Abstract
This study aimed to evaluate prognostic impacts of the number of lymph nodes (LNs) examined and LN ratio on cancer-specific mortality after surgery in patients with right-sided colon cancer (RCC) or left-sided colon cancer (LCC) using the Surveillance, Epidemiology, and End Results database. Number of LNs examined and LN ratio were treated as categorical and/or continuous. Competing risks proportional hazards regressions adjusted by propensity score were performed. All included patients had stage I, II, or III disease, and 45.1% of them had RCC. RCC and LCC patients with high level of LNs examined had better prognosis after segmental resection or hemicolectomy. RCC and LCC patients with higher LN ratio had worse prognosis regardless of surgery. Survival benefit of having high level of LNs examined was observed in RCC patients with stage I, II, or III disease, but only in LCC patients with stage II disease. Both higher LN ratio and high level of LN were negative prognostic factors for cancer-specific mortality in stage III patients regardless of tumor sidedness. In conclusion, RCC patients in various conditions had worse or comparable prognosis compared to their LCC counterparts, which reflected the severity of LN metastasis.
Similar content being viewed by others
Introduction
Colorectal cancer originating from the colon or rectum is the third most common cancer and the fourth deadliest cancer worldwide1. Although trends in incidence and mortality of colorectal cancer vary across countries, the global burden of this disease is projected to increase over the next decade2. However, colon cancer and rectal cancer are indeed two discrete tumor entities requiring distinct staging procedures and treatment approaches3,4, because of their different molecular developmental mechanisms and metastatic patterns3,5. It is estimated that there will be approximately 101,420 new cases of colon cancer, which is more than double that of rectal cancer (44,180), in the United States in 20196. Moreover, due to world population growth and aging, the global number of deaths from colon cancer is anticipated to keep rising in the coming decades7. Hence, identifying prognostic factors for colon cancer is of clinical significance.
The prognostic impact of tumor location in colon cancer has been extensively explored with controversial results8,9,10,11,12,13,14,15,16. Compared to patients with left-sided colon cancer (LCC), right-sided colon cancer (RCC) patients had lower overall survival (OS)8,9, disease-free survival (DFS)9, progression-free survival10 and net survival11. A population-based study further revealed that RCC patients had worse OS and cancer-specific survival (CSS) than LCC patients12. However, another population-based study found that after propensity score matching, RCC was significantly associated with better OS and CSS compared to LCC13. Furthermore, several cohort studies reported that RCC patients had similar prognosis regarding OS14, DFS15 and CSS16, in comparison with their LCC counterparts. Further subgroup analyses indicated that the prognostic effects of tumor sidedness varied across different stages of colon cancer9,13,14,15,16.
The extent to which lymph node metastasis affects colon cancer prognosis has been widely investigated for decades. Several lines of evidence suggested that a minimum of 12 lymph nodes (LNs) should be examined pathologically for adequate N staging in colon cancer patients17,18,19, and that the number of LNs examined (≥12) was significantly associated with OS, DSF, and CSS in colon cancer20,21,22. However, the relevant debate has never ended because optimal other than 12 have been implied, such as 2023, 2124, and 2225. Similar to tumor sidedness, the prognostic value of the number of LNs examined varied depending on stages of colon cancer21,22,26. In addition, the prognostic effect of LN ratio, defined as the ratio of the number of positive LNs to the total number of LNs examined, has been demonstrated in stage II and III colon cancer patients27,28. Notably, LN ratio exhibited greater prognostic value than number of LNs examined in patients with stage III disease29.
CSS was significantly improved in RCC patients with a minimum of 15 LNs examined, but the minimum requirement for better CSS could be reduced down to 11 LNs LCC patients30. Moreover, it has been suggested that postoperative survival rates of RCC and LCC patients may be affected by surgical treatment31. Therefore, the present study aimed to explore the prognostic impacts of the number of LNs examined and LN ratio in RCC and LCC patients, especially if patients undergoing different surgical treatments or at distinct cancer stages.
Methods
Data source
This population-based, retrospective cohort study analyzed the United States Surveillance, Epidemiology, and End Results (SEER) database, which is maintained by the National Cancer Institute. The SEER database is representative of approximately 34.6% of the United States population. The permission to access the research data files of the SEER program registries was obtained (reference number, 15309-Nov2017). Because the SEER data are de-identified, institutional review board approval and informed consent by the study subjects were not required for this SEER-based study.
