Abstract
Vanadium dioxide (VO2) is a strongly correlated electronic material with a metal-insulator transition (MIT) near room temperature. Ion-doping to VO2 dramatically alters its transport properties and the MIT temperature. Recently, insulating hydrogenated VO2 (HVO2) accompanied by a crystal structure transformation from VO2 was experimentally observed. Despite the important steps taken towards realizing novel applications, essential physics such as the diffusion constant of intercalated protons and the crystal transformation energy between VO2 and HVO2 are still lacking. In this work, we investigated the physical parameters of proton diffusion constants accompanied by VO2 to HVO2 crystal transformation with temperature variation and their transformation energies. It was found that protons could propagate several micrometers with a crystal transformation between VO2 and HVO2. The proton diffusion speed from HVO2 to VO2 was approximately two orders higher than that from VO2 to HVO2. The long-range propagation of protons leads to the possibility of realizing novel iontronic applications and energy devices.
Similar content being viewed by others
Introduction
Controlling the properties of strongly correlated electronic materials via carrier and impurity doping has gained significant attention over the past years. Doping has an impact on 3d-band filling, which often results in dramatic modification of the orbital states. This results in large changes of electronic properties, such as the metal-insulator transition (MIT). For example, a shift of the MIT temperature (TMI) of VO2 via doping with a variety of elements, such as W, Mo, and Nb has been reported1,2,3,4,5,6,7,8. This is caused by charge transfer from the impurity ions to the vanadium ions through the oxygen ions, which displaces an integral number of 3d1 electrons in V4+ that are Mott insulating states to 3d1+δ, resulting in the formation of more stable metallic states. When using impurity elements with large atomic numbers of W, Mo and Nb, however, it is impossible to dynamically control the number of mobile carriers because of solid-state materials determined by an initial doping level. On the other hand, protons having strongly reductive activity in oxide materials can dynamically move via external fields and function as an electron donor. Recent work has demonstrated that dynamic proton-intercalation results in a large, reversible resistance modulation in oxide thin films, such as SrTiO3, NdNiO3, and VO2 via a catalytic effect9,10,11,12,13, a non-catalytic effect14,15, or an electric field16,17,18,19,20. In general, the intercalation of protons in the insulating VO2 state decreases its resistivity and it approaches a metallic state with a pseudo rutile structure11,19,21,22,23,24. According to Yoon et al.13, heavy doping with protons using Pt catalytic nano-particles transforms VO2 into HVO2 under an H2 + Ar gas atmosphere. HVO2 has a different crystal structure from the tetragonal VO2, and it demonstrates typical-insulating behavior that follows the Arrhenius equation with a higher resistivity than that of VO2. Moreover, a reversible structural deformation is possible between VO2 in air and HVO2 in the H2 + Ar gas atmosphere. This reversible resistance change has the potential to lead to the realization of novel ionic and/or electronic applications. However, there is lack of information regarding essential physical parameters in this system, such as the diffusion constant of the intercalated protons in VO2 and the crystal transformation energy between VO2 and HVO2.
In this work, we demonstrated the long-range propagation of protons in VO2. This was accomplished by investigating the transient electronic transport properties during proton intercalation in VO2 and the associated structural transformation to HVO2 under H2 + Ar gas atmosphere. This was followed by a return to VO2 from HVO2 after proton desorption under an N2 gas atmosphere. The results showed that the diffusion constants and crystal transformation energies differed for the two states.
