Abstract
This study aims to identify the most valid, reliable and practicable indicators of laboratory mouse welfare using the Delphi consultation technique. The effective assessment of laboratory mouse welfare is a fundamental legal and moral requirement as it is critical part of both maintaining and improving the welfare of the most widely used laboratory animal globally. Although many different welfare indicators are routinely used to assess mouse welfare, the validity, reliability and practicability of many of these measures remains unclear. The Delphi consultation technique is designed to gauge expert opinion through multiple rounds of surveys until a consensus is reached. Participants ranked 59 welfare indicators in terms their validity, reliability and practicability for either a half-day unit audit or a daily welfare assessment and for each scenario identified 10 key indicators. The Delphi consultation reached consensus at 72% for the overall list of indicators and over 60% for each individual indicator. From this consensus the key indicators for each mouse welfare scenario (half day audit and daily welfare assessment) were identified and used to create a welfare scoring system for each scenario.
Similar content being viewed by others
Introduction
Mice are the most commonly used species in scientific research, with over 4.6 million mice estimated to be used annually in regulated research globally1. Over 3 million scientific procedures involving animals were carried out in the UK during 2017, 58% of which involved mice2. Due to the large number of mice used in research, refinement of their welfare is critical. This refinement is dependent on our ability to efficiently assess their welfare, as without this assessment we cannot identify instances when a refinement is needed or if any refinement applied has been effective. Consequently, there has been increasing interest in developing new methods to effectively assess the welfare of mice at both an individual and group level within animal facilities3,4,5,6. The welfare of laboratory mice is routinely assessed using a combination of animal-based indicators (e.g. physiological, psychological changes) or resource-based indicators (e.g. environmental conditions, staff training), along with indices derived from the specific procedures or studies (e.g. pain management)7,8,9,10. Resource-based assessment is carried out using indicators that reflect the animals environment and how animals cope with the environmental changes, preserving their biological and psychological functions. Indicators include environmental indices relating to the animals’ housing and husbandry as well as every day husbandry activities (e.g. cleaning cages)11. Animal-based assessment involves the measurement of an animal’s behaviour and physiological reactions. The aggregation of all aspects of laboratory mouse welfare (physical, physiological, behavioural and environmental) into a welfare protocol, is paramount to provide an overall assessment. There have been limited studies gathering information from experts about indicators and methods for assessing animal welfare. These studies were conducted a few years ago (around 2010), and the majority focus on species other than mice, such as cows12, horses13, pigs14 and laying hens15 and their focus was the development of policies and recommendations for welfare16,17,18. Most of these studies use the Delphi method which is a widely used survey technique that seeks information from experts about a specific topic13,19,20. The answers are given anonymously, through a series of rounds with the aim of achieving consensus within the group21. The Delphi methodology has been used in diverse range of animal science fields, including to assess the impact of DEFRA policy on welfare16; the implication of animal diseases on productivity22; and for the selection of a subset of species to have their habitat protected23. This technique has shown to be an effective method of gaining information about welfare assessment in farm animals13,14,15. In these studies, the Delphi consultation process was used in different ways, including the use of vignettes with horse welfare case scenarios13, questions about preferences in animal-based welfare indicators for hens, pigs and cattle15 and with case studies in livestock including dairy and egg production16. The results of these studies provided information about stakeholders attitudes towards methods of improving horse welfare13; formed a foundation for the development of welfare protocols including indicators related to health status, behaviour and records which were selected by the experts (e.g. lameness in dairy cattle)15 and highlighted the need to increase of monitoring compliance regarding welfare standards in dairy and egg production systems16. The Delphi methodology is used as one of the preliminary sources for assessing ‘face’ validity, which is defined as the subjective opinion of experts about the extent to which the measure is meaningful in terms of providing information on the animal’s welfare24,25,26. This face validity is based on the assumption of “safety in numbers” where a group of people are less probable to come to a wrong conclusion than an individual27. This study uses expert opinion about the validity of laboratory mouse welfare indicators.