Study population
The codes of the International Classification of Diseases for Oncology (ICD-O) were used for identification of eligible patients. The SEER database (2004–2013) was searched to identify patients with RCC (ICD code: C180, C182, C183, and C184) or LCC (ICD code: C185, C186, C187, C199, and C209) as previously described12. Furthermore, the inclusion criteria were (i) primary cancer, (ii) adenocarcinoma (ICD code: 8140), (iii) distant metastases could not be evaluated (MX, the TNM staging system), and (iv) received curative surgery (segmental resection, hemicolectomy, or total colectomy). On the other hand, patients without data of age, survival time, tumor size, number of LNs examined, number of positive LNs, tumor grade, surgery, or cancer stage were excluded.
Study variables and outcome
Demographic covariates studied in the present study included age, sex, and race. Race was categorized into white, black, and other races. Other covariates related to tumor characteristics and treatment modalities, such as tumor size, number of LNs examined, number of positive LNs, LN ratio, tumor grade, surgery, and cancer stage, were also included in this study. In addition, the “SEER cause-specific death” was used to identify patients who died of colon cancer, and cancer-specific mortality was then calculated.
Statistical analysis
Categorical variables were presented as frequency (percentage), and continuous variables were reported as mean ± standard deviation. Differences in patients’ characteristics between RCC and LCC were examined by Pearson’s chi-square test for categorical variables, and by two-sample t-test for numerical variables. The medians of the numbers of LNs examined and LN ratios in various study populations were used as cut-off values to divide the corresponding variables in half in the regression analyses (Supplementary Table Sl).
A competing risks proportional hazards regression model was built to evaluate prognostic factors for cancer-specific mortality in patients with colon cancer. The patient who died of colon cancer was identified as an event; the patient who did not have an event during the follow-up period was defined as a censoring. The associations of cancer-specific mortality with number of LN examined, LN ratio, or tumor location were examined using univariate and multivariate competing risks proportional hazards regression analyses. Multivariate model was adjusted for propensity score (PS score) that was calculated by including significant variables identified in univariate analysis. Furthermore, the multivariate models of LCC and RCC were stratified by surgery and cancer stage. The results of regression analyses were presented as hazard ratio (HR) with corresponding 95% confidence interval (95%CI) and P values. All P values were 2-sided, and values of P < 0.05 were considered to indicate statistical significance. All statistical analyses were performed using the statistical software package SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Results
Demographic and clinical characteristics of the total population as well as RCC and LCC patients
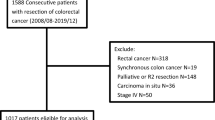
In the present retrospective cohort study, 373,801 patients diagnosed with either RCC or LCC were identified from the SEER database (2004 to 2013). Of them, 130,569 patients, who had primary adenocarcinoma that could not be evaluated for distant metastasis, and underwent curative surgery, were included. However, 19,515 patients were excluded due to missing values of covariates studied. As a result, the data of 111,054 patients were subject to final analysis (Fig. 1).
Among these eligible colon cancer patients, 50,102 (45.1%) patients were diagnosed with RCC and 60,952 (54.9%) had LCC. The demographic and clinical features of the total population and tumor site subgroups are presented in Table 1. The mean age of the total population was 67.2 years. In the total population, 49.3% of them were females, 79.7% were white, and 57.0% were married. There were significant differences in all demographic and clinical characteristics between RCC and LCC patients (all P ≤ 0.021) (Table 1). Briefly, compared to LCC patients, patients with RCC were older and more likely to be females. RCC patients tended to have a larger tumor and a higher number of LNs examined, but a lower number of positive LNs and LN ratio. The majorities of RCC and LCC were grade 2 (68% and 77.8%, respectively), and stage II (45.8% and 41.9, respectively). Regarding curative surgery, 79.2% of RCC patients underwent hemicolectomy, while 72.6% of LCC patients received segmental resection.
