Abstract
This study evaluates the antioxidant activity of Ranunculus muricatus and isolation and structure elucidation of the active constituents. The aerial parts of the plants were shade dried at room temperature and powdered and extracted with methanol. The free radical scavenging activity was evaluated by 1,1-diphenyl-2-picryl-hydrazyl (DPPH) assay. The percentage scavenging activity was determined based on the percentage of DPPH radical scavenged. Column chromatography was used in order to isolate the active compounds. Spectral techniques UV, IR, 1H NMR, 13CNMR and HREI-MS were used for the structure elucidation of the isolated compounds. Two isolated compounds, A (caffeoyl-β-d-glucopyranoside) and B (1,3-dihydroxy-2-tetracosanoylamino-4-(E)-nonadecene), exibited a significant antioxidant activity as showed by DPPH radical scavenging method. Percentage inhibition for compound A (at 0.5 mM) was 82.67 ± 0.19 with IC50 of 93.25 ± 0.12 (μM), and for compound B (at 0.5 mM) was 69.23 ± 0.19 with IC50 of 183.34 ± 0.13 (μM). Quercetin was used as standard control. It was conclued from the present study that caffeoyl-β-d-glucopyranoside and 1,3-dihydroxy-2-tetracosanoylamino-4-(E)-nonadecene isolated from methanol extract of aerial parts of Ranunculus muricatus posses antioxidant activity.
Similar content being viewed by others
Introduction
Buttercup (Ranunculus muricatus L., Family Ranunculaceae) had been used for heart diseases and cancer in folker medicine. Inspection of plant derived drugs included in western pharmacopoeias shows their importance with different pharmacological activity; e.g. quinine from Cinchona, reserpine from Rauwolfia serpentina, vinblastine and vincristine from Catharanthus roseus, taxol from Taxus brevifolia and semisynthetic products such as steroidal hormones depends on plant sources for starting material1. The Genus Ranunculus consists of 600 species. Ranunculus muricatus, a species of buttercup known by the common name Spinyfruit buttercup, is distributed in Atlantic and Southern Europe, West and South West Asia, Crimea, Caucasus, Southern Siberia, India and Pakistan2.
Tricin 7-O-β-d-glucopyranoside, anemonin, β-sitosterol, isoscopoletin and protocatechuyl aldehyde were isolated from Ranunculus muricatus and have shown antibacterial activity against Staphylococcus aureus, Micrococcus luteus and Escherichia coli, Pseudomonas aeruginosa, Enterobacter cloacae and Klebsiella pneumonia. In particular, tricin 7-O-β-d-glucopyranoside showed a potent activity against Staphylococcus aureus with MIC value of 0.156 mg/ml3.
Search for therapeutically useful herbal antioxidants is a hot subject in the field of medical biology. Recently, many antioxidants have been established; nalewkas, cold brew coffee, Solanum xanthocarpum, Zaleya pentandra, and Erythrina superosa are some of them4,5,6,7,8. Caffeoyl derivatives are present in various plants and have been proved to be antioxidants9,10,11.
Objective of the present study was the antioxidant activity evaluation of aerial parts of Ranunculus muricatus methanolic extract.
Results
Isolation of active constituents
Methanolic extract (10 g) of aerial parts of Ranunculus muricatus (RMAPM) was fractionated on column by using silica gel 60 (40–63 μm) as stationary phase, ethyl acetate and methanol was used as mobile phase. Four fractions were obtained (RMAPM1-4). The fraction RMAPM-1(1.093 g) was further separated by column chromatography on silica gel 60 (40–63 μm) and chloroform: methanol: water (80:20:2) as mobile phase. Four fractions were obtained (RMAPM-1a to RMAPM-1d). The fractions RMAPM-1c (40 mg) was further fractionated by gel chromatography using Sephadex LH-20 as stationary phase and methanol as mobile phase and four fractions were obtained. The fraction RMAPM-1c2 was found to be pure compound A (5 mg). The fraction RMAPM-1c3 (6 mg) was purified by column chromatography on silica gel using stepwise elution to afford compound B (RMAPM-1c3b) 2.8 mg. The isolation scheme of compounds A and B is given in Fig. 1.
Structural elucidation of isolated compounds
Compound A (Caffeoyl-β-d-glucopyranoside)
Chemical structure of Compound A elucidated from the data is given in Fig. 2.
