Abstract
Root-knot nematodes (RKNs) are devastating parasites that infect thousands of plants. As RKN infection is facilitated by oesophageal gland effector genes, one such effector gene, Mi-msp2, was selected for a detailed characterization. Based on domain analysis, the Mi-MSP2 protein contains an ShKT domain, which is likely involved in blocking K+ channels and may help in evading the plant defence response. Expression of the Mi-msp2 gene was higher in juveniles (parasitic stage of RKNs) than in eggs and adults. Stable homozygous transgenic Arabidopsis lines expressing Mi-msp2 dsRNA were generated, and the numbers of galls, females and egg masses were reduced by 52–54%, 60–66% and 84–95%, respectively, in two independent RNAi lines compared with control plants. Furthermore, expression analysis revealed a significant reduction in Mi-msp2 mRNA abundance (up to 88%) in female nematodes feeding on transgenic plants expressing dsRNA, and northern blot analysis confirmed expression of the Mi-msp2 siRNA in the transgenic plants. Interestingly, a significant reduction in the reproduction factor was observed (nearly 40-fold). These data suggest that the Mi-msp2 gene can be used as a potential target for RKN management in crops of economic importance.
Similar content being viewed by others
Introduction
Attacking more than 2000 plant species and leading to crop losses of 173 billion USD annually in worldwide agriculture, root-knot nematodes (RKNs; Meloidogyne spp.) are among the most injurious plant parasitic nematodes (PPNs)1. With the challenges of the ever-increasing global population and meeting food requirements, it has become important to control the damage to various crops caused by nematodes. However, the above-ground symptoms of RKN infections are not conspicuous, and these sedentary endoparasites are thus mostly unnoticed, resulting in large-scale damage. Due to their wide host range, their dynamic distribution and the associated large economic losses, RKNs are considered to be among the most threatening PPN pests. RKN second-stage juveniles (J2s) infect plant roots and form multinucleated polyploid feeding cells, termed giant cells, which are required for nematode development and reproduction2.
The development of specialized structures in nematodes that enable parasitism has been observed during evolution3. These specialized structures, such as the stylet, amphids and oesophageal glands, are required for penetration and feeding to promote parasitism4,5. In PPNs, parasitism is enabled by the secretion of effector proteins that suppress the host defence system. Effector proteins that are produced in a granulated form in the oesophageal glands (secretory proteins) are transferred to plant cells via the stylet6. These proteins are essential for the onset and maintenance of parasitism and are mainly associated with three functions: (i) facilitation of migration, (ii) defence against plant responses and (iii) establishment and maintenance of permanent feeding sites through manipulation of the host cellular machinery7. There are two types of oesophageal secretory glands in RKNs: subventral glands, which are responsible for the early onset of parasitism; and a single dorsal gland, which is active in later stages for maintenance of parasitism. Hence, significant changes in secreted and associated components occur during the nematode parasitic life cycle4,8,9. Indeed, maintenance of successful parasitism depends on interactions and molecular communication between pathogen effector proteins and host metabolic processes. Various effector genes have been reported to be involved in modulating host signalling pathways, providing evidence that endoparasitic nematodes hijack host cellular machinery to expand feeding sites. Additionally, host defence responses have been shown to be effectively altered by nematodes10. The roles of several effector genes in PPNs have been demonstrated; for instance, effectors such as 16D10 reportedly increase host plant vulnerability after nematode infection11. Additionally, overexpression of the effector protein Mi-MSP18 in onion cells led to increased susceptibility to M. incognita, M. javanica and M. graminicola infection12. The calreticulin effector gene of M. incognita has been found to suppress basal immunity within the host plant13, and another effector gene, Mj-FAR-1, has been reported to affect various defence-related plant responses, including on cell wall-related genes14. Another nuclear-localized effector protein, Mj-NULG1, is reportedly localized to the plant host nucleus and to possibly be involved in cell cycle modifications, and 8D05 is involved in water transport by interacting with the host protein TIP215,16.
With the discovery of gene expression control via small interfering RNA (siRNA) and microRNA (miRNA) molecules, biologists have been exploring genes and development from a new perspective. For example, ingestion of double-stranded RNA (dsRNA) by C. elegans, and subsequently cyst nematodes, was reportedly able to induce posttranscriptional silencing of the complementary gene in the nematode17,18. Methods for in vivo dsRNA delivery have proven to be useful in functional analyses of nematode parasitism genes, providing a feasible gene silencing approach for investigating nematode effector function, especially for parasitism genes that are expressed only when nematodes are present in the host plant. In addition, host-delivered RNAi (HD-RNAi)-mediated resistance has been employed to target secretory proteins such as 8D05 in Arabidopsis and 16D10 in grape hairy roots19,20. Other specific genes encoding putative M. incognita oesophageal gland cell secretory proteins (msps) have also been successfully silenced via HD-RNAi, including Misp12, Mimsp40, Mimsp18, Mimsp20 and Mi-msp-121,22,23,24,25. Possible future applications of functional genomics may include the control of PPNs by validation of these putative target genes and their utilization in a loss-of-phenotype strategy through a reverse-genetic approach. In general, characterization of the effector proteins that a nematode secretes into its host during infection using the HD-RNAi technique is necessary for developing an effective way to control this devastating pest.
Previous studies on effector genes have led to the conclusion that these genes comprise good targets for HD-RNAi-mediated silencing in terms of the reduced infection rates and gene expression levels that are achieved. Secretory gland genes are crucial for the secretion of several effector molecules that have been implicated in nematode parasitism. To date, 37 putative effectors have been identified and localized subcellularly as proteins that are secreted by the subventral and dorsal oesophageal glands in M. incognita26.
