Abstract
Throughout all kingdoms of life, highly conserved transport proteins mediate the passage of ammonium across membranes. These transporters share a high homology and a common pore structure. Whether NH3, NH4+ or NH3 + H+ is the molecularly transported substrate, still remains unclear for distinct proteins. High-resolution protein structures of several ammonium transporters suggested two conserved pore domains, an external NH4+ recruitment site and a pore-occluding twin phenylalanine gate, to take over a crucial role in substrate determination and selectivity. Here, we show that while the external recruitment site seems essential for AtAMT1;2 function, single mutants of the double phenylalanine gate were not reduced in their ammonium transport capacity. Despite an unchanged ammonium transport rate, a single mutant of the inner phenylalanine showed reduced N-isotope selection that was proposed to be associated with ammonium deprotonation during transport. Even though ammonium might pass the mutant AMT pore in the ionic form, the transporter still excluded potassium ions from being transported. Our results, highlight the importance of the twin phenylalanine gate in blocking uncontrolled ammonium ion flux.
Similar content being viewed by others
Introduction
Ammonium is one of the major inorganic nitrogen sources for plants. In Arabidopsis thaliana, other plants and organisms from all kingdoms of life, ammonium is transported across membranes by proteins of the AMT (AMmonium Transporter) / Rh (Rhesus protein)/Mep (Methylammonium permease) family.
The uptake of ammonium (here referring to the sum of NH3 and NH4+) was comprehensively studied in model organisms, where it has been shown that ammonium can be taken up against its concentration gradient. This results in a significant accumulation of ammonium within cells1,2,3,4. At acidic pH, the NH4+ ion is by orders of magnitude more abundant than NH3 (pKa = 9.25). The uptake of ammonium by plants is biphasic and is constituted of a high-affinity transport system component (HATS) and a low-affinity transport system component (LATS). AMT proteins build the plant ammonium HATS, while the molecular identity of the ammonium LATS remains elusive5. Plant AMTs further split into transport proteins conducting net ammonium (NH3 + H+) transport (AMT1s) and AMT2-like transporters, conducting NH36,7,8,9,10.
Protein structures of AMT proteins from bacteria and archaea allow comparisons of the pore structures of different AMTs11,12,13. It is striking that otherwise diverse AMTs like AtAMT1;2 and EcAmtB (E. coli Ammonium transporter B) share very high identity in their pore-lining residues7,9. A close look at the pore structure of EcAmtB shows a conserved outer recruitment site for NH4+ with the amino acid residue Asp160 serving as countercharge to stabilize the recruited NH4+14,15,16,17. This aspartic acid residue was further proposed to hold an important function in stabilizing the AMT protein structure11,12,14,15,18. A conserved serine11,16,19 and alanine17 appear to be involved in replacing the water in the hydration shell of NH4+ before it enters the AMT pore12,15,20.
After the recruitment of NH4+ at the external vestibule, deprotonation to ammonia was proposed by studies on structural constraints and molecular dynamics simulation on the ammonium transport mechanism of the AMT/Rh/Mep proteins. Different sites of deprotonation were suggested14,15,16,18,20,21,22,23,24,25,26,27,28. Directly adjacent to the NH4+ recruitment site, a pore-occluding double phenylalanine gate appears to block further transport of NH4+ into the pore. Some computational studies proposed that deprotonation of the substrate might occur during the translocation from the external vestibule to the inside of the pore, while passing the phenylalanine gate15,16,18,20,26. Other studies suggested that the ammonium ion is guided into the channel lumen, crossing the phenylalanine gate before it is deprotonated at the position of a conserved twin-His structure14,21. Mutational studies of the two phenylalanines in EcAmtB highlighted their importance in the ammonium transport mechanism. Exchange of F107 by alanine and leucine yielded a completely active transporter19,29, but the second Phe was important for the transport function. Replacement of the second phenylalanine by leucine strongly reduced ammonium uptake, as well as methylammonium transport19,29. Change of this inner phenylalanine to alanine inactivated the transporter. Most double mutants of the phenylalanines in EcAmtB e.g. the F107A F215A mutant were completely inactive in ammonium transport19. This inactive double phenylalanine to alanine mutant potentially mimics the configuration of an “open” transport pathway. Molecular dynamics simulations of ammonium transport by this mutant proposed that NH4+ is stabilized by the protein backbone at the position of the phenylalanine gate. As a consequence, ammonium deprotonation during its transportation occurs beyond the Phe-gate19 possibly at the adjacent conserved twin-His structure. This implies that the phenylalanine gate might not directly be involved in substrate deprotonation.