Physical Data:
White crystalline solid
Yield: 5 mg
M.P.: 326 °C
UVλmax: 206 (3.1) nm
[α]D25: +45(c = 0.022, MeOH)
IR: (KBr) νmax cm−1:3423, 1663 and 1600–1400
1H-NMR: (500 MHz)
Proton appeared at δ 7.05 (1H, d, J = 2.0 Hz, H-2), δ 6.96 (1H, dd, J = 8.0, 2.0 Hz, H-6), δ 6.77 (1H, d, J = 8.0 Hz, H-5), δ 7.64 (1H,d, J = 16 Hz), δ 6.29 (1H,d, J = 16 Hz), δ 5.56 (1H, d, J = 8.0 Hz, H-1′), δ 3.35–3.46 (1H, m, H-2′), δ 3.35–3.46 (1H, m, H-3′), δ 3.35–3.46 (1H, m, H-4′), δ 3.35–3.46 (1H, m, H-5′), δ 3.84(1H, dd, J = 12.5, 1.5 Hz, H-6a′) (1H, dd, J = 12.0, 5.0 Hz, H-6b′).
13C-NMR: (150 MHz)
Carbon at δ 127.57(C-1), δ 114.33 (C-2), δ 148.4 (C-3), δ 149.94 (C-4), δ 115.22 (C-6), δ 123.26 (C-7), δ 146.87 C-7 and δ 116.52 (C-8), δ 167.79 (C-9), δ 95.75(C-1′), δ 78.79 (C-2′), δ 77.99 (C-3′), δ 74.02 (C-4′), δ 71.07(C-5′) and δ 62.31 (C-6′).
EI-MS:
342 (76), 180 (100), 164 (13), 148 (37), 165 (26), 104 (18) and77 (47) m/z with (rel. int.)
HR-EI-MS:
342.0689 (calculated for C15H18O9, 342.0695)
Compound B (1,3-dihydroxy-2-tetracosanoylamino-4-(E)-nonadecene)
Chemical structure of Compound B elucidated from the data is given in Fig. 3.
Physical Data:
Colorless amorphous powder
Yield: 2.8 mg
[α]D25: −19.1° (c 0.021, pyridine)
IR; 3300 (O-H), 1660 (C=C), 1642 (C=O) cm−1 by KBr methods
EI-MS: 284 (31), 269 (16), 225 (21), 195 (12), 169 (10) m/z with (rel. int.)
HR-EI-MS: 663.6512 (calculated for C43H85NO3, 663.6529)
1H-NMR: (500 MHz)
Proton resonated at δ 1.28 (brs, singlet), δ 4.17 (dd, J = 3.1, 11.1 Hz, H-1), δ 4.12 (dd, J = 3.1, 11.1 Hz, H-1), δ 5.25 (H-5) (dt, J = 7, 17 Hz), δ 4.96 (H-4) (dd, J = 7, 17 Hz), δ 0.84 (6H, t, J = 6.8 Hz, CH3-24′, 19), δ 4.26 (m, H-3), δ 3.96 (m, H-2), δ 7.51 (1H, d, J = 8.7 Hz, NH).
13C-NMR: (125 MHz)
Carbon at δ 165.2 (C-1′), δ 124.4 (C-4) δ 123.9 (C-5) δ 76.2 (C-3), δ 68.3 (C-1), δ 48.6 (C-2) δ 31.1, all other methylene in the range of δ 29.7–31.4, δ 20.5 (C-19), δ 14.2 (C-24′).
Antioxidant Activity (DPPH radical scavenging activities of isolated compounds)
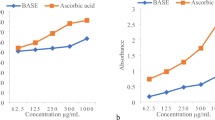
In vitro free radical scavenging activities of isolated compounds A & B were performed using the standard techniques. The inhibition (%) at 0.5 mM was measured and IC50 (μM) values were calculated. The results are shown in Table 1. Data shows that compounds A and B exhibited a significant antioxidant activity as measured by DPPH radical scavenging method with a percent inhibition value of 82.67 ± 0.19 and 69.23 ± 0.19 respectively at 0.5 mM. Quercetin was used as control.
Discussion
Different active constituents have been isolated from Ranunculus species12,13,14,15. Ranunculus species have been evaluated to have antibacterial16,17, cytotoxic18, antiviral19 and antifungal20 activity.
