems neurophysiology approach to voluntary event coding. Neuroimage 135, 324–332 (2016).
Stein, B. E. & Stanford, T. R. Multisensory integration: current issues from the perspective of the single neuron. Nat. Rev. Neurosci. 9, 255–266 (2008).
Managing Tourette syndrome: a behavioral intervention for children and adults: therapist guide. (Oxford University Press, 2008).
Deckersbach, T. et al. Neural correlates of behavior therapy for Tourette’s disorder. Psychiatry Research: Neuroimaging 224, 269–274 (2014).
Ganos, C., Roessner, V. & Münchau, A. The functional anatomy of Gilles de la Tourette syndrome. Neurosci. Biobehav. Rev. 37, 1050–1062 (2013).
Worbe, Y., Lehericy, S. & Hartmann, A. Neuroimaging of tic genesis: Present status and future perspectives. Mov. Disord. 30, 1179–1183 (2015).
Makino, H. Top-down control: A unified principle of cortical learning. Neurosci. Res. 141, 23–28 (2019).
Dippel, G., Chmielewski, W., Mückschel, M. & Beste, C. Response mode-dependent differences in neurofunctional networks during response inhibition: an EEG-beamforming study. Brain Struct Funct 1–11, https://doi.org/10.1007/s00429-015-1148-y (2015).
Helton, W. S. Impulsive responding and the sustained attention to response task. J. Clin. Exp. Neuropsychol. 31, 39–47 (2009).
Helton, W. S. et al. Signal regularity and the mindlessness model of vigilance. Br. J. Psychol. 96, 249–261 (2005).
McVay, J. C. & Kane, M. J. Conducting the train of thought: working memory capacity, goal neglect, and mind wandering in an executive-control task. J. Exp. Psychol. Learn. Mem. Cogn. 35, 196–204 (2009).
Quetscher, C. et al. Striatal GABA-MRS predicts response inhibition performance and its cortical electrophysiological correlates. Brain Struct. Funct. 220, 3555–3564 (2015).
Stevenson, H., Russell, P. N. & Helton, W. S. Search asymmetry, sustained attention, and response inhibition. Brain Cogn. 77, 215–222 (2011).
Faul, F., Erdfelder, E., Lang, A.-G. & Buchner, A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 39, 175–191 (2007).
Masson, M. E. J. A tutorial on a practical Bayesian alternative to null-hypothesis significance testing. Behav. Res. Methods 43, 679–690 (2011).
Acknowledgements
This work was supported by Grants from the Deutsche Forschungsgemeinschaft (DFG) MU1692/4-1, BE4045/19-1 and FOR 2698.
Author information
Authors and Affiliations
Contributions
V.P., B.B., A.B., A.M., V.R. and C.B. designed the study and wrote the protocol. Author V.P., B.B. collected the data and Authors A.B. and C.B. undertook the statistical analysis and wrote the first draft of the manuscript. V.P., B.B., A.B., A.M., V.R. and C.B. contributed to and have approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
V.P., B.B. and A.B. have nothing to disclose. V.R. has received payment for consulting and writing activities from Lilly, Novartis, and Shire Pharmaceuticals, lecture honoraria from Lilly, Novartis, Shire Pharmaceuticals, and Medice Pharma, and support for research from Shire and Novartis. He has carried out (and is currently carrying out) clinical trials in cooperation with the Novartis, Shire, and Otsuka companies. A.M. has received commercial research support and honoraria from Pharm Allergan, Ipsen, Merz Pharmaceuticals, Actelion, GlaxoSmithKline, Desitin and Teva. C.B. has received payment for consulting from Bayer, GlaxoSmithKline, Novartis, Teva and Genzyme.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Petruo, V., Bodmer, B., Bluschke, A. et al. Comprehensive Behavioral Intervention for Tics reduces perception-action binding during inhibitory control in Gilles de la Tourette syndrome. Sci Rep 10, 1174 (2020). https://doi.org/10.1038/s41598-020-58269-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-58269-z
This article is cited by
-
Group comprehensive behavioral intervention for tics contribution to broader cognitive and emotion regulation in children
European Child & Adolescent Psychiatry (2023)
-
Enhanced habit formation in Tourette patients explained by shortcut modulation in a hierarchical cortico-basal ganglia model
Brain Structure and Function (2022)
-
Tourette Syndrome Treatment Updates: a Review and Discussion of the Current and Upcoming Literature
Current Neurology and Neuroscience Reports (2022)
-
European clinical guidelines for Tourette syndrome and other tic disorders—version 2.0. Part II: psychological interventions
European Child & Adolescent Psychiatry (2022)
-
Short-term Focused Attention Meditation Restricts the Retrieval of Stimulus-Response Bindings to Relevant Information
Mindfulness (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.