As characterization of these effectors will lead to improved understanding of the molecular basis of M. incognita parasitism, in this study, we characterized one such M. incognita effector gene, Mi-msp2 (for Meloidogyne secretory protein), as previously reported by Huang et al.26. The protein encoded by the Mi-msp2 gene has been designated 2G0226, and herein we refer to this gene as Mi-msp2. Using HD-RNAi technology, transgenic Arabidopsis thaliana lines expressing Mi-msp2 dsRNA were employed to promote nematode resistance.
Results
In silico analyses of the Mi-msp2 gene and protein sequence
A genome-wide NCBI-BLAST analysis of the Mi-msp2 gene sequence revealed it to be specific to M. incognita, with no sequence orthologues found in the NCBI redundant database. However, WormBase analyses led to the identification of Mi-MSP2 orthologues in three RKN Meloidogyne species, namely, M. hapla (Contig2148.frz3.gene8), M. arenaria (Scaff9036g069424) and M. graminicola (NXFT01000603.1.1405_g), with up to 46% of the amino acids being identical (97 of 210 amino acids) and 18% being similar (38 of 210 amino acids). Furthermore, nearly 86% of the residues towards the amino-terminal end of the protein are identical or similar, indicating extensive sequence conservation (Fig. 1a).
In silico analyses of Mi-MSP2 protein sequences. (a) Multiple sequence alignment of predicted orthologues of the Mi-MSP2 protein across different plant parasitic nematodes. An asterisk (*) indicates a position of a single, fully conserved residue, a colon (:) indicates conservation between groups of strongly similar properties, and a period (.) indicates conservation between groups of weakly similar properties. (b) The neighbour-joining phylogenetic tree was constructed using MEGA7 of Mi-MSP2. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (2000 replicates) is shown next to the branches. (c) Illustration of the Mi-MSP2 protein structure and domains predicted by SMART software.
Structural analyses of the Mi-MSP2 protein revealed the presence of two structural classifications of proteins (SCOP) domains, SCOP:d0c3a and SCOP:d1|1|a, and one ShK toxin domain comprising 37 amino acids (residues 33–69) (Fig. 1c). The signal peptide sequence is predicted by both Signal 4.0 and SMART software to correspond to amino acids 1–18. Transmembrane domains are absent in the Mi-MSP2 protein, as depicted by TMHMM server v. 2.0 analysis, suggesting the secretory nature of the protein.
Upregulation of the Mi-msp2 gene in the early parasitic stage of M. incognita
Quantitative real-time PCR analysis was performed to quantify the expression levels of the Mi-msp2 secretory gene in three different developmental stages during the life cycle of M. incognita, namely, egg masses, J2s and adult females, isolated from RKN-infected tomato (Pusa Ruby) plants. The highest level of Mi-msp2 expression was observed in J2s, the parasitic stage, followed by egg masses and, finally, adult females (Fig. 2). The differential levels of expression at different developmental stages illustrate the functional pattern of the secretory gland gene and its origin. The maximum expression levels found in early parasitic stage (J2s) and the subsequent decline in the adult stage suggest that Mi-msp2 plays an important role during the early stages of parasitism.
Infection assay of transgenic Arabidopsis thaliana plants expressing Mi-msp2
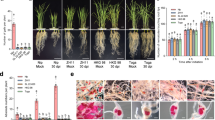
A nematode infection assay was carried out using two independent homozygous T3 transgenic RNAi lines (Mi-msp2 E1 and Mi-msp2 E2) expressing Mi-msp2 dsRNA; the number of galls, females and egg masses per gram root fresh weight were compared with those of control Arabidopsis plants (Fig. 3). A decrease in infection was clearly evident in the roots of both independent RNAi lines compared to the control plants (Fig. 4). The reductions in the number of galls, females and egg masses were found to be in the ranges of 52–54%, 60–66% and 84–95%, respectively. Thus, a significant reduction in nematode parasitism was observed with Mi-msp2 gene RNAi, indicating an important role of this gene in nematode development.
Two independent RNAi lines (Mi-msp2 E1 and Mi-msp2 E2) showing reductions in the numbers of galls, females and egg masses relative to control plants in response to M. incognita infection. Each bar represents the mean ± SE. Double asterisks indicate statistically significant differences according to one-way ANOVA and Tukey’s test (p ≤ 0.01).
Comparative morphology of nematode females feeding on control and transgenic Arabidopsis
A comparative analysis of the size of adult female nematodes feeding on the control (wild-type) and transgenic lines was performed by measuring the width (μm) (Fig. 5a) and surface area (μm2) of feeding females (Fig. 5b). Twenty-five females were collected from control or transgenic plants (cumulative females from the two independent lines, Mi-msp2 E1 and Mi-msp2 E2), and their sizes were measured. In comparison with the control, the average width and area of females feeding on the RNAi lines were significantly reduced, with decreases in the average width and area by 32.2% and 54.8%, respectively (Fig. 5c,d), highlighting the importance of the Mi-msp2 gene in the normal development of RKN.
Morphological size comparison of female nematodes isolated from control and transgenic plants (cumulative females from both the Mi-msp2 E1 and Mi-msp2 E2 lines). (a) Average female width (μm), (b) average female area (μm2). Each bar represents the mean ± SE. Double asterisks indicate statistically significant differences according to one-way ANOVA and Tukey’s test (p ≤ 0.01). Representative images of adult females isolated from (c) control and (d) Mi-msp2-RNAi lines.