Ammonium transport in mutants lacking both phenylalanines could partially be rescued by introducing another mutation, W148L19,29. This rescue was not due to charge stabilization in the external vestibule, as W148 was computed to be only of minor importance for stabilizing the ammonium charge in the binding site14. Introduction of the W148L mutation strongly increased methylammonium transport in AmtB. Mutations in the corresponding residue in an AMT1;1 from tomato reduced transport rates for NH4+ and MeA6. In consequence, it was argued that the double phenylalanine gate is dispensable for transport by EcAmtB29.
When addressing the function of the Phe-gate in the human RhCG transporter, already a mutation of one of the Phe residues resulted in a dramatic activity loss. Interestingly, the double mutant, which is non-functional in EcAmtB, was active in RhCG30. The combined results point to a species-specific importance of the external vestibule and the Phe-gate in distinct ammonium transporters.
The Phe-gate function has not yet been investigated for the functionally unique AMT1 net ammonium transporters from plants. We hypothesized that the Phe-gate is dispensable for function. Here we analyzed the role of the Phe-gate and the conserved residues in the external vestibule in substrate recruitment, deprotonation and selectivity of AtAMT1;2.
Results
The function of the NH4 + recruitment pocket residues is conserved between plant and bacterial transporters
Multiple studies highlighted three highly conserved amino acid residues to be essential in electrostatic stabilization of the charged substrate in the NH4+ recruitment pocket11,12,13. Based on a structural alignment and homology modeling, previous studies identified the respective residues in AtAMT1;27,10. Asp211, Ala213 as well as Ser275 (Fig. 1) were proposed to either coordinate the substrate or stabilize the substrate charge within the external vestibule14,15,16,17. We addressed the importance of these residues in AtAMT1;2 by changing them into glycine residues and tested functionality of the mutants in yeast. AtAMT1;2 or AtAMT2 rescued yeast growth of an ammonium transporter-deficient yeast strain (ΔΔΔmep) with ammonium as sole nitrogen source8,31. Asp211 and Ser275 have side chains possibly involved in direct hydrogen bonding with NH4+. While Asp211 was essential for transport activity, the S275G mutant showed negligible residual activity (Figs 1; S1). Albeit, the A213G mutation did not affect transporter activity.
Functional assessment of recruitment pocket mutants by complementation of the ΔΔΔmep yeast. Homology model of AtAMT1;2 (top left) based on the ScMEP2 protein structure37. The model shows the location and orientation of the amino acid residues investigated in this study (D211, A213, S275 in the recruitment pocket, the Phe gate F147 and F271, W188 and the twin His motif H219 and H386). ΔΔΔmep yeast was transformed with empty vector (pDR199) or pDR199 containing wildtype or mutant AtAMTs as well as NeRh1. The transformed yeast was spotted in 10-fold dilutions beginning with an OD595 = 2 on media containing arginine (control = bottom right), ammonium (3 mM = top right) as sole nitrogen source or Arginine with 60 mM MeA (bottom left). Yeast growth on ammonium indicates ammonium transport, while no growth on toxic MeA indicates MeA uptake. Three independent repetitions have been performed, the figure shows one representative experiment.
The highly conserved pore-occluding Phe-gate is not essential for transport
The Phe-gate (F147 and F271) (Fig. 1) is highly conserved throughout the AMT/Rh/Mep protein family, as it is exhibited by plant AMTs from important model and crop plants like Arabidopsis thaliana (AtAMT), Medicago truncatula (MtAMT), Lycopersicon esculentum (LeAMT), Oryza sativa (OsAMT) and Triticum aestivum (TaAMT). The neighbouring amino acids residues are highly conserved as well, implying a conserved function in the pore region (Fig. S2). Phenylalanine mutants were analyzed by mutation and growth complementation in ΔΔΔmep yeast (Fig. 2).
