Abstract
The role of enlarged subarachnoid space (ESS) in preterm infants has not been described in concrete. We aimed to evaluate whether ESS should be considered a risk factor potentially associated with adverse neurodevelopmental outcomes in prematurity. Electronic medical records of 197 preterm infants (median 32.1 weeks' gestation) including cranial ultrasound (cUS) images, head circumferences, and Korean Developmental Screening Tests for Infants and Children (K-DST) results at 18–24 months corrected age were reviewed. The clinical characteristics and K-DST results were compared in infants with and without ESS (sinocortical width > 3.5 mm). A multivariable logistic regression analysis was performed to identify potential risk factors associated with positive K-DST results. At a median corrected age of 39.0 weeks, 81/197 (41.1%) infants presented ESS. A significantly greater percent of infants in the ESS group screened positive on the K-DST than in the no ESS group (27.2% vs 12.1%, p = 0.007). Within the ESS group, micro-/macrocephaly at term-equivalent age was not different with regard to the K-DST results. From the multivariable logistic regression analysis, gestational age (p = 0.016, OR = 0.855, 95% CI = 0.753–0.971) and ESS (p = 0.019, OR = 1.310, 95% CI = 1.046–1.641) were two significant risk factors associated with positive K-DST results. ESS identified on cUS at term-equivalent age in preterm infants is associated with possible developmental delays. Macrocephaly at term-equivalent age does not guarantee a benign prognosis. Future studies are required to verify ESS as a potential marker for neurodevelopmental delay in preterm infants.
Similar content being viewed by others
Introduction
Ultrasonography (US) is a frequently used imaging tool in the neonatal intensive care unit (NICU) because it is noninvasive and portable. Cranial US (cUS) is readily used for assessing preterm infants’ brain lesions such as intraventricular haemorrhage (IVH) or periventricular leukomalacia (PVL)1. However, cUS has a relatively lower accuracy than brain magnetic resonance imaging (MRI)2,3,4,5 in defining diffuse white matter lesions or PVL without cyst or cavitation formation, which is a more common type of non-haemorrhagic intracranial disease compared to cystic PVL6.
White matter injuries on MRI at term-equivalent age in very preterm infants are reportedly associated with grey matter abnormalities (including reduction in cerebral cortical grey matter volume) and future neurological impairment7,8. However, periventricular echogenicity greater than that of the choroid plexus, or inhomogeneous flares, which is often considered a possible US finding of non-cystic PVL9,10, has presented inconsistent association with punctate or diffuse white matter injuries or decreased brain volumes on MRI at term-equivalent age11,12,13 or future adverse neurological outcomes14,15,16. Rather, other combined cUS findings supportive of cerebral atrophy showed an association with neurodevelopmental outcome in previous literature14, with enlarged subarachnoid space (ESS) being one of them.
Structural changes around the subarachnoid space17,18 can be assessed easily using cUS images. An ESS may often be encountered in otherwise normally developing infants19,20 but is at times deemed pathologic for its potential association with adverse neurodevelopmental outcomes. Whether ESS is merely a normal variant (benign ESS) or not (e.g., brain atrophy) may be distinguished with supportive information such as the head circumference. For instance, infants with benign ESS often have macrocephaly, but those with cerebral atrophy would have blunted growth of head circumference21. However, the trend of head growth may not be evident in the very early days, when ESS is first identified. Furthermore, the independent role of ESS in prematurity has not been studied extensively. Therefore, we aimed to evaluate whether ESS should be considered a risk factor potentially associated with adverse neurodevelopmental outcomes in prematurity.
Materials and Methods
Patient selection and data collection
Electronic medical records including cUS images of all preterm infants who had been admitted to the NICU of our institute from March 2014 to June 2017 were retrieved. Infants who died during NICU stay or after hospital discharge, who did not visit for neurodevelopmental assessment at 18–24 months corrected age at our hospital, or who had major congenital anomaly or chromosomal anomaly were excluded. Infants who did not undergo repeat cUS beyond the 4th week of life were excluded, because we judged that images obtained from such infants could not be qualified for evaluation of subarachnoid space changes over time. Infants with severe IVH (grade 3 or 4), cystic PVL, and other parenchymal lesions were excluded to avoid the possible confounding effects of such lesions when evaluating the association with neurodevelopmental outcomes. This study was approved by the institutional review board of Seoul St. Mary’s Hospital. The research was performed in accordance with the Declaration of Helsinki. The need for informed consent was waived due to the retrospective nature of the study.