The aim of this study was to determine, through a modified Delphi consultation, which indicators of mouse welfare are considered valid, reliable and practical for a half-day audit and daily welfare assessment of laboratory mice. This study uses the Delphi consultation technique as a tool to identify potential measures for assessing mouse welfare. In this consultation, a level of 70% global consensus (i.e. across all the indicators) and over 60% individual indicator consensus was required. There are no specific guidelines offering a definition of consensus in Delphi studies, as it is argued it depends on the nature of the research that is carried out (e.g. medical decision, development of new policies etc.)28. Studies using this technique in nursing and animal welfare contexts have used a level of 70% consensus as a standard29,30,31,32. However, many studies do not provide any information about the level of consensus needed13,16,23 or if they are required to have 100% agreement22. Since there are no guidelines to set a consensus level in Delphi studies a level of 70% was used as this is aligned with other peer-reviewed, animal welfare research18,33.
Results
Demographics
Of the 98 participants who completed both rounds of the Delphi consultation, 30% were veterinarians, 20% were researchers who used animals in their research, 19% were laboratory facility managers, 11% were technicians, 10% were Named Animal Care Welfare Officers, and 8% were Animal Welfare researchers. The majority of the respondents were working in the United Kingdom (41%), followed by USA (13%), Australia (12%), Canada (10%) and Switzerland (8%). Participant expertise was based on the number of years of experience working with laboratory animals, qualifications and job position. Most of the participants had a PhD (35%), other qualifications related to animal welfare (e.g. IAT, Diploma in animal science) (20%) or a Masters in Animal Behaviour and Welfare degree (15%).
Half day welfare audit
A total of 98 participants completed the second-round questionnaire. Twenty-nine percent of these participants agreed to the initial rank order (from round one) and seventy-one percent of participants made minor changes to the indicators rank order from round one (Fig. 1). The rank position of the indicators was not modified significantly as the order of the indicators did not change from one extreme position to another, although a lower level of agreement can be seen in some of the indicators (e.g. alertness and staff training, both with 62% agreement). The overall consensus for the rank order of indicators used in a half-day audit assessment was 77.2%. Based on these results, a consensus among the participants was reached in the second round so no further consultation was deemed to be necessary. The indicators with the highest level of consensus were hunched position and coat condition ranked first and second with over 90% of agreement between participants. The indicators with the lowest consensus were staff training, alertness, empathetic attitude of staff towards animals, and facial expressions of pain with 62% of agreement.
Everyday welfare assessment
Participants were asked to agree or disagree with the rank order of the most important indicators for an everyday welfare assessment by technical staff (Fig. 2). A consensus was reached with 85.7% of agreement between the participants. There were few indicators with consensus level over 95%. These included hunched position, coat condition, food type, substrate type and light source. Humidity, room temperature and gait were the indicators with the lowest consensus level with 66%, 67% and 68% respectively.
Top ten indicators to be used in a half day audit assessment and in an everyday welfare assessment
The final top ten list of indicators with the information regarding the percentage of validity, practicability and reliability from both half day animal welfare audit and everyday welfare assessment are provided in Figs 3 and 4 respectively. The percentage validity, reliability and practicability of the indicators included in both half-day and everyday welfare assessment vary between the indicators. All of the indicators show validity over 80% in both, half-day and every day, however, reliability and practicability were different. In the half day welfare assessment, most of the indicators had over 80% reliability except exhibition of normal (74.6%) and abnormal behaviour (71.6%) and usage of nesting material (68.4%). Practicability was under 80% for Body Condition Score (76.3%), weight change (59.7%) and exhibition of abnormal behaviour (78.5%). In the everyday welfare scenario, reliability was under 80% for exhibition of abnormal behaviour (71.6%), usage of nesting material (68.4%), pups outside the nest (67.4%) and alertness (67.4%). Practicability was under 80% for exhibition of abnormal behaviour (78.5%) and Alertness (76.1%). The selection of some of these final top ten indicators for the half day audit or everyday welfare assessment (Fig. 5) were associated with participant’s experience in working with laboratory animals. There is a positive association between the selection of body condition score as top ten indicator and the length of time that participants have been working with laboratory animals (x2 = 14.4; p = 0.02). This indicator was chosen more by people with over 6 years of experience (48%). Similarly, hunched position (x2 = 12.2; p = 0.01) and mortality rate (x2 = 10.4; p = 0.03) seems to be positively associated with the length of time that participants have been working with laboratory mice. These indicators were chosen more frequently (50%) by participates with over 6 years of experience working with laboratory animals.