Single Phe mutants complement the growth of the ΔΔΔmep yeast on ammonium as the sole nitrogen source. ΔΔΔmep yeast was transformed with empty vector (pDR199) or pDR199 containing wildtype or mutant AtAMTs. The transformed yeast was spotted in 10-fold dilutions beginning with an OD595 = 5 on media containing arginine (control) or media containing ammonium (1 mM and 3 mM) as the sole nitrogen source. Yeast growth indicates ammonium transport. Four independent repetitions have been performed, the figure shows one representative experiment.
Single alanine replacements of individual phenylalanines to alanine were tolerated, which in part is in contrast to the situation in EcAmtB, while the simultaneous exchange of both Phe-gate residues abolished ammonium transport (Figs 2; S3). A further amino acid residue, W188, was included in the analysis by introducing a W188A or W188L mutation. Mutation of this residue was able to rescue a Phe double mutant in EcAmtB29.
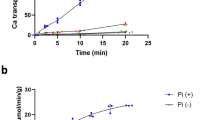
The transport activity of these mutants was then quantified in liquid culture using an ammonium uptake assay. Negligible residual flux of ammonia into the cells was observed in empty vector controls, while both single Phe mutants retained wildtype AtAMT1;2 transport capacity (Fig. 3). The double mutant did not show significant ammonium uptake, but some residual transport was conferred by the additional W188L mutation. Thus, a single Phe is sufficient to maintain plant AMT1;2 ammonium transport function, while double mutants lacking both pore Phe residues were largely inactive.
Single mutations in the Phe-gate do not affect the ammonium transport activity of AtAMT1;2. ΔΔΔmep yeast expressing the empty vector (pDR199), AtAMT2, AtAMT1;2 or the mutant versions of AtAMT1;2 was grown in liquid media, washed and re-suspended with an OD595 = 5 in media containing 3 mM NH4+. The removal of ammonium from the medium was measured photometrically as described in the methods. The lower line in the graph indicates the background uptake by the empty vector control. The upper line indicates the transport rate of wild type AtAMT1;2. The experiment was performed in four repetitions. Data are shown as means with the error bars indicating the standard error. The data is showing the ammonium uptake by the yeast over time in µM/min x OD595. Significant differences with p ≤ 0.05 are indicated by letters.
Mutation of W188 to Ala or Leu established profound MeA transport by the double mutant
In the AMT1;2 single and double Phe mutants, the ability to transport ammonium directly correlated with the capacity to import the toxic analog MeA, which then impaired yeast growth (Figs 4; S3). While the triple mutants F147A F271A W188A/L failed to transport ammonium (Figs 2, 3), they transported MeA, in contrast to the F147A F271A double mutant (Fig. 4).
Methylammonium transport by AtAMT1;2 can be partially rescued by combining a W188A/L mutation to the double Phe-gate mutant. ΔΔΔmep yeast was transformed with empty vector (pDR199) or pDR199 containing wildtype or mutant AtAMTs. The transformed yeast was spotted in 10-fold dilutions beginning with an OD595 = 5 on media containing arginine as a nitrogen source and different concentrations of methylammonium (0 mM, 60 mM and 120 mM). Yeast growth indicates low or no methylammonium transport. Four independent repetitions have been performed, the figure shows one representative experiment.
The combined results identify similarity to the situation in EcAmtB and suggest that loss of both Phe in AtAMT1;2 can be compensated by an additional mutation in W188 for the substrate MeA but not for ammonium.
Mutation of Phe271 may result in ion slippage through the AtAMT1;2 pore
In the proposed AMT transport mechanism with NH4+ recruitment, deprotonation and finally conduction of NH3 in the hydrophobic pore19, the fate of the proton remained controversial. The basic mechanism of deprotonations was, however, strongly supported by the identification of δ 15N shifts caused by ammonium uptake into yeast cells32. Discrimination against the heavier isotope was interpreted as a direct indication for deprotonation of the substrate in AMT proteins32. The AtAMT1;2 wildtype, as well as the F147A single mutant, showed strong isotope discrimination. Interestingly, all variants bearing the F271A mutation showed a decreased discrimination of the heavier isotope (Fig. 5), potentially indicating a reduced NH4+ deprotonation and an increase in ion slippage through the mutant AtAMT1;2 pore.