Three hundred and twenty-seven preterm infants who survived and visited for neurodevelopmental assessment at 18–24 months corrected age were included. Infants (n = 129) with the following were excluded: insufficient brain image data (n = 65), severe IVH (n = 36), cystic PVL (n = 14), focal parenchymal lesions (n = 12), agenesis of septum pellucidum (n = 1), and neurofibromatosis type 1 (n = 1). One infant was further excluded because the neurodevelopmental assessment was incomplete. Thus, 197 infants (108 male, median 32.1 weeks’ gestation) were finally included in the study.
Basic clinical characteristics such as gestational age (GA), birthweight, 1- and 5-minute Apgar scores, sex, mode of delivery, preterm premature rupture of membrane >18 h, mother’s age and underlying maternal morbidities (diabetes, hypertensive disorder), neonatal morbidities [respiratory distress syndrome, patent ductus arteriosus requiring treatment, necrotizing enterocolitis ≥stage 2 based on the modified Bell’s criteria22, moderate to severe bronchopulmonary dysplasia (BPD) based on a previous definition23, culture-proven sepsis], and length of NICU stay were recorded. Head circumferences at birth, term-equivalent age, and 18–24 months corrected age were collected. The measurements were categorised into percentile groups to assess the presence of microcephaly (<10th percentile) and macrocephaly (>90th percentile) based on the growth curves derived from Korean infants (head circumferences at birth and at term-equivalent age)24 and the 2017 Pediatrics and Adolescents Growth Standards provided by the Korea Centre for Disease Control and Prevention (KCDC) (head circumferences at 18–24 months corrected age)25.
cUS image and measurement obtainment
The cUS images were acquired and reviewed by one paediatric radiologist with 17 years of experience. During the study period, US examinations were performed using a 4- to 10-MHz linear probe and an Acuson Sequoia 512 (Siemens Medical Engineering group, Mountain View, CA) or a 5- to 8-MHz curved probe and an Affiniti 50 G scanner (Philips Ultrasound, Bothell, Seattle, WA). The anterior fontanelle was used as an acoustic window, and images were recorded in at least six coronal and five sagittal planes. cUS examinations with additional mastoid fontanelle views were performed between the 1st and 14th day of life. The routine practice for cUS assessments in our NICU was to obtain the first cUS image within the first week of life, and follow-up schedules were arranged depending on the previous cUS results or the clinical condition of the infant, at the on-duty neonatologists’ discretion. In general, the preterm infants were followed up at a 1–2 week(s)' intervals until 4 weeks of life, and then followed up at 2 or more weeks’ intervals if IVH was less than grade 3. Follow-up cUS was withheld at approximately term-equivalent age or 1-month corrected age provided that there was no IVH or grade 1–2 IVH was involuting. Whenever a massive blood loss was suspected or when an infant underwent critical procedures such as surgery under general anaesthesia, an additional follow-up cUS was performed.
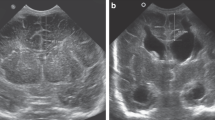
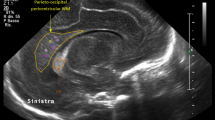
IVH was graded according to Papile’s criteria26, and periventricular echogenicity was considered to be present when it was greater than the choroid plexus echogenicity or was inhomogeneous9,10,27. The subarachnoid space was measured on the coronal plane at the level of the foramen of Monro (Fig. 1) and ESS was defined based on the work by Armstrong et al. as sinocortical width (SCW) >3.5 mm18 at any age at which cUS took place. Along with SCW, interhemispheric width (IHW) and craniocortical width (CCW) – the criteria originally described previously28 – were also measured (Fig. 2). SCW was selected as the parameter to define ESS in our study because we deemed that CCW measurements, more easily influenced by the pressure of the probe delivered by the examiner on the anterior fontanelle, would provide inconsistent measurements. One paediatric radiologist at our institute reviewed the archived images to obtain measurements at two separate time points at least 2 weeks apart, and the mean value was selected for analyses in order to minimise intraobserver variability. An example of the series of cUS images and a brain MRI at term-equivalent age reviewed in one patient is presented on Fig. 3(a–d).