Results
Device structures and basic transport properties
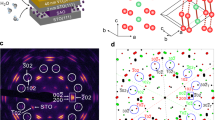
For VO2 microwire devices prepared on TiO2 (001) substrates, the width (w) was fixed at 2 μm and the distance between catalytic Pt electrodes (d) was from 2 μm to 10 μm, as shown in the schematic in Fig. 1a. The optical image on the right of Fig. 1a shows a VO2 microwire with Pt electrodes as an example. Figure 1b shows the temperature dependence of the resistivity curves in a VO2 microwire with d = 4 μm. The pristine VO2 shows typical electronic transport properties with hysteresis due to the MIT in a N2 gas atmosphere (solid black dots). Continuous annealing under H2(5%) + Ar(95%) gas at 380 K for ~5 h resulted in a marked change to the insulating phase that followed the Arrhenius equation (solid red dots). The resistivity increased by ~5 orders of magnitude, which is in agreement with ref. 13. They determined that the heavily hydrogenated VO2 had a structural deformation to HVO2, which resulted in a ~10% expansion in the [100] direction of the rutile VO2 structure and opened a band gap calculated by a first-principle method13. The band gap (Eg) of the hydrogenated HVO2 was 0.71 eV, as derived from the inset of Fig. 1b using the equation as a pure semiconductor \(\mathrm{ln}\,\sigma =-\frac{{E}_{g}}{2{k}_{B}}\frac{1}{T}+\,\mathrm{ln}\,{\sigma }_{0}\), where σ, σ0, and kB are the conductivity of the thin film, a constant, and the Boltzmann constant, respectively. The Eg takes the half value with the calculated bandgap of the HVO2 with 2 electron doping unit cell in ref. 13. Furthermore, these results indicate that the VO2 microwires also become fully hydrogenated VO2 in the 4 μm gap between the Pt source and drain catalytic electrodes. Figure 1c shows the reversibility of the transport properties under H2+Ar or N2 gases at 380 K. The VO2 microwire was initially metallic at 380 K in a N2 atmosphere. As soon as H2 gas was introduced into the measurement cell, the resistivity promptly increased with the intercalation of protons into VO2. Eventually, the H2 gas annealing resulted in the heavily hydrogenated VO2, i.e., it formed the insulating HVO2 phase. Compared with the time required for the transformation from VO2 to HVO2, the HVO2 phase returned to the initial metallic VO2 more quickly on N2 gas annealing. This trend was in agreement with the data from ref. 13. The results of this experiment revealed the micro-scale diffusion of protons with a crystal change from VO2 to HVO2. Regarding repeatability of electronic property of VO2 thin films after N2 annealing at 380 K from HVO2, the temperature dependence of resistance curves is almost same as the pristine curve, keeping the framework of VO2 (Please see the section E in Supplementary Information in detail).
VO2 microwire device with Pt electrode with catalytic effect and Basic transport properties of VO2 and heavily proton intercalated VO2. (a) A schematic illustration (left) and an optical microscope image (right) of a VO2 microwire for the proton intercalation experiment. (b) Temperature dependence of the resistivity curves of a pristine 4-μm-length VO2 wire (the black solid dots) and of hydrogenated VO2 (HVO2) (the red solid dots). The inset shows the 1/T dependence of ln σ for the Arrhenius plots (c) The reversibility of the transport properties under H2 + Ar or N2 gases at 380 K.
Analysis of the transient resistance behavior
Based on the H2 or N2 atmosphere dependent reversible reaction, the evolution of the proton concentration in VO2 with time was investigated. As a boundary condition between the Pt and VO2 interface, the chemical reaction rate as a differential equation with respect to time (t) was introduced according to:
where k1 and k2 are the forward and reverse reaction rate constants, respectively, nH+ is the proton concentration at the contact area between the Pt catalytic electrodes and VO2, and ninter is the proton concentration at the VO2 interface. From Eq. (1), the behavior of Fig. 1c can be understood. In the beginning, protons are intercalated into VO2 at the contact points between Pt, VO2, and the H2 + Ar gas phase, as seen in an upper illustration of Fig. 2a, because k1nH+ > k2ninter, and ninter = 0 during the initial stage. The value of k2ninter gradually approaches that of k1nH+, until the two values are finally equal and reach the equilibrium state. As the H2 + Ar gas is changed to N2, nH+ approximately becomes zero, thus the protons in VO2 are removed and the insulating HVO2 returns to the metallic VO2 form. This dynamic reaction at the interface was taken as a boundary condition with a dynamic time-dependence in the simulation below.