Increase in δ 15N values implies a partial loss of deprotonation in F271A mutants. ΔΔΔmep yeast transfected with wildtype and mutant transporters were grown with 3 mM ammonium chloride in liquid culture and δ 15N values were assessed by isotope ratio mass spectrometry. The experiment was performed four times with two technical replications for mass spectrometry. Data are given as means ± SD. Significant different values are indicated by different letters p ≤ 0.01.
Reduced isotope discrimination is not accompanied by a compromised selectivity against K+
Deprotonation of the substrate elegantly explains the high ammonium selectivity of AMT proteins against the similarly-sized K+. Therefore, the possibility of K+ conduction by the mutants harboring the F271A mutation was considered in this study. We hypothesized that if the F217A mutant allows NH4+ ion slippage through the central pore, it may as well allow K+ transport. Potassium transport capacity was tested in the WΔ3 yeast strain which lacks two endogenous potassium transporters. This yeast was transformed with all active transporter variants and grown on media containing low mM K+ concentrations. Improved growth of the yeast on this medium would indicate facilitated K+ transport. None of the transformed transporter variants was able to promote yeast growth (Figs 6, S4). By contrast all constructs slightly impaired growth on high K+. This result probably indicates unspecific negative effects by each construct, as the non-functional mutant had the largest impairment relative to the empty plasmid control.
Growth complementation and functionality of AtAMT1;2 Phe-gate mutants in K+-uptake deficient WΔ3 yeast. Single, double and triple Phe-gate mutant plasmids were transformed into WΔ3 yeast. 10-fold dilutions of an OD595 = 2 culture were spotted on plates containing 0.5, 1 or 50 (control) mM potassium. The experiment was performed three times independently, representative data of one experiment are shown.
Discussion
The protein structures of several ammonium transporter proteins from various organisms indicate a conserved pore-occluding twin Phe-gate directly adjacent to the external ammonium recruitment site14,15,16,18,20,21,22,23,24,25,26,27,28. Based on sequence identity and homology modeling5,9, we propose a similar orientation of these two highly conserved phenylalanines in plant AMT1 transporters as in AMT crystal structures. Members of the plant AMT1 family appear to transport net ammonium (as NH3 + H+)6,7, whereas plant AMT2-like proteins seem to transport ammonia after NH4+ deprotonation8,9. While the transport mechanism differs between individual AMT/Mep/Rh proteins (NH3, NH4+ or NH3 + H+ transport), the pore-lining residues and the basic mechanism with NH4+ deprotonation after NH4+ recruitment appear highly conserved9,10. The external ammonium recruitment site including Asp211, Ala213 and Ser275 (Fig. 1) and the function of the pore-occluding twin Phe-gate were addressed here. The D211G and S275G mutations strongly reduced or even impaired AMT activity, in line with a proposed role of substrate acquisition to the external recruitment site or for protein stability11,12,13,14,15,16,17,18,19,20.
Knowing that the transport mechanism between EcAmtB and AtAMT1s may differ, the Phe-gate might vary in its importance for each individual transporters. In AtAMT1;2, exchange of individual phenylalanines to alanine yielded full transport function (Figs 2–4). For the first (outer) phenylalanine, this is in accordance with previous results in EcAmtB19,29. The F271A mutant in AtAMT1;2 was completely active while F215 of EcAmtB was essential for ammonium and methylammonium transport19,29. The double mutants F147A F271A in AtAMT1;2 and F107A F215A in EcAmtB were both inactive in ammonium and methylammonium transport. It is still interesting though, that in molecular dynamic simulations of this EcAmtB mutant (mimicking an open phenylalanine gate) the ammonium ion was still stabilized by the protein backbone in the position of the twin-His motif following the Phe-gate19. Notably, the introduction of the W148L mutation in EcAmtB (W188L in AtAMT1;2, respectively) partially rescued the transport function of the EcAmtB F215L mutant and the F107A F215L double mutant, respectively. W148 is located in the NH4+ recruitment site of the transporter and not directly part of the phenylalanine gate. It seems to play a minor role in stabilizing the ammonium charge in the recruitment pocket. The W188L mutation will significantly increase the free space within this pocket, possibly facilitating the binding of the more bulky methylammonium14. This follows the observations from EcAmtB, in which the individual W148L mutation strongly increased methylammonium transport29. In AtAMT1;2 triple mutants (F147A F271A W188A or F147A F271A W188L) methylammonium transport was also restored (Fig. 4). Replacements in W188, however, did not restore ammonium transport in the double mutant (Figs 2 and 3).