(a–d) Serial brain images of a preterm infant enrolled in our study. The infant was born at a gestational age of 29 + 5 weeks, with a birthweight of 1306 g. The initial cranial ultrasound done on the 4th day of life reveals inhomogeneously increased periventricular echogenicity (arrow) to the same degree as choroid plexus (a). In the subsequent cranial ultrasound done at the 20th day of life, the periventricular increased echogenicity is regressed without cystic formation (b). Enlarged subarachnoid space is first noticed on the follow-up cranial ultrasound done at 45th day of life (arrow), at a corrected age of 36 + 0 weeks (sinocortical width = 8.3 mm) (c). On the brain magnetic resonance image (coronal T2-weighted image, TR/TE 5482/100) done at term-equivalent age, the enlarged subarachnoid space (arrow) is also noticed (d). The infant was screened positive in the gross motor function field at the neurodevelopmental assessment done at the follow-up visit.
Neurodevelopmental assessment
The neurodevelopmental assessment of the infants at 18–24 months corrected age was performed using the Korean Developmental Screening Test for Infants and Children (K-DST), developed in September 2014 by the Korean Pediatric Society and presided by the KCDC29. The K-DST was developed to take into account the components of the culture and infant care environment in Korea and improve detection of suspected developmental delay30. The K-DST is composed of questionnaires for six domains (gross motor function, fine motor function, cognition, language, social interaction, and self-help). The summed score for each domain is categorised into one of the following four: higher-than-peer level (≥1 SD), peer level (<1 SD and ≥−1 SD), follow-up evaluation is recommended (<−1 SD and ≥−2 SD), and detailed evaluation is warranted (<−2 SD). If the score for any one of the six domains is <−2 SD, the infant is considered ‘screen positive’ for the K-DST, signifying that the infant must be further evaluated for possible developmental delay. Two and five supplemental key questions associated with abnormal features (e.g., No independent walking – yes or no?) are provided at the end of the K-DST for 18 and 24 months corrected age, respectively. If the response is positive (=‘abnormal’) in any one of the key questions, the infant is considered ‘screen positive,’ regardless of the scores in the six domains. Additionally, the gross motor function status was assessed using the Gross Motor Function Classification System (GMFCS)31. If an infant had visited for assessment more than once during the period of 18–24 months corrected age, the most recent assessment result was included.
Statistical analysis
The enrolled infants were divided into two groups according to the presentation of ESS. Baseline characteristics, other brain lesions identified from cUS, and the K-DST results at 18–24 months corrected age were compared between the two groups.
The infants were also divided into two groups according to the K-DST result [‘screen positive’ (score < −2 SD in any domain) vs ‘screen negative’ (score > −2 SD in all domains)]. The two groups were compared for baseline characteristics, differences in the frequency of ESS, periventricular echogenicity, or IVH, and the specific parameters of ESS were also compared.
Continuous variables were analysed using the Mann-Whitney U test, and categorical variables were analysed using the chi-square test or Fisher’s exact test, as appropriate. A multivariable logistic regression analysis was carried out in a backward conditional manner to evaluate the usefulness of parameters associated with positive results from the K-DST. p < 0.050 was considered statistically significant.
Results
Eighty-one infants (41.1%) presented with ESS while 36 infants (18.3%) were ‘screen- positive’ on the K-DST.