Fecundity of M. incognita females feeding on RNAi lines and control plants
To examine the level of susceptibility of M. incognita to HD-RNAi, egg masses isolated from control and transgenic plants (cumulative egg masses from the two independent lines, Mi-msp2 E1 and Mi-msp2 E2) were counted, and the number of eggs was reduced by 74.3% in the case of the Mi-msp2 RNAi lines (Fig. 6a). Compared to that of nematodes that developed from the females feeding on control plants, the reduction in the number of eggs indicated a stalling of nematode development due to the relative inactivity of the secretory gene. In addition, the reproduction factor was calculated by measuring the number of eggs that hatched in the next generation, with values for Mi-msp2 E1 and Mi-msp2 E2 of 0.43 and 0.137, respectively, compared to 15.85 for control plants (Fig. 6b).
Fecundity of M. incognita. (a) The average number of eggs per egg mass isolated from control and transgenic plants (cumulative egg masses from both Mi-msp2 E1 and Mi-msp2 E2 lines). Each bar represents the mean ± SE. Double asterisks indicate statistically significant differences according to one-way ANOVA and Tukey’s test (p ≤ 0.01), (b) Reproduction factors for the control, Mi-msp2 E1 and Mi-msp2 E2.
Gene expression analysis of nematode females feeding on RNAi lines expressing dsRNA and northern hybridization analysis
qRT-PCR was performed to examine Mi-msp2 gene expression in females isolated from transgenic plants compared to control plants, and a reduction in gene expression was observed in terms of the fold change calculated using ΔΔCt values, as evaluated against nematode actin27. The decrease in gene expression was 0.882-fold for Mi-msp2 E1 and 0.855-fold for Mi-msp2 E2 (Fig. 7) in comparison to the control. siRNA specific for Mi-msp2 was also detected in the RNAi lines by northern hybridization (Fig. 8), further confirming successful RNAi in these lines. Thus, the decreased infection rate may be attributed to silencing of the Mi-msp2 gene, highlighting the ability to use HD-RNAi to promote resistance as an important strategy for managing RKNs.
qRT-PCR-based Mi-msp2 expression analysis of M. incognita females isolated from infected roots of control plants and two independent RNAi lines, Mi-msp2 E1 and Mi-msp2 E2. Each bar represents the mean ± SE. Double asterisks indicate statistically significant differences according to one-way ANOVA and Tukey’s test (p ≤ 0.01).
PAGE gel image. (a) Ethidium bromide-stained PAGE gel (before transfer) showing the total RNA fraction and (b) the corresponding northern blot for detecting the presence of Mi-msp2 siRNA in control plants and RNAi lines. The full-length gel and blot images are provided in Supplementary Fig. S3.
Discussion
PPNs are a consistent threat to agricultural crops. However, the increasing amount of information regarding PPN genomes and mechanism(s) of parasitism that has become available in the past 15 years has enabled new strategies for combating one of the most threatening crop parasites. Indeed, the advent of RNAi technology has provided a very promising tool for combatting these menacing nematodes28. In addition, with the aim of conferring nematode resistance, genome mining has led to the characterization and isolation of various nematode genes and promoters involved in successful parasitism. Various nematode genes involved in parasitism have been identified and employed using an HD-RNAi-mediated approach to develop nematode-resistant plant8,13,29,30. In addition to effector genes, other nematode genes that have been targeted include housekeeping genes, such as a splicing factor and integrase, transcription factors, ribosomal protein 3a, ribosomal protein 4, and developmental genes encoding spliceosomal SR proteins, such as glp-1 and col1, a dual oxidase gene31,32,33,34,35,36,37. Although targeting these genes has reduced the level of PPN parasitism, greater and more effective reductions in infection have been achieved by silencing effector genes. The genes targeted include effector genes such as 16D10, 8D05, Mimsp40, Hs4G06, Hs3B05, Hs8H07, and Hs10A06 and those encoding cell wall-degrading enzymes such as cellulose-binding proteins and β-1,4-endoglucanase11,19,22,27,38,39. Interesting and encouraging results have been obtained by targeting the 16D10 and Hg30C02 genes, with up to a 90% reduction in nematode infection observed11,40.
The present study involved the utilization of Mi-msp2 (AF531161), an effector gene, as a potent target for an HD-RNAi-mediated approach to manage nematode parasitism. Mi-msp2 was previously reported by Huang et al. to be an effector protein of subventral gland origin in M. incognita26. BLAST analysis using WormBase led to the identification of orthologues for the Mi-MSP2 protein in all four Meloidogyne species. However, this result was in contrast to that of a similar analysis using the NCBI database, which identified no orthologues. The Mi-MSP2 protein sequence shows extensive conservation with the MSP2 proteins of other Meloidogyne species.