Isotope fractionation during ammonium transport into yeast cells had been proposed to result from easier deprotonation of the lighter N isotope substrate. Ultimately this would result in a higher abundance of the lighter isotope in total assimilated yeast N32. The isotope fractionation was reduced by the F271 mutation, despite an unchanged transport rate, potentially indicating a partially lacking substrate deprotonation (Fig. 5). These results indicate an important role of F271 in the deprotonation mechanism and thus selection against uncontrolled ion flux. Although the Phe-gate was suggested not to be directly involved in deprotonation19, F271 might be important for stabilizing NH4+ or its charge during deprotonation. Deprotonation is then likely fulfilled by the adjacent twin-His motif. If slippage of NH4+ ions occurs, one may expect that ions of similar charge and size, such as K+ are transported. However, none of the mutants or the wild type facilitated potassium uptake (Fig. 6). Instead, expression of all mutants impaired growth of the yeast on high K+, compared to the empty plasmid control, pointing to a general fitness decrease by AMTs in this yeast strain. Interestingly, the non-functional F147A F271A mutant was most inhibitory, showing that the transport function of the protein was not the cause of growth impairment.
Taken together, single mutations of the phenylalanines did not affect the transport activity of AtAMT1;2. Mutation of the inner Phe residue resulted in a partial loss of the AMT1;2 mediated isotope fractionation, indicating a partial loss of deprotonation. This as well indicates that the substrate is still protonated at the position of the phenylalanine gate, which would contradict a mechanism of ammonium deprotonation in the external vestibule15,16,18,20,26. While deprotonation was partially lost in the mutants, all transporter versions were still selective against potassium.
In conclusion, not both Phe of the pore-occluding Phe-gate in the electrogenic AtAMT1;2 can simultaneously be replaced. While selective transport depends on substrate deprotonation32 (Fig. 7A), especially the inner Phe residue avoids NH4+ ion slippage through the pore. Mutation of F271 may partially allow NH4+ ions to pass the pore without fractionation into H+ and NH3 (Fig. 7B). The loss of the aromatic structure in the F271A mutant might decrease the NH4+ coordination in this position and thereby reduce the deprotonation dependence. Mutation of W188 provides a pore recruitment constitution that improves methylammonium entrance to the pore. Therefore, W188 recruits NH4+, but prevents methylammonium entry into the AMT pore.
Schematic model of the transport mechanism in the AtAMT1;2 wildtype and the F271A mutant. (A) In the wildtype transporter, NH4+ passes the pore and is deprotonated after crossing about 40% of the membrane electric field. On the cytoplasmic side, it is re-protonated to NH4+ by a co-transported proton. (B) In the F271A mutant, the transport – deprotonation coupling is partially lost. NH4+ might directly slip through the AMT pore (red arrows). The red bidirectional arrow indicates reduced coordination of the ammonium ion. This possible loss of ion stabilization might reduce deprotonation efficiency. The schematic model is based on a homology model of AtAMT1;27. The structural fitting for the homology model was done using the crystal structures of AfAMT-138 and EcAmtB11,12.
Material and Methods
Plasmid constructs
Briefly, the open reading frames of AtAMT1;2 (At1g64780), AtAMT2 (At2g38290) and NeRh1 (gi_30248465) were amplified from genomic DNA or cDNA of Arabidopsis thaliana Col-0 wildtype and cDNA of Nitrosomonas europaea respectively. Using specific restriction enzymes they were then subcloned into the yeast expression vector pDR1997,9,31. The mutant constructs were based on this original construct. To introduce the mutations, forward and reverse mismatch primers were designed and mutant constructs were produced by mutagenesis PCR using S7 Fusion High-Fidelity DNA Polymerase (Biozym Scientific, Hessisch Oldendorf, Germany). Template DNA was digested by DpnI. To verify the mutations and exclude other mutations the constructs were Sanger sequenced (Eurofins Genomics, Ebersberg, Germany).