Transient electronic transport behaviors of VO2 microwires in 4-μm-, 6-μm- and 10-μm-length. (a) Illustration of the chemical kinetics with forward (k1) and reverse (k2) reaction rate constants with the Pt catalytic effect (upper) and the serial resistor model via the FMD simulation. (b) Experimental and simulation results for the time dependence of the resistivity in 4-μm-, 6-μm-, and 10-μm-length VO2 wires after starting hydrogen intercalation under the H2(5%) + Ar(95%) gas atmosphere at 380 K. The inset shows the spatiotemporal map of the proton ratio for the case of DHVO2 = 150 nm2/s, which was the fitting value for the doted lines of the 4-μm-, 6-μm-, and 10-μm-length VO2 wires in Fig. 2b. (c) Spatial maps of the proton ratio using D = 150 nm2/s in the 4-μm-, 6-μm-, and 10-μm-length VO2 wires for 4 h after start from hydrogen intercalation.
Next, we consider how the intercalated protons diffuse in VO2. Theoretically, for ion diffusion, the ionic fluxes likely arise from a gradient in the ion concentration in the solid-state material. Thus, since nHVO2 is the proton ratio in a VO2 unit cell, the hydrogen ion flux (JHVO2) can be described as:JHVO2 = −D∇nHVO2, where D is the diffusion coefficient. To conduct the transient state analysis, we use Fick’s second law in the one dimensional case, namely, \(\frac{\partial {n}_{HVO2}}{\partial t}=-\frac{\partial {J}_{HVO2}}{\partial x}\), which predicts the spatiotemporal evolution of the ion concentration. With this the following equation was obtained:
To evaluate the spatiotemporal evolution of the proton concentration in VO2, numerical analysis using the finite difference method (FDM) was carried out based on the boundary condition of Eq. (1) and the transient diffusion equation of Eq. (2) (see Section A in Supplementary Information).
The inset of Fig. 2b shows the spatiotemporal mapping of the proton density in VO2 from the simulation with D = 150 nm2/s. Here, x = 0 represents the interface of the VO2 contacted with the Pt electrodes. We divided the 2-μm-length VO2 wire by 100 in the FDM calculation, thus the integral i goes from 1 to 100 and the divided length (Δx) is 20 nm (further details in Section A of the Supplementary Information). The inset of Fig. 2b represents the proton diffusion behavior of a 4-μm-length VO2 wire because the intercalation and diffusion behavior becomes symmetric at a 2 μm distance from the Pt electrodes. Through this analysis, we can clearly understand the transient behavior of proton diffusion in the VO2 wires.
For conversion of the proton concentration in VO2 to resistivity, a serial resistor model was used as shown in the lower illustration of Fig. 2a, which is represented by the following equation:
The ρi(T) can be simply defined as \((1-{n}_{HVO2}){\rho }_{i}^{VO2}+{n}_{HVO2}{\rho }_{i}^{HVO2}(T)\), where \({\rho }_{i}^{VO2}\) is the resistivity of metallic VO2 and \({\rho }_{i}^{HVO2}(T)\) is the temperature dependent resistivity of the insulating HVO2.