A detailed computational analysis of the Mi-msp2 gene structure, as well as the protein structure, was performed. The absence of a transmembrane subunit revealed the secretory nature of the Mi-MSP2 protein, similar to other secretory proteins; these proteins are directly secreted from the nucleus into the oesophageal gland cell lumen in the form of membrane-bound granules derived from the Golgi apparatus3. Protein domain structure predictions for the Mi-MSP2 protein carried out using SMART software revealed the presence of two SCOP domains, SCOP:d0c3a and SCOP:d1|1|a. The SCOP:d0c3a domain belongs to the C3a anaphylatoxin family of the complement system; the SCOP:d1|1|a_domain belongs to the pyruvate formate-lyase-like (PFL) glycyl radical superfamily, which is involved in oxidation-reduction reactions in several metabolic processes. We were unable to find any previous reports of the existence of the C3a complement domain in nematodes. Another domain present in the Mi-MSP2 protein is the Stichodactyla toxin (ShKT; from the sea anemone Stichodactyla helianthus) domain, which has an ancient origin in both plants and animals. The function of this domain in worms (Caenorhabditis elegans, Brugia malayi, Ancyclostoma ceylanicum, Schistosoma mansoni) is specifically related to the modulation of ion channels41. In C. elegans, the ShKT domain is found in the astacin/adamalysin family of metallopeptidases, which function in blocking voltage-gated K+ channels (Kv). The ShK toxin is heat resistant and binds to the phospholipase A subunit of K+ channels, blocking the influx of K+. As K+ channels are reported to be indirectly involved in plant defence responses through the action of reactive oxygen species (ROS) (biotic and abiotic stress responses)41, it is plausible that the ShK toxin has a similar effect on Kv channels in plants. Two 37-aa putative C-terminal ShKt domains are also present in another sub-ventral gland gene, Mi msp40, of M. incognita, which suggests that the functionality of these domains in host infection is mediated by blocking Kv channels22. In the case of animal-parasitic worms, ShK toxin has been found to exhibit an immunomodulatory effect, and this toxin has been employed as a selective inhibitor of Kv channels to treat autoimmune disease42.
Huang et al. reported the subcellular localization of putative effector proteins in M. incognita26. Consistent with their findings, our study on stage-specific gene transcription revealed that Mi-msp2 (subventral oesophageal gland origin) was expressed at higher levels during the initial parasitic developmental stages than in the other stages investigated, with maximal expression was observed in J2s and egg masses43,44.
HD-RNAi-mediated silencing of Mi-msp2 resulted in a reduction in the number of galls (up to 54%) in transgenic Arabidopsis RNAi lines compared to the control, illustrating the importance of the Mi-msp2 secretory gene for successful parasitism. Similar results regarding a reduction in the number of galls were observed for two housekeeping genes encoding secretory splicing and integrase proteins, 16D10 (63–90% reduction) and 8D0511,19,31. In our analyses, the number of gall-inhabiting females that were able to successfully parasitize plants was reduced by 60–66%. Completion of the nematode life cycle is marked by the egg masses laid by adult females, and the number of egg masses was drastically reduced by 84–95% in the RNAi lines. This finding provides useful insight into the mode of M. incognita parasitism and the relevance of secretory genes in host invasion. Furthermore, comparison of the reductions in the numbers of females and egg masses revealed a dramatic decrease in the number of egg masses, which indicates the importance of secretory genes in reproduction and fecundity. The results reported by Kumar et al. regarding RNAi targeting of splicing and integrase genes showed the same pattern of a decreased number of egg masses compared to the number of females32. The reduction in fecundity was further confirmed by analysis of the number of eggs per egg mass in the RNAi lines, demonstrating a strong decline in the number of eggs by up to 74%, similar to the decrease reported for 16D1011.
As Mi-msp2 expression was reduced by up to 88% in the transgenic RNAi lines, we can infer its importance in M. incognita parasitism. Further studies on the protein function of Mi-MSP2 should be performed to assess its relevance regarding parasitism and development. Moreover, comparison of female size (in terms of width and area) indicated that along with hampering parasitism, RNAi knockdown of this effector gene impeded development. This observation further demonstrates the relevance of investigating secretory gene function Additionally, gene expression analyses conducted to elucidate the role of Mi-msp2 in the normal progression of nematode parasitism revealed a reduction in transcript levels in the RNAi lines. Thus, the reduction in infection can be linked to the reduction in transcript levels, suggesting a lack of functional redundancy. In support of this observation, the detection of siRNA in the RNAi lines confirmed that the decrease in nematode infection was due to successful HD-RNAi-mediated knockdown of the Mi-msp2 gene. Thus, HD-RNAi knockdown of the Mi-msp2 gene may be considered successful, as demonstrated by a reduction in the levels of gene expression. Based on our results, Mi-msp2 appears to be an important candidate gene, reducing nematode infection by as much as 88%, and may be employed for promoting nematode resistance in economically useful plants. Furthermore, because of the significant sequence conservation, the gene may be applied for the management of different RKNs (Meloidogyne spp.).
Several candidate genes have been identified, many of which have exhibited the ability to reduce nematode infection, with varying efficiencies, after being targeted by the HD-RNAi-mediated approach. This result is truly encouraging, further identification and extensive characterization of gene(s) in different plant/crop systems should be performed to obtain and utilize the best combination for addressing nematode infection. Undoubtedly, effector genes are the most important targets in the arsenal against nematode infection, and their utilization should be a successful approach to managing PPNs.
Materials and Methods
Gene and protein sequence analysis of Mi-msp2
The 590-base pair sequence of Mi-msp2 (AF531161), reported by Huang et al., was subjected to BLAST analysis across the NCBI redundant database11 as well as a WormBase BLAST/BLAT search (https://wormbase.org/tools/blast_blat). Multiple sequence alignment was performed for the orthologues of the Mi-MSP2 protein across Meloidogyne spp. using CLUSTAL Omega software; and simple phylogeny analysis was achieved with MEGA-X software45,46. Domain analysis of the Mi-MSP2 protein sequence was performed using the SMART database to search for different structural domains47. The signal peptide sequence was identified using SignalP3.0, and the TMHMM server v. 2.0 was employed to predict the presence of transmembrane structures48,49.