Basic clinical characteristics were compared between the infants with and without ESS (Table 1). The ESS-group infants were born at a shorter GA (30.1 [27.9–33.4] vs 32.9 [30.5–34.4] weeks, p < 0.001) and a lower birthweight (1360 [1029–2033] vs 1800 [1256–2195] g, p = 0.003), with a significantly lower 1-minute Apgar score. The proportion of infants born via caesarean delivery was significantly greater in the ESS group (74 [91.4%] vs 93 [80.2%], p = 0.032). Maternal characteristics did not differ. The prevalence of moderate to severe BPD (35.8 vs 19.8%, p = 0.012) and the length of hospital stay (42 [21–61] vs 24 [12–48] days, p < 0.001) were higher and longer in the ESS group.
The cUS findings compared depending on presence of ESS are described in Table 2. ESS presented at a median [interquartile range] corrected age of 39.0 [36.6–41.4] weeks. Overall, the IVH grade did not differ between the two groups, but periventricular echogenicity was more frequently found in the ESS group (12.3% vs 2.6%, p = 0.007). The IHW, CCW, and SCW were all significantly greater in the ESS group.
The K-DST results assessed at 18–24 months corrected age were compared between the infants depending on the presence of ESS (Table 3). The proportion of ‘screen-positive’ infants was more than twice higher in the ESS group than in the no ESS group (27.2% vs 12.1%, p = 0.007). The difference was most evident in the gross motor function domain (23.5% vs 6.0%, p < 0.001). Concerning the GMFCS, a greater percentage of infants in the ESS group showed level ≥2 (7.4 vs 0.9%, p = 0.020), of which one infant in the ESS group was at level 3. All infants with positive responses on the supplementary questions or GMFCS had a score <−2 SD in at least one of the six domains of the K-DST.
The infants were compared for baseline characteristics depending on the K-DST result (Table 4). The screen-positive group showed significantly shorter GA (29.7 [26.4–32.6] vs 32.7 [29.9–34.1] weeks, p < 0.001), lower birthweight (1152 [860–1803] vs 1734 [1205–2152] g, p = 0.001), and lower 1-minute Apgar score (4 [2–6] vs 5 [3–6], p = 0.021). Moderate to severe BPD was the only neonatal morbidity that showed significant difference in prevalence (47.2 vs 21.7%, p = 0.002) between the screen-positive vs negative groups. The length of hospital stay was also significantly longer in the screen-positive group.
Table 5 describes the prevalence of ESS and periventricular echogenicity, and the IHW, CCW, and SCW compared between the ‘screen-positive’ and ‘screen-negative’ groups. Significantly more infants had ESS in the ‘screen-positive’ group (61.1% vs 36.6%, p = 0.007), initially presenting at term-equivalent age in both groups. IHW, CCW, and SCW were significantly greater in the ‘screen-positive’ group.
Head circumferences measured at birth, term-equivalent age, and 18–24 months corrected age were compared between infants with and without ESS, further subdivided according to the K-DST results (Table 6). A significantly greater proportion of infants had microcephaly in the K-DST-positive subgroup (27.3 vs 7.3%, p = 0.028) in the ESS group but not in the no-ESS group. The prevalence of macrocephaly at neither term-equivalent age nor 18–24 months corrected age showed statistically significant difference between the subgroups within the ESS and no ESS groups.
A multivariable logistic regression analysis was performed to evaluate the association with positive results on the K-DST (Table 7). Significant variables from univariable analyses and clinically important factors were included. GA [p = 0.016, odds ratio (OR) = 0.855, 95% confidence interval (CI) = 0.753–0.971] and ESS [p = 0.019, OR = 1.310, 95% CI = 1.046–1.641] were two factors that maintained statistical significance.
Discussion
We evaluated the association between ESS and neurodevelopmental test results in preterm infants. Based on our study results, ESS was associated with positive results on the K-DST, which was most evident in the gross motor function domain. Although it failed to maintain statistical significance in the multivariable analysis, microcephaly at 18–24 months corrected age tended to be more prevalent in the K-DST screen-positive infant subgroup in the ESS group.