Yeast transformation
The plasmids containing the respective open reading frames were heat shock-transformed in the ura- ammonium transporter defective yeast strain (31019b; ΔΔΔmep)33 and the potassium transporter defective Saccharomyces cerevisiae strain W∆3 (MATa, ade2, ura3, trp1, trk1Δ::LEU2, trk2Δ::HIS3)34. Selection for transformed yeast was done on solid arginine media (20 g × l−1 Agar, 1.7 g × l−1 YNB w/o amino acids and ammonium sulfate (Difco), supplemented with 20 g × l−1 glucose and 1 g × l−1 arginine (Arg) as nitrogen source) or on Arg phosphate minimal media35 containing 100 mM K+.
Yeast growth assays
Yeast was grown in liquid Arg medium until OD595 (optical density at 595 nm) reached 0.6–0.8. Cells were harvested, washed three times and resuspended in water to a final OD595 of 2 or 5. 10 µl of these cells and 10-fold dilutions were spotted on Arg medium with or without MeA (60 mM or 120 mM), or media containing no Arg, but 1 mM or 3 mM NH4Cl as sole nitrogen source. For growth on selective potassium media, yeast was spotted as 10 fold dilutions of OD595 of 2, on Arg phosphate minimal media containing different concentrations of K+. Pictures were taken after 3 to 5 days of growth, the experiments were performed in three or four independent repetitions.
Ammonium uptake assay
To determine ammonium uptake rates of the ΔΔΔmep yeast expressing the different ammonium transporter constructs, an ammonium uptake assay was conducted. Cells were grown overnight in 50 ml Arg medium supplemented with 1 g × l−1 arginine at 28 °C, washed three times in water, and resuspended in Arg medium with 3 mM ammonium chloride at OD595 of 3. The cultures were incubated with shaking (200 rpm) at 28 °C in a 30 ml volume and 500 µl samples were taken every 30 min until 3 h. The cells were pelleted and 300 µl supernatant was separated from the pellet and stored at 4 °C. 40 µl of the supernatant was added to 760 µl OPA (o-phthaldialdehyde) solution (540 mg o-phthaldialdehyde, 10 ml ethanol, 50 µl β-mercaptoethanol, 0.2 M phosphate buffer, pH 7.3 ad 100 ml) to quantify the remaining ammonium36. After 20 min of incubation in the dark, the extinction at 420 nm was measured. As a reference, 760 µl OPA plus 40 µl water was used. The system was calibrated with ammonium chloride concentrations from 0 to 5 mM. The experiment was performed in four independent repetitions.
Change in the natural δ15N
Discrimination of the transporters against the heavy 15N isotope was determined in yeast. ∆∆∆mep yeast was transformed as described above. Cells were grown overnight in 50 ml SD medium with 1 g × l−1 arginine at 28 °C. OD595 of the overnight culture was determined and a new 150 ml culture in SD medium with 3 mM NH4+ as sole nitrogen source was inoculated with OD595 0.01. Cultures were incubated at 28 °C with shaking at 120 rpm. Cultures were harvested when OD595 reached approx. 0.3. Cells were washed two times, pelleted, freeze-dried and δ 15N ratios were determined by isotope ratio mass spectrometry. For IRMS analysis approx. 0.5 mg of yeast or standard material war balanced into tin cups (HEKAtech, Wegberg, Germany). Standard material used was USGS41, L-glutamic acid with δ 15 = 47.6‰ air N2. For measurement an element analyzer (EuroVector, HEKAtech, Wegberg, Germany) coupled to a mass spectrometer (Delta plus Advantage, Thermo Scientific, Bremen, Germany) was used. δ15 values are calculated vs. Air-N2 using the following equation: δ ‰ = (Isotope ratio of sample/Isotope ratio of air - 1) × 1000. Data are given as means from two independent experiments with two biological replicates each ± SD. Significance was calculated by one-way ANOVA followed by a Tukey HSD test.
Data availability
All data are part of the manuscript.
References
Smith, F. A. & Walker, N. A. Entry of methylammonium and ammonium ions into Chara internodal cells. J. Exp. Bot. 29, 107–120 (1978).
Pelley, J. L. & Bannister, T. T. Methylamine uptake in the green alga Chlorella pyrenoidosa. J. Phycol. 15, 110–112 (2008).
Kleiner, D. The transport of NH3 and NH4 + across biological membranes. Biochim. Biophys. Acta - Rev. Bioenerg. 639, 41–52 (1981).
Boussiba, S., Resch, C. M. & Gibson, J. Ammonia uptake and retention in some cyanobacteria. Arch. Microbiol. 138, 287–292 (1984).