Median numbers of LNs examined and LN ratios as cut-off points
The median values of number of LNs examined and LN ratio of the total population and various subgroups are shown in Supplementary Table S1, which were used as cut-off values in the subsequent regression models. For example, the total population were classifed into two groups, low level (less than 16 LNs examined) and high level (16 or more LNs examined). The cut-off values of number of LNs examined for RCC and LCC patients were 17 and 15, respectively. Furthermore, the total population was also stratified by surgery and cancer stage, yielding distinct medians. Generally speaking, RCC patients had a higher number of LNs examined than LCC patients, regardless of surgery and cancer stage.
On the other hand, the median values of LN ratio in the total population and all subgroups were zero, except those of stage III patients (RCC: 0.15 and LCC: 0.17). Hence, LN ratio was calculated as a continuous variable in the present study unless otherwise specified.
Association of mortality with low level of LNs examined, higher LN ratio, and RCC in the total population
Univariate analyses revealed that cancer-specific mortality was significantly associated with age, sex, race, marital status, tumor size, tumor grade, surgery, and cancer stage. To reduce the effect of confounding variables, multivariate Cox regression model was adjusted for PS score that was calculated by including the above-mentioned significant variables. Multivariate analyses revealed that among all patients, patients with high level of LNs examined were significantly associated with a lower risk of cancer-specific mortality compared to patients with low level of LNs examined (adjusted HR = 0.83) (Table 2). Patients with higher LN ratio had a significantly higher risk of cancer-specific mortality (adjusted HR = 3.46, 95% CI = 3.24, 3.69, P < 0.001). Furthermore, compared to LCC, RCC was significantly associated with elevated risk of cancer-specific mortality (adjusted HR = 1.08) (Table 2).
Surgery-stratified analyses of mortality in RCC and LCC patients
To investigate the extent to which distinct surgical treatments affect hazard ratios for cancer-specific mortality in RCC and LCC patients, surgery-stratified Cox regression analyses were performed. After adjusted for PS score, multivariate analyses revealed that in reference to low level of LNs examined, high level of LNs examined was significantly associated with reduced cancer-specific mortality in RCC patients undergoing segmental resection and hemicolectomy (adjusted HR: 0.78 and 0.83, respectively) (Table 3). Significantly decreased risks of cancer-specific mortality were also obseved in LCC patients undergoing segmental resection and hemicolectomy (adjusted HR: 0.81 and 0.83, respectively), which were comparable to those of RCC patients (Table 3). While, no significant associations between number of LNs examined and mortality in patients undergoing total colectomy, regardless of tumor sidedness.
In addition, multivariate analyses revealed that compared to lower LN ratio, higher LN ratio was significantly associated with elevated cancer-specific mortality in RCC patients undergoing segmental resection, hemicolectomy, and total colectomy (adjusted HR: 4.25, 4.70, and 3.53, respectively) (Table 3). Similarly, LCC patients with higher LN ratio also had significantly elevated cancer-specific mortality after segmental resection, hemicolectomy, and total colectomy (adjusted HR: 2.76, 2.51, and 3.11, respectively) (Table 3). RCC patients, who had higher LN ratio and underwent segmental resection or hemicolectomy, had more than 1.5 times risks of cancer-specific mortality than their LCC counterparts; however, the mortality risk was similar in RCC and LCC patients with higher LN ratio and undergoing total colectomy (Table 3).
Cancer stage-stratified analyses of mortality in RCC and LCC patients
RCC and LCC patients were then stratified by cancer stage followed by Cox regression analyses to evaluate the effect of cancer stage on cancer-specific mortality in RCC and LCC patients. After adjusted for PS score, multivariate analyses revealed that in reference to low level of LNs examined, high level of LNs examined was significantly associated with reduced cancer-specific mortality in patients with stage I, II, and III RCC (adjusted HR: 0.77, 0.68, and 0.93, respectively) (Table 4). In contrast, significant association between high level of LNs examined and mortality was only obseved in tage II LCC patients (adjusted HR: 0.69) (Table 4).