Summary of top ten indicators selected by participants to be used in an audit assessment. The x-axis represents the three items that were assessed by participants, validity (red), reliability (green) and practicability (blue). The y-axis represents the percentage of participants who scored each item as valid and very valid in the Delphi consultation.
Summary of the top ten indicators selected by participants to be used in an everyday welfare assessment. The x-axis represents the three items that were assessed by participants, validity (red), reliability (green) and practicability (blue). The y-axis represents the percentage of participants who scored each item as valid or very valid in Delphi consultation.
Discussion
The aim of this study was to determine, through expert opinion, which laboratory mouse welfare indicators would be valid to use in a half-day welfare audit of a laboratory mouse facility and in an everyday welfare assessment carried out by technical staff.
Delphi methodology has been shown to be a valuable tool for aggregating information from laboratory mouse welfare experts across the world, allowing experts to exchange opinions and come to a consensus. The Delphi consultation process focused on the rank order of 59 indicators in each specific context (see material and methods Fig. 6). Consensus was reached with an agreement of 70% for the top ten indicators for a half-day welfare audit assessment (see Fig. 5). The highest ranked three indicators with the highest agreement (over 84%) did not change position from round one, supporting participant’s opinion about the high validity of these three indicators. Most of the indicators are animal-based (8 out of 10) demonstrating the high credibility (or the high level of confidence) that this type of indicators has between the participants. It is interesting to note that the top four indicators are physiological, followed by indicators relating to behaviour (normal and abnormal), social interaction and the environment. These results further support the idea of the importance of physiological indicators in welfare assessment34. These physiological responses which constantly adapt to maintain animal’s welfare can be measured in a non-invasive manner, which might provide a high level of validity35. Behavioural indicators are also important as they are easy to measure, and they show an animal’s adaptations to present environmental conditions7,36,37.
Conversely, mortality rate and staff training are the only two resource-based indicators included in this list. Staff training can have a significant impact on laboratory mouse welfare as inadequate training can lead to improper care of animals, e.g. handling which can cause fear affecting animal’s performance and welfare38,39. Despite the small number of resource-based indicators selected, their inclusion in welfare assessment protocols is important as they include procedures, treatments and management which can have a high welfare impact, especially in laboratory animals (e.g. room temperature preferences, environmental enrichment in the cages)11. This is contrary to other authors suggestions that the assessment of welfare should focus on only animal-based indicators40.
The indicators with the lowest percentage of agreement (62%): staff training, alertness, empathetic attitude of staff towards animals and facial expressions of pain were still highly ranked (9, 11, 12 and 16 out of 59 respectively). These indicators are considered to be subjective as it is the observer who gives a score based on observations. This subjectivity might explain their high rank but low general agreement. The fact that participants considered these indicators as important, despite not yet being fully validated, as some of them are relatively new (e.g. facial expression of pain – method initially published by Langford, et al.41 in 2010) might also have a role in their rank position. In addition many of the studies of the MGS conducted to date, have used it for retrospective scoring from either video or still images. The limited number of studies that have used the MGS for ‘live’ scoring have shown conflicting results, with some finding similar scores to retrospective scoring42 and some finding retrospective scores to be higher than those of live scores43. This potential inconsistency when scoring live compared to retrospectively may have lead the participants to be concerned about the practicability and reliability of this method at the cage-side and so influenced the ranked position it was given by the participants41,44,45.