Preparation of M. incognita effector gene RNAi constructs
The Mi-msp2 dsRNA construct was prepared using Gateway technology. For bacterial recombination, attB1 and attB2 sites were added to gene-specific primers. PCR amplification of the Mi-msp2 effector gene (590 bp) was conducted using M. incognita cDNA, and the product was cloned into the pGEM-T Easy vector (Promega, USA). Subsequently, the size and sequence of the fragment were confirmed through Sanger DNA sequencing, and the fragment was subcloned into the pDONR vector pRK2031 using BP clonase (Invitrogen, USA)50. Positive clones were then transferred into a plant expression vector, pYSB (this vector was generated as part of the current study in collaboration with IIT Kanpur, and it drives expression of sense and antisense genes using the 35S promoter), using the Gateway LR clonase II enzyme mix (Invitrogen, USA) to obtain the recombinant expression vector. The recombinant pYSB RNAi (Fig. S1) constructs were transferred into Agrobacterium tumefaciens strain GV3101 by the freeze-thaw method according to the standard procedure51. For the selection of recombinant transformants, Agrobacterium was grown on YEP medium containing 50 mg/L hygromycin and 25 mg/L rifampicin. Colony PCR was performed to confirm positive clones, with introduction into Arabidopsis thaliana via Agrobacterium-mediated transformation52. The constructs were screened using MS medium containing 50 mg/L kanamycin. PCR confirmed transgenic RNAi plants expressing the Mi-msp2 dsRNA constructs (Fig. S2), followed by propagation to the T3 homozygous generation.
Obtaining a pure M. incognita culture and maintenance of the culture in the tomato cultivar Pusa Ruby
A pure culture of the RKN M. incognita (Kofoid & White) Chitwood race 1 was obtained from the Division of Nematology, Indian Agricultural Research Institute (IARI), New Delhi and maintained in Pusa Ruby tomato (Solanum lycopersicum L.) plants. Females that developed from the pure culture obtained from a single egg mass were isolated to identify M. incognita using sequence-characterized amplified region (SCAR) primers and the perineal pattern of the female53,54. J2s were hatched from the egg mass obtained from an M. incognita female and used to propagate the pure culture. Ten-day-old tomato seedlings were transferred to 6-inch-diameter pots containing a soil medium mixture of sand, soil rite and vermiculite in a 1:1:1 ratio. Hoagland solution was administered to the plants regularly, and the plants were infected with 500 M. incognita juveniles. After 6 to 7 weeks of infection, the roots were observed for gall development; the roots were observed for egg masses after completion of the M. incognita life cycle. The egg masses were then hand-picked with the help of a dissection needle; to allow for hatching for 3 days at 28 °C, the egg masses were placed on tissue paper over a wire gauge on Petri plates in 20–30 mL of sterile water following the modified Baermann funnel technique55. J2s were collected in the Petri plates to maintain a continuous culture of M. incognita.
Development of transgenic Arabidopsis thaliana lines containing the effector gene Mi-msp2
Arabidopsis thaliana (Col-0) plants were transformed with the Mi-msp2 RNAi construct using the floral dip method52. T1 seeds of transgenic plants expressing dsRNA for the Mi-msp2 gene were sterilized and grown on MS medium supplemented with 1% sucrose and 0.8% agarose. The transformants were selected on growth medium supplemented with 50 µg/mL kanamycin. Seedlings that were resistant to kanamycin and produced healthy green leaves and well-established root systems were selected and transferred onto an autoclaved soilrite mixture and grown to maturity in a greenhouse at 22 °C with an 8-hour light/16-hour dark photoperiod. The seeds were harvested, and molecular analysis was carried out. T2 seeds were used to produce T3 lines; all nematode infection assays were carried out on T3 homozygous transgenic lines. The RNAi-transgenic plants were subjected to phenotypic comparison with control plants to exclude variables related to nematode infection. The controls and transgenic RNAi lines were observed phenotypically to assess changes in growth patterns, which can indirectly alter nematode infection. The RNAi lines were visually compared with the control on agar medium for root length and growth patterns. No gross morphological differences were observed in root growth between the RNAi lines and control plants or among the RNAi lines.
The control and RNAi plants were also assessed morphologically for any differences in terms of root and shoot growth, flowering response and life cycle, and no differences were observed. Furthermore, no phenotypic differences among the plants of different RNAi lines were found.
Sterilization and growth of transgenic Arabidopsis thaliana plants
Seeds of the A. thaliana control line (wild-type Col-0) and two independent T3 transgenic lines harbouring Mi-msp2 RNAi were sterilized with 70% ethanol (1 min) and with 0.1% SDS and mercuric chloride (8 min) and then washed three times with sterile double-distilled, autoclaved water. The sterilized seeds were vernalized on 0.1% agarose at 4 °C for 72 hours and sown on autoclaved MS medium (pH 5.8) (HiMedia). Plants were grown at 21 °C with a 16-h light/8-h dark photoperiod. Two-week-old seedlings were transferred to 9-cm-diameter round Petri dishes containing 1/2 MS and CleriGel (HiMedia), with eight plants per plate. The Petri dishes were placed at a slight angle of 45° to promote unidirectional root growth for approximately one week until secondary roots appeared.
Sterilization of nematodes and infection assay
M. incognita J2s were hatched at 28 °C and suspended in M9 buffer before being further subjected to surface sterilization using nematode sterilization buffer, followed by several washes with autoclaved double-distilled water56. Three-week-old plants were infected with the sterilized M. incognita J2s (approximately 300 J2s per plant). For phenotypic analysis and collection of adult females and egg masses, the plants were carefully harvested from MS media (CleriGel) at 35 days postinoculation (dpi) and weighed to determine the fresh weight of the root mass. The numbers of galls, females and egg masses per gram of root fresh weight were counted manually by dissecting the roots and isolating the females and egg masses under light microscopy. The data obtained from the RNAi lines were used as a measure of relative infection in comparison with the control plants. The mean numbers of galls, females and egg masses per gram of root fresh weight were calculated from 10–15 biological replicates per RNAi line. The susceptibility of the control and RNAi lines was assessed by comparing the gene-silencing efficiencies.