Since none of eligible stage I colon cancer patients had positive LNs in the present study, LN ratio was not available for stage I RCC and LCC patients (Table 4). Multivariate analyses revealed that higher LN ratio was significantly associated with cancer-specific mortality in stage III RCC and LCC patients (adjusted HR: 6.30 and 3.84, respectively), but not in stage II RCC and LCC patients (Table 4). The risk of mortality in stage III RCC patients was 1.64 times higher than that of stage III LCC patients. Furthermore, the median LN ratio was used as the cut-off point to divided LN ratio into low and high levels, and then the prognostic effect of LN ratio calculated as a categorial variable on cancer-specific mortality was re-evaluated exclusively in stage III colon cancer patients. The median LN ratios for RCC and LCC were 0.15 and 0.17, respectively (Supplementary Table S1). After adjusted for PS score and level of LNs examined (low vs. high), multivariate analyses revealed that both RCC and LCC patients with high level of LN ratio had significantly higher risks of cancer-specific mortality than those with low level of LN ratio (adjusted HR for RCC and LCC: 2.02 and 1.71, respectively) (Table 5).
Discussion
The results of this population-based study disclosed that after adjusted for PS, multivariate analyses indicated that low level of LNs examined, higher LN ratio, and RCC were significantly associated elevated cancer-specific mortality in stage I-III colon cancer patients undergoing curative surgery. In addition, stratified analyses demonstrated that the prognostic impact of number of LNs examined and LN ratio varied depending on tumor sidedness, surgical treatments and cancer stages. After segmental resection or hemicolectomy, both RCC and LCC patients with high level of LNs examined had better prognosis than those with low level of LNs examined. While, no survival benefit of having high level of LNs examined was observed in RCC or LCC patients undergoing total colectomy. RCC and LCC patients with higher LN ratio (treated as continuous) had worse prognosis regardless of surgical treatment. On the other hand, RCC patients with high level of LNs examined had better prognosis regardless of cancer stage, but survival benefit of having high level of LNs examined was observed in stage II LCC patients. Finally, both higher LN ratio and high level of LN (considered as categorial) were negative prognostic factors for cancer-specific mortality in patients with stage III RCC or LCC. Overall, RCC patients in various conditions had worse or comparable prognosis compared to their LCC counterparts.
Growing evidence indicated that RCC and LCC are two distinct tumor entities in terms of embryology, histology, genetics, and clinical outcomes32,33,34,35. RCC and LCC had flat and polypoid-like morphology, respectively34. DNA mismatch repair pathway mutations were often occurred in RCC, while mutations in chromosomal instability pathway were commonly observed in LCC33. A molecular study demonstrated differential expression and translation profiles between RCC and LCC, suggesting two overlapping but distinct carcinogenesis mechanisms underlying RCC and LCC35. RCC patients responded better to immunotherapies, but LCC patients benefited more from chemotherapies and targeted therapies34. Hence, it was suggested that RCC and LCC should be treated with different treatment strategies33,34,35. In addition, the impact of tumor sidedness on postoperative survival has been demonstrated in colon cancer patients worldwide, including Italy8, south Korea9, China10, Japan11, and the United States26. The prognostic factors for cancer-specific mortality in RCC and LCC patients undergoing curative surgery were, therefore, investigated separately in the present SEER-based study.
Near 96% of colorectal cancers were adenocarcinomas in the United States36, so the present sudy focused on colon adenocarcinoma. According to the AJCC staging system (6th edition), prognostic uncertainty of MX colon cancer patients, whose cancers cannot be evaluated for distant metastasis, is likely to be higher than that of M0 colon cancer patients without distant metastasis. Investigation of prognostic factors in MX colon cancer may provide insights into the development of efficiout therapeutic strategies. Furthermore, since tumor characteristics are correlated with one another to some degree, PS has been included in several survival analyses by colon cancer sidedness9,13,25. To eliminate confounding effects, multivariate models in the present study were therefore adjusted for PS. However, unlike Warschkow et al.13 reporting that after PS matching RCC patients had better prognosis, the present study found that RCC patients had worse prognosis after adjusted for PS.
The number of LNs examined and LN ratios are two variables frequently used to assess the impact of lymph node metastasis on postoperative survival in colon cancer20,21,22,27,28,29. It was suggested that at least 12 LNs should be examined pathologically to determine the absence of lymph node metastasis in colon cancer patients17,18,19. Theoretically, colon cancer patients who have a sufficient number of LNs being examined will have a lower risk of lymph node metastasis, therefore they will have a better chance of survival. Despite “the 12 LNs rule,” several studies suggested that more than 12 LNs should be examined for adequate N staging in colon cancer23,24,25. By using the median number of LNs examined as the cut-off point, the present study found that colon cancer patients with high level of LNs examined (≥16) had significantly reduced cancer-specific mortality. Nevertheless, whether the minimum number of LNs examined in colon cancer could be reduced down to 15 or less remains to be investigated.