The compound A was obtained as white crystalline solid from the methanol soluble fraction, mp 326 °C. It gave violet colour when treated with FeCl3 (recognition test for phenol). The absorption bands in the UV spectrum were observed at 335, 273 and 202 nm while in the IR spectrum showed absorption bands at 3423, 1663 and 1600–1400 cm−1. The low resolution EI mass spectrum showed the molecular ion peak at m/z 342 while the HR-EIMS showed the [M]+ peak at m/z 342.0689 which deduced the molecular formula C15H18O9. The 1H-NMR spectrum showed signal at substituted phenyl ring (ABX system) at δ 7.05 (1H, d, J = 2.0 Hz, H-2), δ 6.96 (1H, dd, J = 8.0, 2.0 Hz, H-6) and δ 6.77 (1H, d, J = 8.0 Hz, H-5). The signals appeared at δ 7.64 (1H, d, J = 16 Hz) and δ 6.29 (1H, d, J = 16 Hz) were assigned due to olefinic protons attached to C-7 and C-8 respectively. The methine protons of glucosidal moiety were observed in the range of δ 3.35–3.46 while the anomeric proton resonated as doublet at δ 5.56 (1H, d, J = 8.0 Hz, H-1′). The 13C NMR (BB and DEPT) spectra showed the 15 carbon signals consisting of one methylene, ten methine and four quaternary carbon atoms. The 13C-NMR spectrum showed characteristic pattern of cinnamoyl moiety while the signals at δ 95.75 and δ 79.2–62.3 were of glucose moiety. On the basis of spectral data compound A could be identified as caffeoyl- β-D-glucopyranoside21.
Compound B was obtained as a colorless amorphous solid belongs to ceramide family. Its IR spectrum gives absorptions at 3300 cm−1 for a hydroxyl moiety, 1642 cm−1 for a carbonyl group. EIMS gives M+ ion peak at m/z 663 (calculated for C43H85NO3, 663.6529) corresponding to the molecular formula C43H85NO3. The large numbers of methylene groups at δ 1.28 were observed in 1H-NMR spectrum. It further showed nonequivalent methylene proton (H-1) signals at δ 4.17 (dd, J = 3.1, 11.1 Hz) and δ 4.12 (dd, J = 3.1, 11.1 Hz), two olefinic proton signals at δ 5.25 (H-5) (dt, J = 7, 17 Hz) and δ 4.96 (H-4) (dd, J = 7, 17 Hz) and two methine protons at δ 4.26 (m, H-3) and δ 3.96 (m, H-2). The large coupling constants (J = 17 Hz) of two methine protons at δ 5.25 and δ 4.96 revealed the (E) configuration of the double bond. The 13NMR DEPT spectrum showed five methine carbon groups resonated at δ 124.8, 123.1, 76.2, 48.6, 31.1, one oxy methylene carbons appeared at δ 68.3 and two methyl carbons appeared at δ 20.5 and δ14.2. There connectivities were determined by using 2D NMR techniques including HMBC and HMQC. On the basis of reported data compound B could be identified as 1,3-dihydroxy-2-tetracosanoylamino-4-(E)-nonadecene22.
Ranunculaceae are generally well known for their antioxidant properties. Many active compounds have been isolated from these species that have been evidenced to posses’ antioxidant activity. Ranunculus acris, Ranunculus platanifolius, Ranunculus repens, and Ranunculus serpens were characterized by a high percentage of hexadecanoic acid, followed by phytol, octadecadienoic and octadecatrienoic acids, which have antioxidant activities23,24. Caffeoyl derivatives have been demonstrated to have antioxidant properties. 3,4-di-O-caffeoylquinic acid, methyl 3,4-di-O-caffeoyl quinate, 3,5-di-O-caffeoylquinic acid, methyl 3,5-di-O-caffeoyl quinate, 4,5-di-O-caffeoylquinic acid and methyl 4,5-di-O-caffeoyl quinate present in Dipsacus asper showed antioxidant activity11. Caffeoyl malate anhydride and its derivative also showed antioxidant activity25. Caffeoylaltraric acid and caffeoylquinic acid present in Smallanthus sonchifolius also showed antioxidant property9. Therefore, presence of caffeoyl derivative in Ranunculus muricatus gave a clue to have antioxidant activity and finaly, the present study verified the anti-oxidant activity of Ranunculus muricatus by using DPPH radical scavenging method.