The ESS criteria we used in our study were based on a previous work by Armstrong et al.18 including preterm infants at <37 weeks’ GA. The traditional criteria for ESS – IHW > 6 mm, CCW > 4 mm, and SCW > 3 mm – were based on values derived from infants at 1–12 months of age28. Meanwhile, Lam et al.32 suggested their regression curves as the new criteria for normal upper limit values including term infants. Neither reference value has been validated in preterm infants; thus, we decided to adhere to the value proposed by Armstrong et al.18.
The ESS course has been previously described as benign19,20,33. However, some studies have reported the possibility of transient and, less frequently, permanent developmental delays associated with ESS34,35. Our study results also revealed an association with developmental delay. Whether or not these delays would be transient or permanent could not be confirmed at this stage, but it does not change the fact that these infants may potentially not be able to “grow out” of the delays and may require rehabilitative intervention of some type. Therefore, ESS in preterm infants deserves the attention of clinicians.
To our knowledge, no studies have examined how to distinguish the suspected course of ESS at the early stages of presentation in preterm infants. A concomitant presentation of ESS and macrocephaly is considered to be associated with a lower possibility of adverse neurological outcomes in full-term infants. However, based on our study results, the same cannot be determined in preterm infants. The initial presentation of ESS was identified at term-equivalent age and postnatal age of 57 [38–77] days, which is earlier than that in full-term infants who present with ESS at 3 to 8 months of life. The prevalence of macrocephaly at term-equivalent age or 18–24 months corrected age did not differ depending on the neurodevelopmental screening assessments in infants presenting with ESS. Therefore, a concomitant presentation of ESS and macrocephaly at term-equivalent age does not guarantee a normal future neurodevelopmental course in preterm infants. Rather, microcephaly at 18–24 months corrected age was significant at least in the univariable analysis. Therefore, we postulate that ESS in preterm infants is a neglected condition that deserves more attention from both clinicians and radiologists. A larger-scale prospective study is warranted to validate our results and draw specific conclusions for the role of ESS in future neurodevelopmental outcomes in preterm infants.
Our study is restricted by several factors. The retrospective design and the small number of included patients is the first limitation. Infants who resided at far distances and were lost to follow-up at our hospital were excluded. Some infants would have received follow-up evaluations at other institutes, but such information was inaccessible. Secondly, we did not analyse the results from examinations such as the Bayley Scales of Infant and Toddler Development (BSID) but used the K-DST for outcome assessment. Our unit protocol is to carry out follow-up neurodevelopmental evaluations largely based on the K-DST. If screened positive, the infant is referred for further examinations such as the BSID to the department of rehabilitative medicine, unless the infant is judged to be at a very high risk for adverse neurodevelopmental outcome (such as most extreme prematurity, severe IVH, cystic PVL, and hypoxic-ischemic encephalopathy). However, the K-DST reflects the cultural and environmental facets of our country29,30 and is easy to use, so complete assessment was achieved in most infants who visited the outpatient clinic at 18–24 months corrected age, which is the strength of our study. We also used the GMFCS to further complement our assessment. Another limitation is that we did not include cerebellar injury in analysing the cUS findings, because there was insufficient data in the archive. However, since large cerebellar haemorrhagic injuries often present with concomitant severe supratentorial haemorrhagic lesions36, those with such lesions would have been excluded per our study enrollment scheme excluding severe IVH. Furthermore, small cerebellar haemorrhages are difficult to detect with cUS37, so there is a high probability that patients with isolated cerebellar microhaemorrhages would not have been readily identified, even if appropriate images had been obtained. In addition, because the cUS were done at several weeks’ intervals in the later stages of life in some infants, it is possible that in some cases, the cystic stage of PVL may have been missed (as in the case of Fig. 3, in which there was a long delay between the first (a) and second (b) cUS scan). Finally, we did not analyse brain MRI findings due to lack of data. However, cUS is the most generally-used imaging modality with a fair cost-effective character. In contrast, MRI is expensive, often requires sedation, and is frequently difficult to perform in infants with critical pulmonary conditions. Therefore, using cUS findings to assess ESS is meaningful for its high applicability at clinical settings similar to our NICU.