The \({\rho }_{i}^{VO2}\) was fixed at 0.0008 Ω cm between 300 K and 380 K because of its nearly constant value with reference to the VO2 resistivity curve in Fig. 1c, while \({\rho }_{i}^{HVO2}(T)\) varies with temperature. The experimental values of \({\rho }_{i}^{HVO2}(T)\) were taken at the required temperature by referring to the temperature vs resistance curve of HVO2 in Fig. 1b. Figure 2b shows the transient resistivity behavior with time for the 4-μm-, 6-μm-, and 10-μm-length VO2 wires at 380 K. The red dot curves show the simulation results using Eqs. (1) to (3) with the appropriately selected diffusion constant for the proton that transforms the crystal structure from VO2 to HVO2; DHVO2 = 150 nm2/s at 380 K determined by the wire lengths of VO2 with 4 μm, 6 μm, and 10 μm. The simulation curves could fit to the experimental curves well in their lengths. The diffusion value was compared with proton diffusion in the [001] direction of rutile-type TiO2 without a structural transformation25. It is considered that the slower diffusion constant of VO2 [110] than that of TiO2 [001] would be due to difference of two oxygen distance (do-o) in [001] and [110] directions, respectively, because the diffusion constant of protons hopping to next oxygen sites is dependent on square length of do-o. The do-o in TiO2 [001] direction is approximately 1.48 Å25, whereas the do-o in VO2 [110] and HVO2 [110] are approximately 2.66 Å and 2.68 Å13, respectively. Figure 2c shows the spatial mapping of the proton ratio using DHVO2 = 150 nm2/s with 4-μm-, 6-μm-, and 10-μm-length VO2 wires for 4 h after the start of proton intercalation. The occupancy of the protons was high at >80%/unit cell, even at the center of the 4-μm-length VO2 wire, while the center of the 10-μm-length wire was still unoccupied by protons. The spatial density of HVO2 clearly reflects the resistance behavior shown in Fig. 2b.
Discussion of the physical picture
Next, we estimated the activation energy (EHVO2) for the structural transformation from VO2 to HVO2 using the experimental results of the 4-μm-length VO2 wire and simulation fittings. Figure 3a–d show the time dependence of the resistivity behaviors in the 4-μm-length VO2 wire at 320 K, 340 K, 260 K, and 380 K, respectively. The simulation curves (red dot curves) were fitted by appropriately selecting the DHVO2 values. The dashed blue lines in Fig. 3a–d indicate the resistivity of HVO2 with the measurement temperature taken from the 4-μm-length VO2 wire in Fig. 1b. The values of the experimental resistivity at each temperature were used as the simulation values of \({\rho }_{i}^{HVO2}(T)\). From these fittings, we estimated the DHVO2 values, which are 5 nm2/s at 320 K, 10 nm2/s at 340 K, 50 nm2/s at 360 K, and 150 nm2/s at 380 K, respectively. In general, the variation of the diffusion constant (D) with temperature takes the form of a thermally activated-type equation as below:
where ED is the diffusive activation energy and D0 is the frequency factor.
Transient electronic transport behaviors of the 4-μm-length VO2 microwire at a variety of temperatures. (a–d) Time dependence of the resistivity behaviors in the 4-μm-length VO2 wire at 320 K, 340 K, 360 K and 380 K, respectively. (e) the red dots represent experimental DHVO2 values in the VO2 wire with structural transformation to HVO2, estimated from the simulated fitting in Fig. 3a–d (the red dot lines). The red line is the fitting curve using Eq. (4). In comparison with the experimental DHVO2, the proton diffusion constant of the parallel direction along the c axis of TiO2 as a function of temperature in ref. 21 (black line). The inset shows the 1/T dependence of ln DHVO2 for the Arrhenius plots.