Conclusion
It was concluded from the present study that caffeoyl-β-d-glucopyranoside and 1,3-dihydroxy-2-tetracosanoylamino-4-(E)-nonadecene isolated from methanol extract of aerial parts of Ranunculus muricatus posses antioxidant activity.
Materials and Methods
Plant material
The plant material of Ranunculus muricatus was collected from the fresh water of the Lower Bari Doab Canal flowing in the area of Khanewal in the month of April 2012. The plant was identified by Prof. Dr. Altaf Ahmad Dasti and Dr. Zafarullah Zafar, Institute of Pure and Applied Biology, Bahauddin Zakariya University, Multan, Pakistan. The voucher specimen has been deposited in herbarium with reference No. R.R. STEWART 271-7.
Extraction
The plants parts were shade dried at room temperature and powdered and extracted with methanol at room temperature for 24 hours. The extracts were concentrated under vacuum on Rota vapor model No. Buchi-rotavapor R.200.
Antioxidant activity
The free radical scavenging activity was measured by 1,1-diphenyl-2-picryl-hydrazyl (DPPH) assay7. The DPPH stock solution was prepared by dissolving 20 mg DPPH in 100 ml 95% methanol. This stock solution was stored at −20 °C until needed not more than 10 days. DPPH working solution was prepared by diluting the stock of DPPH solution by adding methanol and absorbance was adjusted about 0.980 ± 0.02 at wave length 517 nm using the spectrophotometer. 3 ml aliquot of this working solution mixed with 100 µl of the plant samples at five different varying concentrations (4–322 µg/ml). The solutions in the test tubes shacked well and put in dark for 15 minutes at room temperature. Then again the absorbance was measured at 517 nm. The percentage scavenging activity was determined based on the percentage of DPPH radical scavenged by using the following equation.
Spectral analysis
Column chromatography was used for the active compounds isolation. Ultraviolet (UV) spectras (MeOH, solvents) were recorded on Perkin Elmer Lambda-25 spectrophotometer. Infrared (IR) spectra were measured on Alpha Bruker Infrared instruments. Proton nuclear magnetic resonance (1H-NMR) spectra were recorded in CD3OD and CDCl3 using TMS as internal standard at 300 MHz, 400 MHz, 500 MHz and 600 MHz on Bruker NMR spectrometers. The 13C-NMR spectra at (75, 100, 125 and 150 MHz) were also recorded using same instruments in same solvents as in 1H-NMR. The 2D NMR (HMBC, HSQC) spectra were recorded in CD3OD, and CDCl3 at 300 MHz, 400 MHz, 500 MHz and 600 MHz on the same instruments.
Low resolution EIMS spectra were recorded on a Finnigan MAT 311 with MassPec data system. Peak matching, fields desorption (FD) and field ionization (FI) were performed on the Finnigan MAT 312 mass spectrometer. Jeol JMS (HX 110) MS were used for the measurement of HRMS.
Statistical analysis
The statistics applied was the student t-test and p < 0.05 was considered as significant.
Ethical approval
Experimental protocols were approved by the Institutional Ethical Review Committee. Furthermore, all methods were performed in accordance with the relevant guidelines and regulations.
Data availability
All the data is available in the manuscript.
References
Riedl, H., Nasir, Y. J. Ranunculus muricatus, Flora of Pakistan, Naturhistorisches Museum, Botanische Abteilung, Wien, Austria and national herbarium, Pakistan agricultural research council, Islamabad, Pakistan, pp. 125–129 (1988).
Evans, W. C. Trease and Evans. Pharmacognosy, 15th edition, WB Saunders, New York. pp. 15–40, 176 (2002).
Nazir, S. et al. Antimicrobial activity of five constituents isolated from Ranunculus muricatus. J Med Plants Res 7, 3438–3443 (2013).
Polak, J., Bartoszek, M. & Bernat, R. Comprehensive comparison of antioxidant properties of tinctures. Sci Rep 9, 6148 (2019).
Rao, N. Z. & Fuller, M. Acidity and antioxidant activity of cold brew coffee. Sci Rep 8, 16030 (2018).
Saleem, S. et al. Cytomorphological, phytochemical, antimicrobial and antioxidant investigations of different morphological parts of Solanum xanthocarpum Schrad & Wendl. Lat Am J Pharm 37, 839–848 (2018).