The top ten indicators selected for an every-day welfare assessment can be seen in Fig. 5. The consensus reached for all the indicators was higher (87.5%) compared to the half-day audit assessment rank order. One possible explanation for the difference in the agreement for both scenarios could be due the nature of the assessment, one for auditing purpose (half-day welfare assessment) and the other for daily check-ups (every day welfare assessment).
An important finding of this study is the differences between the final lists for each scenario. Even though they come from the same initial list of 59 indicators they differ by 4 indicators. The top ten half-day audit welfare assessment indicators include: body condition score, weight change, mortality rate and staff training which are not present in the every-day welfare assessment top ten. These differences could be explained in part by the nature of the assessment (i.e. the scenario) proposed in the questionnaire (see material and methods: Fig. 6). Even if the scenario was the same for these two assessments, there are differences in the assessment duration and the individual who is performing the assessment. An everyday welfare assessment, for example, is usually performed by technical staff, who have knowledge about the facility and the animals being assessed. In order to comply with the time limit for a half day audit (4 hours) the indicators used need to be accurate, practical and rapid to score therefore indicators such as body condition score, mortality rate and staff training were deemed relevant by the experts. Body condition score, for example, provides information about mouse health status in a more practical manner than assessing body weight, where a scale and comparison of previous weight is needed46. Mortality rate is a resource-based indicator used as a retrospective assessment of welfare as provided information about the number of animals found dead (i.e. Diseases, environmental problems)47. However, this indicator is not considered as a welfare measure because it is performed at facility level thus it is not an indicator of individual welfare48 that would be used to assess the welfare on daily basis. In contrast to farmed animals where mortality rate is considered a useful indicator as the level of productivity is directly affected49, in laboratory mice mortality rate is not valued in the same manner as it harder to measure at individual level and there are no practical implications for improving welfare state of that individual. Staff training is also an important indicator for a half-day welfare audit where a longitudinal approach to welfare is considered. Although there is limited research about the real impact of the staff training on the welfare of laboratory mice, recommendations about laboratory animal welfare consider the ability to handle, train and observe mice in the laboratory can be very important to reduce negative impacts on welfare as experienced and trained staff can identify problems promptly50.
Alternatively, room temperature, wounds (excluding bite wounds), pups outside the nest and alertness are included in the every-day welfare assessment top ten list but not in the half-day audit assessment. As discussed, room temperature, wounds and alertness are important for the assessment of laboratory mouse welfare. The usage of these indicators in every-day welfare assessment is likely relevant as the assessment is made daily using records (room temperature) or observing the animals by technical staff in the daily welfare check (pups outside the nest, wounds and alertness). Due to the fact that the staff who perform this assessment are in contact with the animals every day, they are likely to be effective at noticing subtle changes such as these more quickly. The staff are already familiar with the species, the strain, the individuals, and in many cases the protocol procedures, therefore they are more experienced in assessing these indicators.
It is important to emphasise that even though this study uses a rank order to define the level of face validity, considering expert opinion, rank order is not relevant for the indicators in terms of defining their individual level of importance over other indicators (i.e. meaning that 10 is not less important than 9). The importance of this study is in identifying the final list of indicators, considering the type of assessment scenario, and not the assessment of each individual indicator. As it has been stated before, it is an aggregation of different indicators into a protocol which determines the value of the final welfare assessment and not a single indicator alone48,51,52,53,54. It is important also to highlight the variation in validity, practicability and reliability between the final top ten indicators for both assessments. The percentage of validity for all the indicators was over 80% which supports their inclusion in the final list as the most valid indicators taking into account expert opinion. However, the reliability and practicability of the indicators was variable between the different measures. Reliability was under 80% for exhibition of normal and abnormal behaviour and usage of nesting material for the half-day welfare assessment. In the everyday welfare assessment, reliability was low for exhibition of abnormal behaviour, usage of nesting material, pups outside the nest and alertness. Although the values of reliability were over 70%, which was the threshold for accepting the agreement between the experts, their lower values of reliability show that these indicators are considered valid and relevant, but their reliability needs to be taken into account and potentially investigated further. The assessment of laboratory mouse behaviour (including normal, abnormal and usage of nesting material) can be a valuable tool for the assessment of welfare and it has been used in other protocols before40, but requires a lot of practice and knowledge for the assessor to use effectively and so the results may not be viewed as entirely reliable, particularly as laboratory mouse behaviour can be affected by the presence of an observer38. Practicability was considered as low for exhibition of abnormal behaviour, body condition score and weight change in the half-day welfare assessment and for exhibition of abnormal behaviour and alertness in the everyday welfare assessment. This low percentage of practicability (under 80%) for these indicators in both assessments, shows that even if these indicators are considered valid and so important, they are not viewed as very practical. This may be related to the need for baseline recordings to make these indicators truly effective which could have affected how the practicability of these indicators was viewed by the assessors.