Figure 3e shows temperature dependence of DHVO2. The red dots represent the experimental DHVO2 values in the VO2 wire with structural transformation to HVO2, estimated from the simulated red dot lines in Fig. 3a–d. The red line is the fitting curve using Eq. (4). Compared with our experimentally determined DHVO2 values, the diffusion constant of protons in the direction parallel to the c axis of rutile-TiO2 (black line)18 are reasonably similar. Considering the diffusion constant of protons in TiO225, which is same crystal structure as VO2, and the direct observation of proton diffusion in VO2 by a microscope15. In addition, at the section D in Supplementary Information, resistance once insulating monoclinic-state of VO2 at 290 K decreases in an insulating monoclinic-state of VO2 at 290 K under a H2(5%) + Ar(95%) gas atmosphere. This is due to carrier doping from OH group without crystal transformation, that is, carrier density increases keeping the tetragonal VO2 structure. With further developing proton-intercalation, the heavy proton dopants promote transformation to the different crystal structure of insulating HVO213. This transient response appears in Fig. S4. Thus, the origin of resistance changes in Figs. 2b, 3a–d and 4a can be more reasonably explained as proton diffusion in VO2 than formation of contact resistance and/or small fractions having proton-puddles. The inset shows the 1/T dependence of ln DHVO2 for the Arrhenius plots. Figure 3e shows 1/T dependence of the DHVO2 values derived from the fitting curves using Eq. (4). The solid red line was obtained by the least-square method. From the slope, we determined that the diffusive activation energy for transformation from VO2 to HVO2 was 0.61 eV. For the diffusive activation energy for the reverse transformation from HVO2 to VO2, we estimated the diffusion coefficients (DVO2(T)). Figure 4a shows the transient normalized-resistance curves for the reverse HVO2 to VO2 transformation in a N2 atmosphere at 300 K, 320 K, 340 K, 360 K, and 380 K, respectively. Simulation curves using the FDM calculations could be well fitted to the experimental curves by incorporating the temperature-dependent coverage of hydrogen adatoms on Pt surface26 (Section B of the Supplementary Information provides further details). The DVO2 was 4600 nm2/s at 300 K, 10000 nm2/s at 320 K, 20000 nm2/s at 340 K, 45000 nm2/s at 360 K, and 90000 nm2/s at 380 K, which were more than two orders higher than those of DHVO2. From the fitted DVO2 at various temperatures, the activation energy for the transformation from HVO2 to VO2 (EVO2) was determined to be 0.37 eV, as obtained from the slope in Fig. 4b. Figure 4(c) shows the transformation energy between VO2 and HVO2 derived from the above data. VO2 structure is more stable than that in HVO2 structure.
Transient electronic transport behaviors returned from HVO2 to VO2. (a) Time dependence of normalized-resistive behaviors from HVO2 to VO2 under a N2 gas atmosphere in the 4-μm-length wire at 300 K, 320 K, 340 K, 360 K, and 380 K, respectively. The red dot lines show simulation curves derived by Eqs. Eq. (1–4) including the inhabitant effect in Section B of Supplementary Information. (b) The 1/T dependence of ln DVO2 for the Arrhenius plots. (c) Schematic of the crystal transformation energies between VO2 and HVO2.
Discussion
We clarified the physical parameters of heavily proton-intercalated VO2 through investigation of proton diffusion. Single-crystal VO2 thin films with a layer-by-layer growth were used to describe the crystal transformation energies from VO2 to HVO2 and from HVO2 to VO2. This was accomplished through investigation of the proton diffusion using the basic equations listed in Eq. (1) to Eq. (3). Data from in-plane poly-crystalline VO2 thin films with grains on Al2O3 (0001) substrates27 were not well-fitted to the ideal simulations using these equations. This was because a different crystal direction in-plane and grain boundaries disturb the ideal proton diffusion (Section C in Supplementary Information provides further details). It was found that the proton diffusion speed from HVO2 to VO2 was approximately two orders higher than that for VO2 to HVO2. The long-range micro-meter proton propagation, differences in the proton diffusion constants, and the asymmetry transformation energy between HVO2 and VO2 offer opportunity for the realization of novel iontronic applications and for energy devices, such as hydrogen storage.
Methods
Microwire preparation
Single crystal VO2 thin films were epitaxially grown on TiO2 (001) substrates using the pulsed laser deposition technique (ArF excimer laser, λ = 193 nm), with a substrate temperature of 420 °C, an oxygen pressure of 0.95 Pa, a laser repetition rate of 2 Hz, and with an energy fluency of 10 mJ/cm2. A V2O5 pellet was used as the target. The deposition rate was ~0.3 nm/min. The thickness of the VO2 thin films were fixed at ~10 nm. VO2 grown on the TiO2 (001) substrate had a tetragonal (001) plane, which could be verified by the X-ray diffraction pattern and SEM images of the VO2 thin films prepared under the same fabrication conditions in Ref. 28,29,30. The films and Pt catalytic electrodes were patterned via photolithography. A 40-nm-thick Pt electrode was deposited on the patterned VO2 microwires. The width (w) was fixed at 2 μm and the Pt electrode distance (d) was from 2 μm to 10 μm, as shown in the schematic in Fig. 1(a). The right image in Fig. 1a shows a VO2 microwire with Pt electrodes as an example.