It has been suggested that RCC and LCC had distinct requirements for minimal number of LNs examined for better prognosis, with higher number of LNs examined in RCC30. Consistently, the present analysis of SEER data disclosed that RCC patients had significantly more LNs examined than LCC patients (18.9 vs. 16.1), and the median numbers of LNs examined in RCC patients in various conditions (range: 15–19) were higher than those of the corresponding LCC patients (range: 13–15). In addition, a Polish cohort study reported that the total number of LNs examined was significantly higher in RCC patients than LCC patients (11.7 ± 6 vs. 8.3 ± 5)37. The fact that more LNs were examined pathologically in RCC not only implied that in clinical practice more LNs should be examined pathologically in order to rule out lymph node metastasis in RCC, but also agreed with the “two tumor entities” theory32,33,34,35.
The stratified analyses of this study indicated that survival benefits of high level of LNs examined were comparable in RCC and LCC patients regardless surgery. In contrast, the relationship between number of LNs examined and tumor sidedness in patients at different disease stages was more complicated. A study of Taiwan Cancer Database revealed that survival benefits of a minimum of 12 LNs examined in colon cancer were observed in the total population and stage II colon cancer patients, but not in patients with stage III disease26. The present study further demonstrated that survival benefits of high level of LNs examined were cancer stage- and tumor sidedness-dependent. Again, further investigation is warrant to identify the optimal cut-off values of numbers of LNs examined for RCC and LCC in various conditions.
On the other hand, the prognostic effect of LN ratio is limited to patients with advanced colon cancer, because by definition stage I patients did not have metastasis in regional lymph nodes. The majority of patients with positive LNs were stage III patients in the present SEER-based study, which might explained why prognostic significance of LN ratio was emphasized in stage III colon cancer patients28,29,38. Consistently, the present study also found that the prognostic effect of LN ratio was significantly in patients with stage III disease regardless of tumor sidedness.
In order to assess the prognostic impact of LN ratio, LN ratio could be treated as a continuous or categorical variable in the statistical models27,29. Both approaches were applied in the present study, because the median LN ratio was zero in the total population and almost all subgroups. In the continuous approach, the current results indicated that in the total population, patients with higher LN ratio had worse prognosis. Similarly, RCC and LCC patients who had higher LN ratio had worse prognosis regardless of surgery, although RCC patients undergoing segmental resection or hemicolectomy had a greater risk of mortality than LCC patients. In addition, stage III RCC and LCC patients with higher LN ratio had increased mortality, with a relatively high mortality rate in RCC.
Regarding the categorial approach, cut-off point(s) of LN ratio might be set at the median LN ratio39 or determined using statistical methods28,40. The present SEER-based study disclosed that stage III RCC and LCC patients with high level of LN ratio (RCC ≥ 0.15, LCC ≥ 0.17) had elevated risks of mortality. A prospective study utilized the median LN ratio (0.25) as the cut-off value of LN ratio to assess prognostic value of LN ratio in colon cancer39. While, a French multicenter study found that stage III colon cancer patients with LN ratio ≥ 0.10 had worse OS and DFS28. Hence, the optimal LN ratio cut-off values for stage III RCC and LCC patients in various conditions remained to be explored.
Taken together, the present study demonstrated the prognostic value of LN ratio in stage III RCC and LCC patients in both continuous and categorical approaches. Higher LN ratio implies greater number of metastatic lymph nodes, so it is reasonable to assume that the likelihood of subsequent distant metastasis will increase with LN ratio, raising the risk of mortality. In agreement with this hypothesis, a longitudinal cohort study found that 5-year OS rate decreased with increasing LN ratio in patients with colorectal cancer40.
The present study had some strengths and limitations. The data analyzed in this study were derived from the SEER database, a nationwide population-based database that has been demonstrated to be representative of the cancer population of the United States41. Nevertheless, the findings of this SEER-based study should be confirmed by large-scale, longitudinal cohort studies of colon cancer patients from different geographic regions. In the present study, 19,515 patients were excluded because of missing values of covariates; therefore, the possibility of causing selection bias could not be ruled out. Although LCC patients had better response to chemotherapies than RCC patients34, the SEER database does not contain information about chemotherapy. It has been suggested that the selection of the optimal number of LNs examined and LN ratio were subject to the influence of surgeons, pathologists, and hospitals42,43; however, the relevant information was not available in the SEER database.