Calculation of the reproductive capacity of M. incognita after Mi-msp2 gene silencing
To assess the reproductive capacity of M. incognita after gene silencing, a comparison of Oostenbrink’s reproduction factor was performed between the control and transgenic lines57. The reproduction factor provides a measure for assessing variation in nematode responses to host plants. The number of J2s that hatched from the next generation of egg masses was calculated. Photographs of infected roots and M. incognita females were acquired at a suitable magnification with a NIKON® microscope and associated camera software NIS element D (Nikon Instruments Inc., New York, USA). The lengths and widths of 25 nematode females were recorded and averaged using the same microscope software.
RNA isolation and gene expression analysis of RNAi females
To study expression of the Mi-msp2 gene, total RNA was isolated from three developmental stages of the nematode, eggs, J2s and adult females, using PureLink RNA Mini Kit (Thermo Fisher Scientific) according to the manufacturer’s protocol. RNA integrity was assessed by formaldehyde gel electrophoresis with ethidium bromide. A NanoDrop spectrophotometer 8000 (Thermo Fisher Scientific, Massachusetts, USA) was used to calculate purity ratios and quantify total RNA. The RNA (500 ng) obtained was used to prepare cDNA using SuperScript III First-Strand Synthesis System (Thermo Fisher Scientific, Massachusetts, USA), and the cDNA was used as a template for qRT-PCR (Applied Biosystems StepOnePlus™ Real-Time PCR). RT primers were designed based on the cDNA sequence of Mi-msp2 using the online ‘Primer3’ portal (http://frodo.wi.mit.edu/primer3/) (Table S1)58. Gene expression analysis was performed by comparing δδCt values between females feeding on control plants and transgenic Arabidopsis plants expressing dsRNA targeting the secretory gene27. Three independent transgenic lines were evaluated using two housekeeping genes: nematode actin and nematode 18S (accession numbers: Mi-actin, Minc06769; Mi-18S, P901057.2). The results obtained using the nematode actin gene are reported here. Statistical analysis using ANOVA and Tukey’s test was performed to assess significance.
Northern hybridization for siRNA detection
Total RNA was isolated from the two RNAi lines and control plants using the TRIzol method. Samples of total RNA (80 μg) were resolved by 15% polyacrylamide/0.5 X TBE urea gels and then blotted onto a BrightStar™-Plus positively charged nylon membrane (15 cm × 15 cm). For probe preparation, primers for the complete sense strand were used to isolate the gene sense strand suitable for siRNA. To generate specific DNA probes (590 bp), digoxigenin labelling was performed using a DIG-RNA labelling kit (SP6/T7) (Roche). Hybridization was performed using a DIG Northern starter kit (Roche) according to the manufacturer’s instructions, which involved blocking the membrane and immunological detection with an anti-DIG-AP conjugate. Signals were detected by the chemiluminescence method using CDP Ready-To-Use solution (Applera Corporation) as the substrate.
References
Elling, A. A. Major emerging problems with minor Meloidogyne species. Phytopathology 103, 1092–1102 (2013).
Gheysen, G. & Mitchum, M. G. How nematodes manipulate plant development pathways for infection. Curr. Opin. Plant Biol. 14, 415–21 (2011).
Hussey, R. S., Davis, E. L. & Baum, T. J. Secrets in secretions: genes that control nematode parasitism of plants. Braz. J. Plant Physiol. 14(3), 183–194 (2002).
Davis, E. L. et al. Nematode parasitism genes. Annu. Rev. Phytopath. 38, 341–372 (2000).
Baum, T. J., Hussey, R. S. & Davis, E. L. Root-knot and cyst nematode parasitism genes: the molecular basis of plant parasitism. Genet. Eng. 28, 17–41 (2007).
Hussey, R. S., Davis, E. L. & Ray, C. Advances In Molecular Plant Nematology. (ed. Lamberti, F., De Giorgi, C. & Bird, D. Mck.) 233–249 (New York: Plenum Press, 1994).
Rehman, S., Gupta, V. K. & Goyal, A. K. Identification and functional analysis of secreted effectors from phytoparasitic nematodes. BMC Microbiol. 16, 48 (2016).
Hussey, R. S. Disease–inducing secretions of plant–parasitic nematodes. Annu. Rev. Phytopathol. 27, 123–141 (1989).
Davis, E. L., Hussey, R. S., Mitchum, M. G. & Baum, T. J. Parasitism proteins in nematode–plant interactions. Curr. Opin. Plant Biol. 11, 360–366 (2008).
Hewezi, T. & Baum, T. J. Manipulation of plant cells by cyst and root-knot nematode effectors. Mol. Plant Microbe. Interact. 26, 9–16 (2013).
Huang, G., Allen, R., Davis, E. L., Baum, T. J. & Hussey, R. S. Engineering broad root-knot resistance in transgenic plants by RNAi silencing of a conserved and essential root-knot nematode parasitism gene. Proc. Natl. Acad. Sci. USA 103, 14302–14306 (2006).
Grossi-de-Sa, M. et al. Rice susceptibility to root-knot nematodes is enhanced by the Meloidogyne incognita MSP18 effector gene. Planta 1–13 (2019).