Some caution should be taken in interpreting the results from this study. The scenarios used involved a specific description of facilities which can affect the indicators selected as well as the purpose of the selection. Due to the nature of the suggested scenarios and the specific information about the facilities (number of animals, racks, room, etc.), a specific list of indicators have been selected which may not be applicable in different circumstances. It is important to highlight that the indicators selected for this study are those that relate to the influence on welfare of housing and husbandry rather than indicators related to the experiments conducted in animals, which were not included. However, these specific procedure indicators are important in laboratory mouse welfare as procedures (e.g. surgeries, treatments, and behavioural tests) have a direct impact on welfare, affecting physical and psychological health which need to be measured using specific indicators (e.g. Body Condition Score for assessing mouse condition in tumours studies)55,56,57,58.
This study has several practical implications. It could be used as a preliminary source of face validation to select indicators for a mouse welfare assessment considering the purpose of the assessment, i.e. a welfare audit or daily welfare check. Furthermore, this study illustrates that more research regarding validation, reliability and practicability of welfare indicators to assess laboratory mouse welfare is needed. It also can be concluded that when assessing stock mice, or those not yet actively enrolled on research protocols, the indicators of welfare in Fig. 5 are deemed the most valid to use, based on expert opinion, considering the nature of the assessment (audit welfare assessment or everyday welfare checks). An example score sheet for the audit welfare assessment and everyday welfare check can be found in the supplementary documents. The indicators in each of the example score sheets, which include both resource-based and animal-based indicators, can be used as a preliminary tool for designing a mouse welfare protocol for stock animals in a laboratory facility. Additionally, these indicators could be used as a preliminary list when assessing the welfare of mice enrolled on scientific studies with the addition of key information from study plans and project licenses. These additional experiment specific indicators can be added to an assessment protocol aligned with a preliminary definition of good welfare for the animals being assessed.
Methods
Ethical statement
This study were conducted at Newcastle University following the registration for unlicensed work (AWERB Project ID: 449), and in accordance with the EU Directive (2010/63/EU), and ASPA (1986). An informed consent was obtained from all participants.
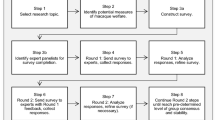
A Delphi consultation process was conducted to determine, through expert opinion, the most valid, reliable and practical indicators to be use in a half day and every day assessment of laboratory mice welfare. It comprised of four distinct sequential phases; [1] a scoping meeting, [2] a pilot survey, [3] Round one of Delphi consultation, and [4] Round two of Delphi consultation (Fig. 7).
The scoping meeting was divided into two sessions. In session one participants were asked to generate as many indicators of mouse welfare as possible, considering their validity, reliability and practicability for the assessment of mouse welfare in a specific context. In session two, the groups were asked to rank the quality of a list of potential questions that could be used in the first round of Delphi consultation. The pilot survey of the first round of the Delphi consultation was launched using the on-line system Qualtrics platform (http://www.qualtrics.com) and was live for 2 weeks (December 2015) with 9 participants completing it. Participants were asked to complete the survey and assess the type of questions, their clarity and to indicate the amount of time needed to complete the questionnaire. This survey provided feedback on the type of questions, the length and the information contained within the questionnaire, and was used to refine the questions and survey format for the first round of the Delphi consultation.