In conclusion, the prognostic impact of number of LNs examined and LN ratio on cancer-specific mortality in colon cancer patients undergoing curative surgery varied depending on tumor sidedness, surgical treatments and cancer stages. In general, patients with inadquate numbers of LNs examined or greater LN ratio had higher risks of LN metastasis and death. RCC patients in various conditions had worse or comparable survival than their LCC counterparts.
Data availability
All data generated within this study are available from the corresponding author on request.
References
Ferlay, J. et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC Cancer Base No. 11. Lyon, France: International Agency for Research on Cancer. https://www.iarc.fr/news-events/latest-world-cancer-statistics-globocan-2012-estimated-cancer-incidence-mortality-and-prevalence-worldwide-in-2012/ (2013).
Arnold, M. et al. Global patterns and trends in colorectal cancer incidence and mortality. Gut 66, 683–691, https://doi.org/10.1136/gutjnl-2015-310912 (2017).
Tamas, K. et al. Rectal and colon cancer: Not just a different anatomic site. Cancer Treat Rev 41, 671–679, https://doi.org/10.1016/j.ctrv.2015.06.007 (2015).
Paschke, S. et al. Are Colon and Rectal Cancer Two Different Tumor Entities? A Proposal to Abandon the Term Colorectal Cancer. Int J Mol Sci 19, https://doi.org/10.3390/ijms19092577 (2018).
Kalady, M. F. et al. Divergent oncogenic changes influence survival differences between colon and rectal adenocarcinomas. Dis Colon Rectum 52, 1039–1045, https://doi.org/10.1007/DCR.0b013e31819edbd4 (2009).
Siegel, R. L., Miller, K. D. & Jemal, A. Cancer statistics, 2019. CA Cancer J Clin 69, 7–34, https://doi.org/10.3322/caac.21551 (2019).
Araghi, M. et al. Global trends in colorectal cancer mortality: projections to the year 2035. Int J Cancer 144, 2992–3000, https://doi.org/10.1002/ijc.32055 (2019).
Signorelli, C. et al. Correlation of Tumor Location to Clinical Outcomes in Colorectal Cancer: A Single-institution Retrospective Analysis. Anticancer Res 39, 4917–4924, https://doi.org/10.21873/anticanres.13679 (2019).
Lim, D. R., Kuk, J. K., Kim, T. & Shin, E. J. Comparison of oncological outcomes of right-sided colon cancer versus left-sided colon cancer after curative resection: Which side is better outcome? Medicine (Baltimore) 96, e8241, https://doi.org/10.1097/MD.0000000000008241 (2017).
Gao, X. H. et al. Differences of protein expression profiles, KRAS and BRAF mutation, and prognosis in right-sided colon, left-sided colon and rectal cancer. Sci Rep 7, 7882, https://doi.org/10.1038/s41598-017-08413-z (2017).
Nakagawa-Senda, H., Hori, M., Matsuda, T. & Ito, H. Prognostic impact of tumor location in colon cancer: the Monitoring of Cancer Incidence in Japan (MCIJ) project. BMC Cancer 19, 431, https://doi.org/10.1186/s12885-019-5644-y (2019).
He, X. K. et al. Different Anatomical Subsites of Colon Cancer and Mortality: A Population-Based Study. Gastroenterol Res Pract 2018, 7153685, https://doi.org/10.1155/2018/7153685 (2018).
Warschkow, R. et al. Better survival in right-sided versus left-sided stage I - III colon cancer patients. BMC Cancer 16, 554, https://doi.org/10.1186/s12885-016-2412-0 (2016).
Fukata, K. et al. Clinical and prognostic differences between surgically resected right-sided and left-sided colorectal cancer. Surg Today, https://doi.org/10.1007/s00595-019-01889-4 (2019).
Moritani, K. et al. Difference in the recurrence rate between right- and left-sided colon cancer: a 17-year experience at a single institution. Surg Today 44, 1685–1691, https://doi.org/10.1007/s00595-013-0748-5 (2014).