Measurements
The electronic properties of the films were measured via a two-probe method using a current-voltage source meter (2614B, Keithley Instruments) under H2(5%) + Ar(95%) or N2 gas atmospheres. The current flow direction was [110] in the rutile VO2 thin films.
References
Fisher, B. Electrical and Seebeck effect measurements in Nb-doped VO2. J. Phys. Chem. Solid. 43, 205–211 (1982).
Takahashi, I., Hibino, M. & Kudo, T. Thermochromic properties of double-doped VO2 thin films prepared by a wet coating method using Polyvanadate-based sols contacting W and Mo or W and Ti. Jpn. J. Appl. Phys. 40, 1391–1395 (2001).
Shibuya, K., Kawasaki, M. & Tokura, Y. Metal-insulator transition in epitaxial V1-xWxO2(0≤x≤0.33) thin films. Appl. Phys. Lett. 96(1-3), 022102 (2010).
Miyazaki, K., Shibuya, K., Suzuki, M., Wado, H. & Sawa, A. Correlation between thermal hysteresis width and broadening of metal–insulator transition in Cr- and Nb-doped VO2 films. Jpn. J. Appl. Phys. 53(1-5), 071102 (2014).
Strelcov, E. et al. Doping-Based Stabilization of the M2 Phase in Free-Standing VO2 Nanostructures at Room Temperature,. Nano Lett. 12, 6198–6205 (2012).
Ardakani, H. A. et al. Atomic Origins of Monoclinic-Tetragonal (Rutile) Phase Transition in Doped VO2 Nanowires. Nano Lett. 15, 7179–7188 (2015).
Shibuya, K., Kawasaki, M. & Tokura, Y. Metal-insulator transition in epitaxial V1-xWxO2(0≤x≤0.33)thin films. Appl. Phys. Lett. 96(1-3), 022102 (2010).
Liu, S. J., Fang, H. W., Su, Y. T. & Hsieh, J. H. Metal–insulator transition characteristics of Mo- and Mn-doped VO2 films fabricated by magnetron cosputtering technique. Jpn. J. Appl. Phys. 53(063201), 1–5 (2014).
Takami, H., Kanki, T., Ueda, S., Kobayashi, K. & Tanaka, H. Filling-controlled Mott transition in W-doped VO2. Phys. Rev. B 85(1-4), 205111 (2012).
Takami, H., Kawatani, K., Kanki, T. & Tanaka, H. High Temperature-Coefficient of Resistance at Room Temperature in W-Doped VO2 Thin Films on Al2O3 Substrate and Their Thickness Dependence. Jpn. J. Appl. Phys. 50(055804), 1–3 (2011).
Wei, J., Ji, H., Guo, W., Nevidomskyy, A. H. & Natelson, D. Hydrogen stabilization of metallic vanadium dioxide in single-crystal nanobeams. Nature Nanotech. 7, 357–362 (2012).
Shi, J., Zhou, Y. & Ramanathan, S. Colossal resistance switching and band gap modulation in a perovskite nickelate by electron doping. Nat. Commun. 5(5860), 1–9 (2013).
Yoon, H. et al. Reversible phase modulation and hydrogen storage in multivalent VO2 epitaxial thin films. Nature Mater. 15, 1113–1120 (2016).
Chen, Y. et al. Non-catalytic hydrogenation of VO2 in acid solution. Nat. Commun. 9(1-8), 818 (2018).
Lin, J. et al. Hydrogen Diffusion and Stabilization in Single-Crystal VO2 Micro/ Nanobeams by Direct Atomic Hydrogenation. Nano Lett. 14, 5445–5451 (2014).
Ohta, H. et al. Field-induced water electrolysis switches an oxide semiconductor from an insulator to a metal. Nat. Commun. 1(1-6), 118 (2010).
Sasaki, T., Ueda, H., Kanki, T. & Tanaka, H. Electrochemical gating-induced reversible and drastic resistance switching in VO2 nanowires. Sci. Rep. 5(1-7), 17080 (2015).