Conclusion
Enlarged subarachnoid space identified on cranial ultrasound at term-equivalent age is associated with possible developmental delays and could be an early ‘warning sign’ of neurodevelopmental delay in preterm infants. Concomitant presentation of enlarged subarachnoid space and macrocephaly at term-equivalent age in preterm infants may not guarantee a benign nature. Future prospective studies with a large number of subjects with sequential follow-up examinations with sufficient time intervals are needed to verify our findings.
Data availability
The data is available only upon a reasonable request to the corresponding author.
References
Diwakar, R. K. & Khurana, O. Cranial Sonography in Preterm Infants with Short Review of Literature. J. Pediatr. Neurosci. 13, 141–149 (2018).
Hinojosa-Rodriguez, M. et al. Clinical neuroimaging in the preterm infant: Diagnosis and prognosis. Neuroimage Clin. 16, 355–368 (2017).
Roelants-van Rijn, A. M. et al. Parenchymal brain injury in the preterm infant: comparison of cranial ultrasound, MRI and neurodevelopmental outcome. Neuropediatrics 32, 80–89 (2001).
de Vries, L. S., Benders, M. J. & Groenendaal, F. Progress in Neonatal Neurology with a Focus on Neuroimaging in the Preterm Infant. Neuropediatrics 46, 234–241 (2015).
Benders, M. J., Kersbergen, K. J. & de Vries, L. S. Neuroimaging of white matter injury, intraventricular and cerebellar hemorrhage. Clin. Perinatol. 41, 69–82 (2014).
Volpe, J. J. Encephalopathy of prematurity includes neuronal abnormalities. Pediatrics 116, 221–225 (2005).
Woodward, L. J., Anderson, P. J., Austin, N. C., Howard, K. & Inder, T. E. Neonatal MRI to predict neurodevelopmental outcomes in preterm infants. N. Engl. J. Med. 355, 685–694 (2006).
Inder, T. E., Warfield, S. K., Wang, H., Huppi, P. S. & Volpe, J. J. Abnormal cerebral structure is present at term in premature infants. Pediatrics 115, 286–294 (2005).
Leijser, L. M. et al. Comparing brain white matter on sequential cranial ultrasound and MRI in very preterm infants. Neuroradiol. 50, 799–811 (2008).
Sie, L. T. et al. Early MR features of hypoxic-ischemic brain injury in neonates with periventricular densities on sonograms. Am. J. Neuroradiol. 21, 852–861 (2000).
Graca, A. M., Cardoso, K., Costa, J. & Cowan, F. Persistent periventricular echogenicities in preterms are not related to smaller brains at term-equivalent age. Neonatology 106, 42–48 (2014).
Inder, T. E., Anderson, N. J., Spencer, C., Wells, S. & Volpe, J. J. White matter injury in the premature infant: a comparison between serial cranial sonographic and MR findings at term. Am. J. Neuroradiol. 24, 805–809 (2003).
Ciambra, G. et al. Accuracy of transcranial ultrasound in the detection of mild white matter lesions in newborns. Neuroradiol. J. 26, 284–289 (2013).
Horsch, S., Muentjes, C., Franz, A. & Roll, C. Ultrasound diagnosis of brain atrophy is related to neurodevelopmental outcome in preterm infants. Acta Paediatri. 94, 1815–1821 (2005).
Goldstein, R. B., Filly, R. A., Hecht, S. & Davis, S. Noncystic “increased” periventricular echogenicity and other mild cranial sonographic abnormalities: predictors of outcome in low birth weight infants. J. Clin. Ultrasound 17, 553–562 (1989).
Chen, C. C., Huang, C. B., Chung, M. Y., Huang, L. T. & Yang, C. Y. Periventricular echogenicity is related to delayed neurodevelopment of preterm infants. Am. J. Perinatol. 21, 483–489 (2004).
Anderson, N. G. et al. A limited range of measures of 2-D ultrasound correlate with 3-D MRI cerebral volumes in the premature infant at term. Ultrasound Med. Biol. 30, 11–18 (2004).
Armstrong, D. L., Bagnall, C., Harding, J. E. & Teele, R. L. Measurement of the subarachnoid space by ultrasound in preterm infants. Arch. Dis. Child. Fetal Neonatal Ed. 86, F124–126 (2002).