Lee, J. M. et al. Impact of tumor sidedness on survival and recurrence patterns in colon cancer patients. Ann Surg Treat Res 96, 296–304, https://doi.org/10.4174/astr.2019.96.6.296 (2019).
Nelson, H. et al. Guidelines 2000 for colon and rectal cancer surgery. J Natl Cancer Inst 93, 583–596, https://doi.org/10.1093/jnci/93.8.583 (2001).
Fan, L. et al. Lymph node retrieval from colorectal resection specimens for adenocarcinoma: is it worth the extra effort to find at least 12 nodes? Colorectal Dis 13, 1377–1383, https://doi.org/10.1111/j.1463-1318.2010.02472.x (2011).
McDonald, J. R., Renehan, A. G., O’Dwyer, S. T. & Haboubi, N. Y. Lymph node harvest in colon and rectal cancer: Current considerations. World J Gastrointest Surg 4, 9–19, https://doi.org/10.4240/wjgs.v4.i1.9 (2012).
Onitilo, A. A., Stankowski, R. V., Engel, J. M. & Doi, S. A. Adequate lymph node recovery improves survival in colorectal cancer patients. J Surg Oncol 107, 828–834, https://doi.org/10.1002/jso.23332 (2013).
Tsai, H. L. et al. Factors affecting number of lymph nodes harvested and the impact of examining a minimum of 12 lymph nodes in stage I-III colorectal cancer patients: a retrospective single institution cohort study of 1167 consecutive patients. BMC Surg 16, 17, https://doi.org/10.1186/s12893-016-0132-7 (2016).
Ma, Y. et al. Prognostic impact of the number of lymph nodes examined in different stages of colorectal mucinous adenocarcinoma. Onco Targets Ther 11, 3659–3670, https://doi.org/10.2147/OTT.S163076 (2018).
Fortea-Sanchis, C., Martinez-Ramos, D. & Escrig-Sos, J. CUSUM charts in the quality control of colon cancer lymph node analysis: a population-registry study. World J Surg Oncol 16, 230, https://doi.org/10.1186/s12957-018-1533-0 (2018).
Choi, H. K., Law, W. L. & Poon, J. T. The optimal number of lymph nodes examined in stage II colorectal cancer and its impact of on outcomes. BMC Cancer 10, 267, https://doi.org/10.1186/1471-2407-10-267 (2010).
Lee, L. et al. Lower survival after right-sided versus left-sided colon cancers: Is an extended lymphadenectomy the answer? Surg Oncol 27, 449–455, https://doi.org/10.1016/j.suronc.2018.05.031 (2018).
Chang, Y. J., Chen, L. J., Chang, Y. J., Chung, K. P. & Lai, M. S. Application of propensity score model to examine the prognostic significance of lymph node number as a care quality indicator. Surg Oncol 21, e75–85, https://doi.org/10.1016/j.suronc.2011.12.003 (2012).
Zekri, J. et al. Lymph node ratio may predict relapse free survival and overall survival in patients with stage II & III colorectal carcinoma. Hepatogastroenterology 62, 291–294 (2015).
Sabbagh, C. et al. A lymph node ratio of 10% is predictive of survival in stage III colon cancer: a French regional study. Int Surg 99, 344–353, https://doi.org/10.9738/INTSURG-D-13-00052.1 (2014).
Sjo, O. H., Merok, M. A., Svindland, A. & Nesbakken, A. Prognostic impact of lymph node harvest and lymph node ratio in patients with colon cancer. Dis Colon Rectum 55, 307–315, https://doi.org/10.1097/DCR.0b013e3182423f62 (2012).
Guan, X. et al. Whether regional lymph nodes evaluation should be equally required for both right and left colon cancer. Oncotarget 7, 59945–59956, https://doi.org/10.18632/oncotarget.11007 (2016).
Zurleni, T. et al. Surgical and oncological outcomes after complete mesocolic excision in right-sided colon cancer compared with conventional surgery: a retrospective, single-institution study. Int J Colorectal Dis 33, 1–8, https://doi.org/10.1007/s00384-017-2917-2 (2018).
Hussain, M. et al. Right-Sided and Left-Sided Colon Cancers are Two Distinct Disease Entities: an Analysis of 200 Cases in Pakistan. Asian Pac J Cancer Prev 17, 2545–2548 (2016).