Ludewig, U. et al. Molecular mechanisms of ammonium transport and accumulation in plants. FEBS Lett. 581, 2301–2308 (2007).
Mayer, M., Dynowski, M. & Ludewig, U. Ammonium ion transport by the AMT/Rh homologue LeAMT1;1. Biochem. J. 396, 431–7 (2006).
Neuhäuser, B., Dynowski, M., Mayer, M. & Ludewig, U. Regulation of NH4 + transport by essential cross talk between AMT monomers through the carboxyl tails. Plant Physiol. 143, 1651–9 (2007).
Guether, M. et al. A Mycorrhizal-Specific Ammonium Transporter from Lotus japonicus Acquires Nitrogen Released by Arbuscular Mycorrhizal Fungi. Plant Physiol. 150, 73–83 (2009).
Neuhäuser, B., Dynowski, M. & Ludewig, U. Channel-like NH3 flux by ammonium transporter AtAMT2. FEBS Lett. 583, 2833–8 (2009).
Neuhäuser, B., Dynowski, M. & Ludewig, U. Switching substrate specificity of AMT/MEP/Rh proteins. Channels 8, 496–502 (2014).
Khademi, S. et al. Mechanism of ammonia transport by Amt/MEP/Rh: structure of AmtB at 1.35 A. Science 305, 1587–94 (2004).
Zheng, L. et al. The mechanism of ammonia transport based on the crystal structure of AmtB of Escherichia coli. Proc. Natl. Acad. Sci. USA 101, 17090–5 (2004).
Lupo, D. et al. The 1.3-A resolution structure of Nitrosomonas europaea Rh50 and mechanistic implications for NH3 transport by Rhesus family proteins. Proc. Natl. Acad. Sci. 104, 19303–19308 (2007).
Nygaard, T. P., Alfonso-Prieto, M., Peters, G. H., Jensen, M. Ø. & Rovira, C. Substrate Recognition in the Escherichia coli Ammonia Channel AmtB: A QM/MM Investigation. J. Phys. Chem. B 114, 11859–11865 (2010).
Nygaard, T. P., Rovira, C., Peters, G. H. & Jensen, M. Ø. Ammonium recruitment and ammonia transport by E. coli ammonia channel AmtB. Biophys. J. 91, 4401–12 (2006).
Ishikita, H. & Knapp, E.-W. Protonation States of Ammonia/Ammonium in the Hydrophobic Pore of Ammonia Transporter Protein AmtB. J. Am. Chem. Soc. 129, 1210–1215 (2007).
Luzhkov, V. B., Almlöf, M., Nervall, M. & Aqvist, J. Computational study of the binding affinity and selectivity of the bacterial ammonium transporter AmtB. Biochemistry 45, 10807–14 (2006).
Bostick, D. L. & Brooks, C. L. Deprotonation by dehydration: The origin of ammonium sensing in the AmtB channel. PLoS Comput. Biol. 3, e22 (2007).
Javelle, A. et al. Substrate binding, deprotonation, and selectivity at the periplasmic entrance of the Escherichia coli ammonia channel AmtB. Proc. Natl. Acad. Sci. USA 105, 5040–5 (2008).
Lin, Y., Cao, Z. & Mo, Y. Molecular dynamics simulations on the Escherichia coli ammonia channel protein AmtB: mechanism of ammonia/ammonium transport. J. Am. Chem. Soc. 128, 10876–84 (2006).
Lamoureux, G., Klein, M. L. & Bernèche, S. A stable water chain in the hydrophobic pore of the AmtB ammonium transporter. Biophys. J. 92, L82–4 (2007).
Akgun, U. & Khademi, S. Periplasmic vestibule plays an important role for solute recruitment, selectivity, and gating in the Rh/Amt/MEP superfamily. Proc. Natl. Acad. Sci. USA 108, 3970–5 (2011).
Wang, J. et al. Molecular dynamics simulations on the mechanism of transporting methylamine and ammonia by ammonium transporter AmtB. J. Phys. Chem. B 114, 15172–9 (2010).
Lin, Y., Cao, Z. & Mo, Y. Functional role of Asp160 and the deprotonation mechanism of ammonium in the Escherichia coli ammonia channel protein AmtB. J. Phys. Chem. B 113, 4922–9 (2009).