Kuruvilla, L. C. Benign enlargement of sub-arachnoid spaces in infancy. J. Pediatr. Neurosci. 9, 129–131 (2014).
Suara, R. O., Trouth, A. J. & Collins, M. Benign subarachnoid space enlargement of infancy. J. Natl. Med. Assoc. 93, 70–73 (2001).
Hadzagic-Catibusic, F., Gavranovic, M. & Zubcevic, S. Ultrasound differentiation between benign enlargement of the subarachnoid space and brain atrophy. Med. Arh. 56, 11–13 (2002).
Walsh, M. C. & Kliegman, R. M. Necrotizing enterocolitis: treatment based on staging criteria. Pediatr. Clin. North Am. 33, 179–201 (1986).
Jobe, A. H. & Bancalari, E. Bronchopulmonary dysplasia. Am. J. Respir. Crit. Care Med. 163, 1723–1729 (2001).
Lee, J. et al. The Study of Growth Measurements at Different Gestational Ages of Korean Newborn the Survey and Statistics. J. Korean Soc. Neonatol. 13, 47–57 (2006).
Kim, J. H. et al. The 2017 Korean National Growth Charts for children and adolescents: development, improvement, and prospects. Korean J. Pediatr. 61, 135–149 (2018).
Papile, L. A., Burstein, J., Burstein, R. & Koffler, H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J. Pediatr. 92, 529–534 (1978).
Maller, V. V. & Cohen, H. L. Neurosonography: Assessing the Premature Infant. Pediatr. Radiol. 47, 1031–1045 (2017).
Libicher, M. & Troger, J. US measurement of the subarachnoid space in infants: normal values. Radiology 184, 749–751 (1992).
Yim, C. H., Kim, G. H. & Eun, B. L. Usefulness of the Korean Developmental Screening Test for infants and children for the evaluation of developmental delay in Korean infants and children: a single-center study. Korean J. Pediatr. 60, 312–319 (2017).
Chung, H. J. et al. The validity of Korean Ages and Stages Questionnaires (K-ASQ) in Korean infants and children. J. Korean Child Neurol. Soc. 22, 1–11 (2014).
Morris, C. & Bartlett, D. Gross Motor Function Classification System: impact and utility. Dev. Med. Child Neurol. 46, 60–65 (2004).
Lam, W. W., Ai, V. H., Wong, V. & Leong, L. L. Ultrasonographic measurement of subarachnoid space in normal infants and children. Pediatr. Neurol. 25, 380–384 (2001).
Hellbusch, L. C. Benign extracerebral fluid collections in infancy: clinical presentation and long-term follow-up. J. Neurosurg. 107, 119–125 (2007).
Zahl, S. M., Egge, A., Helseth, E. & Wester, K. Benign external hydrocephalus: a review, with emphasis on management. Neurosurg. Rev. 34, 417–432 (2011).
Alvarez, L. A., Maytal, J. & Shinnar, S. Idiopathic external hydrocephalus: natural history and relationship to benign familial macrocephaly. Pediatrics 77, 901–907 (1986).
Steggerda, S. J. et al. Small cerebellar hemorrhage in preterm infants: perinatal and postnatal factors and outcome. Cerebellum 12, 794–801 (2013).
Parodi, A. et al. Accuracy of ultrasound in assessing cerebellar haemorrhages in very low birthweight babies. Arch. Dis. Child. Fetal Neonatal Ed. 100, F289–F292 (2015).
Author information
Authors and Affiliations
Contributions
S.K. Yum and S.A. Im conceptualized and designed the study. S.K. Yum, Y.M. Seo and S.A. Im collected data. S.K. Yum wrote the first draft. I.K. Sung and S.A. Im critically revised the manuscript. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yum, S.K., Im, S.A., Seo, Y.M. et al. Enlarged subarachnoid space on cranial ultrasound in preterm infants: Neurodevelopmental implication. Sci Rep 9, 19072 (2019). https://doi.org/10.1038/s41598-019-55604-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-55604-x
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.