Afzal, S. et al. Antibacterial and antioxidant activity of methanolic extract of Zaleya pentandra. Acta Pol Pharm 73, 147–151 (2016).
Janbaz, K. H., Nizsar, U., Ashraf, M. & Qadir, M. I. Spasmolytic, bronchodilator and antioxidant activities of Erythrina superosa Roxb. Acta Pol Pharm 69, 1111–1117 (2012).
Ueda, Y. et al. Increased phenolic content and antioxidant capacity of the heated leaves of yacon (Smallanthus sonchifolius). Biosci Biotech Bioch, 1–10, https://doi.org/10.1080/09168451.2019.1644151. [ahead of print] (2019).
Fraisse, D., Felgines, C., Texier, O. & Lamaison, J. L. Caffeoyl derivatives: major antioxidant compounds of some wild herbs of the Asteraceae family. Food Nutr Sci 2, 181 (2011).
Hung, T. M. et al. Antioxidant activity of caffeoyl quinic acid derivatives from the roots of Dipsacus asper Wall. J Ethnopharmacol 108, 188–192 (2006).
Erdogan, T. F., Kivcak, B., Onur, M. A. & Braca, A. Chemical constituents and cytotoxic activity of Ranunculus pedatus. Chem Nat Compounds 48, 486–488 (2012).
Prieto, J. M., Braca, A., Morelli, I., Barker, A. & Schaffner, U. A new acylated quercetin glycoside from Ranunculus lanuginosus. Fitoterapia 75, 533–538 (2004).
Wang, L. & Gao, X. Studies on the chemical constituents in Ranunculus muricatus L. Chinese J App Pharmacy 6, 460–463 (2009).
Zou, Y., Tan, C., Wang, B., Jiang, S. & Zhu, D. Phenolic compounds from Ranunculus chinensis. Chem Nat Compounds 46, 19–21 (2010).
Hussain, J. et al. Antibacterial activity of the chemical constituents from Ranunculus laetus. Chem Nat Compounds 45, 720–721 (2009).
Rasool, S., Ali, S. & Mughal, T. A. Antimicrobial and synergistic studies of Ranunculus muricatus against some indigenous bacteria. Pak J Botany 46, 345–352 (2014).
Ibrar, M. & Samreen, U. Phytochemical screening and evaluation of cytotoxic and phytotoxic effects of Ranunculus muricatus L. Pak J Plant Sci 18, 35–45 (2012).
Li, H. et al. Evaluation of antiviral activity of compounds isolated from Ranunculus sieboldii and Ranunculus sceleratus. Planta Med 71, 1128–1133 (2005).
Misra, S. B. & Dixit, S. N. Antifungal principle of Ranunculus sceleratus. Economic Botany 34, 362–367 (1980).
Zhu, T. F. et al. Three new caffeoyl glycosides from the roots of Picrorhiza scrophulariiflora. Molecules 13, 729–735 (2008).
Park, Y. C. Chemical investigation of three Antarctic marine sponges. Graduate theses and dissertation, University of South Florida. pp. 218 (2004).
Kelemen, C. D. et al. Chemical composition of the essential oils of aerial parts of Aconitum, Anemone and Ranunculus (Ranunculaceae) species from Romania. J Essent Oil Bear Pl 22, 728–745 (2019).
Bhatti, M. Z., Ali, A., Ahmad, A., Saeed, A. & Malik, S. A. Antioxidant and phytochemical analysis of Ranunculus arvensis L. extracts. BMC Res Notes 8, 279 (2015).
Anankanbil, S., Pérez, B., Cheng, W., Gouveia Ambrosio, G. & Guo, Z. Caffeoyl maleic fatty alcohol monoesters: Synthesis, characterization and antioxidant assessment. J Colloid Interf Sci 536, 399–407 (2019).
Author information
Authors and Affiliations
Contributions
F.A. Performed the experiments. B.A.C. Supervised the project. H.I. Interpret the data & collected materials. M.I.Q. Designed and write the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Azam, F., Chaudhry, B.A., Ijaz, H. et al. Caffeoyl-β-d-glucopyranoside and 1,3-dihydroxy-2-tetracosanoylamino-4-(E)-nonadecene isolated from Ranunculus muricatus exhibit antioxidant activity. Sci Rep 9, 15613 (2019). https://doi.org/10.1038/s41598-019-52166-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-52166-w
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.