Kanki, T. & Tanaka, H. Research Update: Nanoscale electrochemical transistors in correlated oxides. APL Mater. 5(1-11), 042303 (2017).
Ji, H., Wei, J. & Natelson, D. Modulation of the Electrical Properties of VO2 Nanobeams Using an Ionic Liquid as a Gating Medium. Nano Lett. 12, 2988–2992 (2012).
Jo, M. et al. Gate-Induced Massive and Reversible Phase Transition of VO2 Channels Using Solid-State Proton Electrolytes. Adv. Funct. Mater. 28(1-7), 1802003 (2018).
Filinchuk, Y. et al. In Situ Diffraction Study of Catalytic Hydrogenation of VO2: Stable Phases and Origins of Metallicity. J. Am. Chem. Soc. 136, 8100–8109 (2014).
Hong, W.-K. et al. Hydrogen-Induced Morphotropic Phase Transformation of Single-Crystallin Vanadium Dioxide Nanobeams. Nano Lett. 13, 1822–1828 (2013).
Lin, J. et al. Hydrogen Diffusion and Stabilization in Single-Crystal VO2 Micro/Nanobeams by Direct Atomic Hydrogenation. Nano Lett. 14, 5445–5451 (2014).
Warnick, H. K., Wang, B. & Pantelides, T. S. Hydrogen dynamics and metallic phase stabilization in VO2. Appl. Phys. Lett. 104(1-4), 101913 (2014).
Bates, J. B., Wang, J. C. & Perkins, R. A. Mechanisms for hydrogen diffusion in TiO2. Phys. Rev. B 19, 4130–4139 (1979).
Markovic, N. M., Grgur, B. N. & Ross, P. N. Temperature-Dependent Hydrogen Electrochemistry on Platinum Low-Index Single-Crystal Surfaces in Acid Solutions. J. Phys. Chem. B 101, 5405–5413 (1997).
Okimura, K. & Sakai, J. Changes in Lattice Parameters of VO2 Films Grown on c-Plane Al2O3 Substrates across Metal–Insulator Transition,. Jpn. J. Appl. Phys. 48(1-6), 045504 (2009).
Wei, T., Kanki, T., Fujiwara, K., Chikanari, M. & Tanaka, H. Electric field-induced transport modulation in VO2 FETs with high-k oxide/organic parylene-C hybrid gate dielectric. Appl. Phys. Lett. 108(1-4), 053503 (2016).
Sohn, A., Kanki, T., Sakai, K., Tanaka, H. & Kim, D. W. Fractal Nature of Metallic and Insulating Domain Configurations in a VO2 Thin Film Revealed by Kelvin Probe Force Microscopy. Sci. Rep. 5(1-7), 10417 (2015).
Kawatani, K., Kanki, T. & Tanaka, H. Formation mechanism of a microscale domain and effect on transport properties in strained VO2 thin films on TiO2(001). Phys. Rev. B 90(1-5), 054203 (2014).
Acknowledgements
The authors would like to thank S. Sakakihara for carrying out the RIE process, M. Sakuma for advice on proper device-preparation methods, Professor H. Tanaka for giving comments in this paper, and the Nanotechnology Platform Project (Nanotechnology Open Facilities in Osaka University) from the Ministry of Education, Culture, Sports, Science and Technology, Japan (MEXT) (S-18-OS-0020, F-18-OS-0024). This work was performed under the Cooperative Research Program of “Network Joint Research Center for Materials and Devices”.
Author information
Authors and Affiliations
Contributions
K.M. fabricated the devices, conducted the measurements, analyzed the data, and wrote the manuscript. T.K. planned the research direction, supervised the experiment, analyzed the data, and the main manuscript text. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Muraoka, K., Kanki, T. Long-range propagation of protons in single-crystal VO2 involving structural transformation to HVO2. Sci Rep 9, 20093 (2019). https://doi.org/10.1038/s41598-019-56685-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-56685-4
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.