Yang, H., Xu, Y., Zhu, W., Chen, K. & Jiang, H. Detailed mechanism for AmtB conducting NH4 +/NH3: Molecular dynamics simulations. Biophys. J. 92, 877–85 (2007).
Cao, Z., Mo, Y. & Thiel, W. Deprotonation mechanism of NH4 + in the Escherichia coli ammonium transporter AmtB: insight from QM and QM/MM calculations. Angew. Chem. Int. Ed. Engl. 46, 6811–5 (2007).
Bostick, D. L. & Brooks, C. L. On the equivalence point for ammonium (de)protonation during its transport through the AmtB channel. Biophys. J. 92, L103–5 (2007).
Liu, Y. & Hu, X. Molecular determinants for binding of ammonium ion in the ammonia transporter AmtB - A quantum chemical analysis. J. Phys. Chem. A 110, 1375–81 (2006).
Hall, J. A. & Kustu, S. The pivotal twin histidines and aromatic triad of the Escherichia coli ammonium channel AmtB can be replaced. Proc. Natl. Acad. Sci. USA 108, 13270–4 (2011).
Zidi-Yahiaoui, N. et al. Functional analysis of human RhCG: comparison with E. coli ammonium transporter reveals similarities in the pore and differences in the vestibule. Am. J. Physiol. Physiol. 297, C537–C547 (2009).
Weidinger, K. et al. Functional and physiological evidence for a rhesus-type ammonia transporter in Nitrosomonas europaea. FEMS Microbiol. Lett. 273, 260–7 (2007).
Ariz, I. et al. Nitrogen isotope signature evidences ammonium deprotonation as a common transport mechanism for the AMT-Mep-Rh protein superfamily. Sci. Adv. 4, eaar3599 (2018).
Marini, A. M., Soussi-Boudekou, S., Vissers, S. & Andre, B. A family of ammonium transporters in Saccharomyces cerevisiae. Mol. Cell. Biol. 17, 4282–4293 (1997).
Haro, R., Sainz, L., Rubio, F. & Rodriguez-Navarro, A. Cloning of two genes encoding potassium transporters in Neurospora crassa and expression of the corresponding cDNAs in Saccharomyces cerevisiae. Mol. Microbiol. 31, 511–520 (1999).
Rodríguez-Navarro, A. & Ramos, J. Dual system for potassium transport in Saccharomyces cerevisiae. J. Bacteriol. 159, 940–5 (1984).
Benson, J. R. & Hare, P. E. O-phthalaldehyde: fluorogenic detection of primary amines in the picomole range. Comparison with fluorescamine and ninhydrin. Proc. Natl. Acad. Sci. 72, 619–622 (1975).
van den Berg, B. et al. Structural basis for Mep2 ammonium transceptor activation by phosphorylation. Nat. Commun. 7, 11337 (2016).
Andrade, S. L. A., Dickmanns, A., Ficner, R. & Einsle, O. Crystal structure of the archaeal ammonium transporter Amt-1 from Archaeoglobus fulgidus. Proc. Natl. Acad. Sci. USA 102, 14994–9 (2005).
Acknowledgements
We thank D. Schnell for technical assistance and help with the yeast uptake experiments. Further we thank W. Armbruster for help with the 15N measurements. We thank F. Rubio for supplying the WΔ3 yeast strain.
Author information
Authors and Affiliations
Contributions
R.M. and B.N. performed cloning and the initial ammonium yeast experiments. P.G.,T.I. and R.P. repeated experiments. P.G. performed isotope fractionation experiments and potassium growth. U.L. and B.N. planed the experiments. P.G. and B.N. wrote the manuscript. P.G., R.M., T.I., R.P., U.L. and B.N. revised the manuscript. B.N. is the corresponding author.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ganz, P., Mink, R., Ijato, T. et al. A pore-occluding phenylalanine gate prevents ion slippage through plant ammonium transporters. Sci Rep 9, 16765 (2019). https://doi.org/10.1038/s41598-019-53333-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-53333-9
This article is cited by
-
High-affinity ammonium transport by Arabidopsis thaliana AMT1;4
Acta Physiologiae Plantarum (2021)
-
Distinct transport mechanism in Candida albicans methylammonium permeases
Mycological Progress (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.