Jaouannet, M. et al. The root-knot nematode calreticulin Mi–CRT is a key effector in plant defense suppression. Mol. Plant Microbe. Interact. 26, 97–105 (2013).
Iberkleid, I. et al. Fatty acid–and retinol–binding protein, Mj–FAR–1 induces tomato host susceptibility to root-knot nematodes. PLoS one 8(5), e64586 (2013).
Lin, B. et al. A novel effector protein, MJ NULG1a, targeted to giant cell nuclei plays a role in Meloidogyne javanica parasitism. Mol. Plant Microbe. Interact. 26, 55–66 (2013).
Dong, L., Xu, J., Chen, S., Li, X. & Zuo, Y. Mi–flp–18 and Mi–mpk–1 genes are potential targets for Meloidogyne incognita control. J. Parasitol. 102, 208–213 (2016).
Timmons, L. & Fire, A. Specific interference by ingested dsRNA. Nature 395(6705), 8549 (1998).
Urwin, P. E., Lilley, C. J. & Atkinson, H. J. Ingestion of double–stranded RNA by preparasitic juvenile cyst nematodes leads to RNA interference. Mol. Plant Microbe. Interact. 15(8), 747–52 (2002).
Xue, B. et al. The 8D05 parasitism gene of Meloidogyne incognita is required for successful infection of host roots. Phytopathology 103, 175–181 (2013).
Yang, Y. et al. Molecular characteristics and efficacy of 16D10 siRNAs in inhibiting root-knot nematode infection in transgenic grape hairy roots. PLoS ONE 8(7), e69463 (2013).
Xie, J. et al. A novel Meloidogyne incognita effector Misp12 suppresses plant defense response at latter stages of nematode parasitism. Front. Plant Sci. 7, 964 (2016).
Niu, J. et al. Msp40 effector of root-knot nematode manipulates plant immunity to facilitate parasitism. Sci. Rep. 6, 19443 (2016).
Shivakumara, T. N. et al. Host–induced silencing of two Pharyngeal gland genes conferred transcriptional alteration of cell wall–modifying enzymes of Meloidogyne incognita vis–à–vis perturbed nematode infectivity in eggplant. Front. Plant Sci. 8, 473 (2017).
Chaudhary, S., Dutta, T. K., Shivakumara, T. N. & Rao, U. RNAi of esophageal gland-specific gene Mi-msp-1 alters early stage infection behaviour of root-knot nematode, Meloidogyne incognita. J. Gen. Plant Pathol. 85, 232–242 (2019a).
Chaudhary, S. et al. Host-induced silencing of Mi-msp-1 confers resistance to root-knot nematode Meloidogyne incognita in eggplant. Transgenic Res. 1–14 (2019b).
Huang, G. et al. A profile of putative parasitism genes expressed in the esophageal gland cells of the root-knot nematode Meloidogyne incognita. Mol. Plant Microbe. Interact. 16, 376–381 (2003).
Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real–time quantitative PCR and the 2–ΔΔC T Method. Methods 25, 402–408 (2001).
Fire, A. et al. Potent and specific genetic interference by double stranded RNA in Caenorhabditis elegans. Nature 391, 806–811 (1998).
Sindhu, A. S. et al. Effective and specific in planta RNAi in cyst nematodes: Expression interference of four parasitism genes reduces parasitic success. J. Exp. Bot. 60, 315–324 (2009).
Dinh, P. T. Y., Brown, C. R. & Elling, A. A. RNA interference of effector gene Mc16D10L confers resistance against Meloidogyne chitwoodi in Arabidopsis and potato. Phytopathology 104, 1098–1106 (2014).
Yadav, B. C., Veluthambi, K. & Subramaniam, K. Host–generated double stranded RNA induces RNAi in plant–parasitic nematodes and protects the host from infection. Mol. Biochem. Parasitol. 148, 219–22 (2006).
Kumar, A. et al. Host–delivered RNAi mediated root-knot nematode resistance in Arabidopsis by targeting splicing factor and integrase genes. J. Gen. Plant Pathol. 83, 91–97 (2017).
Fairbairn, D. J. et al. Host–delivered RNAi: An effective strategy to silence genes in plant parasitic nematodes. Planta 226, 1525–1533 (2007).
Klink, V. P. et al. A correlation between host–mediated expression of parasite genes as tandem inverted repeats and abrogation of development of female Heterodera glycines cyst formation during infection of Glycine max. Planta 230, 53–71 (2009).
Kohli, D. et al. Host–mediated RNAi of a Notch–like receptor gene in Meloidogyne incognitainduces nematode resistance. Parasitology 25, 1–11 (2018).
Banerjee, S. et al. RNA Interference: A Novel Source of Resistance to Combat Plant Parasitic Nematodes. Front. Plant Sci. 8, 834 (2017).
Bakhetia, M., Charlton, W., Atkinson, H. J. & McPherson, M. J. RNA interference of dual oxidase in the plant nematode Meloidogyne incognita. Mol. Plant Microbe. Interact. 18, 1099–1106 (2005).
Ding, X., Shields, J., Allen, R. & Hussey, R. S. A Secretory Cellulose–Binding Protein cDNA Cloned from the Root-Knot Nematode (Meloidogyne incognita). Mol. Plant Microbe. Interact. 11(10), 952–959 (1998).
Rosso, M. N. et al. Isolation of a cDNA encoding a beta–1,4–endoglucanase in the root-knot nematode Meloidogyne incognita and expression analysis during plant parasitism. Mol. Plant Microbe. Interact. 12, 585–591 (1999).