Yang, S. Y., Cho, M. S. & Kim, N. K. Difference between right-sided and left-sided colorectal cancers: from embryology to molecular subtype. Expert Rev Anticancer Ther 18, 351–358, https://doi.org/10.1080/14737140.2018.1442217 (2018).
Baran, B. et al. Difference Between Left-Sided and Right-Sided Colorectal Cancer: A Focused Review of Literature. Gastroenterology Res 11, 264–273, https://doi.org/10.14740/gr1062w (2018).
Shen, H. et al. Different treatment strategies and molecular features between right-sided and left-sided colon cancers. World J Gastroenterol 21, 6470–6478, https://doi.org/10.3748/wjg.v21.i21.6470 (2015).
Stewart, S. L., Wike, J. M., Kato, I., Lewis, D. R. & Michaud, F. A population-based study of colorectal cancer histology in the United States, 1998-2001. Cancer 107, 1128–1141, https://doi.org/10.1002/cncr.22010 (2006).
Mik, M., Berut, M., Dziki, L., Trzcinski, R. & Dziki, A. Right- and left-sided colon cancer - clinical and pathological differences of the disease entity in one organ. Arch Med Sci 13, 157–162, https://doi.org/10.5114/aoms.2016.58596 (2017).
Kataoka, K. et al. Prognostic significance of number versus location of positive mesenteric nodes in stage iii colon cancer. Eur J Surg Oncol 45, 1862–1869, https://doi.org/10.1016/j.ejso.2019.05.022 (2019).
Dolan, R. D., McSorley, S. T., Horgan, P. G. & McMillan, D. C. Determinants of lymph node count and positivity in patients undergoing surgery for colon cancer. Medicine (Baltimore) 97, e0185, https://doi.org/10.1097/MD.0000000000010185 (2018).
Rosenberg, R. et al. Prognosis of patients with colorectal cancer is associated with lymph node ratio: a single-center analysis of 3,026 patients over a 25-year time period. Ann Surg 248, 968–978, https://doi.org/10.1097/SLA.0b013e318190eddc (2008).
Kuo, T. M. & Mobley, L. R. How generalizable are the SEER registries to the cancer populations of the USA? Cancer Causes Control 27, 1117–1126, https://doi.org/10.1007/s10552-016-0790-x (2016).
Rhoads, K. F. et al. Adequacy of lymph node examination in colorectal surgery: contribution of the hospital versus the surgeon. Med Care 51, 1055–1062, https://doi.org/10.1097/MLR.0b013e3182a53d72 (2013).
Becerra, A. Z. et al. Surgeon-, pathologist-, and hospital-level variation in suboptimal lymph node examination after colectomy: Compartmentalizing quality improvement strategies. Surgery 161, 1299–1306, https://doi.org/10.1016/j.surg.2016.11.029 (2017).
Acknowledgements
The authors acknowledge the efforts of the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER database. The interpretation and reporting of these data are the sole responsibility of the authors.
Author information
Authors and Affiliations
Contributions
H.-W.L., C.-C.L. and J.C.-C.W. performed conception and design; H.-W.L. and H.-C.H. acquired data; H.-W.L., C.-C.L. and J.C.-C.W. analyzed and interpreted data; H.-W.L. and H.-C.H. prepared the manuscript; J.C.-C.W. and C.-C.L. revised it critically for content; H.-W.L. guarantor of integrity of the entire study; H.-W.L. performed statistical analysis; H.-C.H. defined the intellectual content; C.-C.L. performed literature research; J.C.-C.W. performed clinical studies; J.C.-C.W. and C.-C.L. supervised the project. All authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lai, HW., Wei, J.CC., Hung, HC. et al. Tumor sidedness influences prognostic impact of lymph node metastasis in colon cancer patients undergoing curative surgery. Sci Rep 9, 19892 (2019). https://doi.org/10.1038/s41598-019-56512-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-56512-w
This article is cited by
-
Prognostic value of primary tumor location in colorectal cancer: an updated meta-analysis
Clinical and Experimental Medicine (2023)
-
Comparison of Resected Malignant Tumors of the Right- and Left-Sided Colon—Is There a Difference?
Indian Journal of Surgery (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.