Hamamouch, N. et al. The interaction of the novel 30C02 cyst nematode effector protein with a plant beta–1,3 endoglucanase may suppress host defense to promote parasitism. J. Exp. Bot. 63, 3683–3695 (2012).
Dreyer, I. & Uozumi, N. Potassium channels in plant cells. FEBS J. 278(22), 4293–303 (2011).
Chhabra, S. et al. Kv1.3 channel–blocking immunomodulatory peptides from parasitic worms: implications for autoimmune diseases. FASEB J. 28(9), 3952–64 (2014).
Hussey, R. S. & Mims, C. W. Ultrastructure of esophageal glands and their secretory granules in the root-knot nematode Meloidogyne incognita. Protoplasma 156, 9–18 (1990).
Jones, M. G. K. & Payne, H. L. Early stage of nematode–induced giant–cell formation in roots of Impatiens balsamina. J. Nematol. 10, 70–84 (1978).
Madeira, F. et al. The EMBL-EBI search and sequence analysis tools APIs in 2019. Nucleic Acids Res. 47, W597–600 (2019).
Kumar, S., Stecher, G., Li, M., Knyaz, C. & Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 35, 1547–1549 (2018).
Ponting, C. P., Schultz, J., Milpetz, F. & Bork, P. SMART: Identification and annotation of domains from signaling and extracellular protein sequences. Nucleic Acids Res. 27, 229–232 (1999).
Emanuelsson, O., Brunak, S., von Heijne, G. & Nielsen, H. Locating proteins in the cell using TargetP, SignalP and related tools. Nat. Protoc. 2, 953–971 (2007).
Krogh, A., Larsson, B., von Heijne, G. & Sonnhammer, E. L. L. Predicting transmembrane protein topology with a hidden Markov model: Application to complete genomes. J. Mol. Biol. 305(3), 567–580 (2001).
Mainpal, R., Priti, A. & Subramaniam, K. PUF–8 suppresses the somatic transcription factor PAL–1 expression in C. elegans germline stem cells. Dev. Biol. 360, 195–207 (2011).
Höfgen, R. & Willmitzer, L. Storage of competent cells for Agrobacterium transformation. Nucleic Acids Res. 16(20), 9877 (1988).
Clough, S. J. & Bent, A. F. Floral dip: a simplified method for Agrobacterium–mediated transformation of Arabidopsis thaliana. Plant J. 16, 735–43 (1998).
Zijlstra, C., Donkers–Venne, D. T. H. M. & Fargette, M. Identification of Meloidogyne incognita, M. javanica and M. arenaria using sequence characterized amplified region (SCAR) based PCR assays. Nematology 2, 847–853 (2000).
Eisenback, J. D., Sasser, J. & Carter, C. Diagnostic characters useful in the identification of the four most common species of root-knot nematodes (Meloidogyne spp.). An Advanced Treatise On Meloidogyne 1, 95–112 (1985).
Hooper, D. J. Extraction Of Free–Living Stages From Soil, In Laboratory Methods For Work With Plant And Soil Nematodes (ed. Southey J. F.) 5–30 (London: Ministry of agriculture, fisheries and food, 1986).
Kakrana, A. et al. Identification, validation and utilization of novel nematode–responsive root-specific promoters in Arabidopsis for inducing host–delivered RNAi mediated root-knot nematode resistance. Front Plant Sci 8, 2049 (2017).
Oostenbrink, M. Major characteristics of the relation between nematodes and plants. Mededelingen Landbouhoge School, Wageningen 66, 3–46 (1966).
Rozen, S. & Skaletsky, H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol. Biol. 132, 365–386 (2000).
Acknowledgements
The authors gratefully acknowledge Dr. K. Subramaniam of IIT Kanpur for providing the RNAi vector construct and the staff of the National Phytotron Facility (NPF), IARI, New Delhi, India, for providing space to conduct the Arabidopsis experiments. We also thank the University Grants Commission, India, for providing funding in the form of the UGC-JRF fellowship. The present study was also supported by a grant from the ICAR-NASF (formerly known as 526 NFBSFARA, code: NFBSFARA/RNA-3022) project from 2012–2015.
Author information
Authors and Affiliations
Contributions
P.K.J., A.C. and A.S. designed the experiment. I.J., A.K., A.K.S., D.K. and K.V.R. conducted the experiments. I.J. performed the data analyses, and I.J., A.K. and D.K. prepared the figures and/or tables. I.J. wrote the manuscript. P.K.J., A.C. and A.S. edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Joshi, I., Kumar, A., Singh, A.K. et al. Development of nematode resistance in Arabidopsis by HD-RNAi-mediated silencing of the effector gene Mi-msp2. Sci Rep 9, 17404 (2019). https://doi.org/10.1038/s41598-019-53485-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-53485-8
This article is cited by
-
Advances in RNA Interference for Plant Functional Genomics: Unveiling Traits, Mechanisms, and Future Directions
Applied Biochemistry and Biotechnology (2024)
-
In planta RNAi targeting Meloidogyne incognita Minc16803 gene perturbs nematode parasitism and reduces plant susceptibility
Journal of Pest Science (2024)
-
Meloidogyne arenaria candidate effector MaMsp4 interacts with maize (Zea mays L.) proteins involved in host defense response and cell wall modifications
Plant and Soil (2023)
-
Designing Climate-Resilient Crops for Sustainable Agriculture: A Silent Approach
Journal of Plant Growth Regulation (2023)
-
Host-delivered RNAi-mediated silencing of the root-knot nematode (Meloidogyne incognita) effector genes, Mi-msp10 and Mi-msp23, confers resistance in Arabidopsis and impairs reproductive ability of the root-knot nematode
Planta (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.