First-round questionnaire
Participants were recruited using a diverse set of methods, including personal contacts, professional organisations relating to laboratory animal welfare, veterinarians working in laboratory animal facilities, literature search of academics and researchers who have published on mouse welfare in the last 15 years. A total of 206 people agreed to take part in this first round. The questionnaire was then sent via an individual link to each participant using Qualtrics platform (http://www.qualtrics.com). Consenting participants were informed about the aim, methods and duration of the study. Data collected was only used for this specific research project. Ethical approval was granted from Newcastle University (Project ID 449).
The questionnaire was separated into 3 sections. In the first two sections, the participants were given two different theoretical scenarios as a guide to complete the questions relating to the validity, practicability and reliability of potential welfare indicators (Fig. 6). The indicators were divided into two separate groups, animal-based and resourced-based indicators. This was done to facilitate the assessment process in the Delphi consultation round one questionnaire. Validity was defined as ‘an indicator that provides useful information about the animal’s welfare’; practicability was defined as ‘an indicator that can be measured in a reasonable amount of time, incurring a reasonable cost and is feasible within the constraints of a laboratory animal facility’; and reliability was defined as ‘an indicator that produces consistent information when used by different people assessing the same animal and the same person assessing the same animal in the same state on more than one occasion’. This questionnaire was ‘live’ for two weeks (February 2016). The rank order was created from the indicators assessed as ‘valid’ and ‘very valid’ by the participants. Those indicators were then organised into a rank according to how frequently they were selected by participants from 1 to 59.
Second-round questionnaire
The round two questionnaire was sent out to the participants who completed the round one questionnaire using the Qualtrics platform with a personal link via email. This questionnaire was again ‘live’ for two weeks (March 2016). The second-round questionnaire was separated into two sections in which the participants assessed the rank order of the indicators for both scenarios (half-day and everyday assessment). Participants were instructed to agree or disagree with the rank order of all 59 indicators (included both animal- and resource- based measures) taking into account their validity, reliability and practicability for the assessment of laboratory mouse welfare in each scenario. If they disagreed, they were then asked to reorder the indicators into the rank position they considered more appropriate and state the reason for the change (i.e. based on validity, practicability and/or reliability). The above process was repeated for scenario for section 2 (everyday welfare assessment). We chose to include both animal- and resource-based measures together to determine the ones the participants felt were the most important for assessing welfare within the constraints of the time available for carrying out such assessments.
Data analysis
Information about participant’s selection in validity, practicability and reliability of the indicators was analysed using descriptive statistics. The Delphi consultation methodology is a qualitative method use for gathering information about people’s opinion. Most of the research performed using this methodology used descriptive statistics (frequencies, means, median) for analysing the data12,13,14,18,59. There is also research comparing different Delphi techniques and providing advice about the analysis which recommend the usage of frequencies, mean, median for analysing data and provide final results15,17,27,28,60,61,62. Participant’s selection in terms of the validity of the indicators and rank order were compared across participant’s job role and years of experience working with laboratory animals using a Chi-square test. These two factors (job role and years of experience) were chosen out of the 8 factors from the demographic information of the participants because they are representative of the level of experience with laboratory mice which is considered as an important factor for defining participant’s expertise in the area.
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Taylor, K., Gordon, N., Langley, G. & Higgins, W. Estimates for worldwide laboratory animal use in 2005 (2008).
Home Office. Annual Statistics of Scientific Procedures on Living Animals, Great Britain 2017 (2018).
Wemelsfelder, F. How animals communicate quality of life: the qualitative assessment of behaviour. Anim Welfare 16, 25–31 (2007).
Rock, M. L. et al. The time-to-integrate-to-nest test as an indicator of wellbeing in laboratory mice. Journal of the American Association for Laboratory Animal Science 53, 24–28 (2014).
Branchi, I., Santucci, D. & Alleva, E. Ultrasonic vocalisation emitted by infant rodents: a tool for assessment of neurobehavioural development. Behavioural Brain Research 125, 49–56, https://doi.org/10.1016/S0166-4328(01)00277-7 (2001).
Proctor, H. S. & Carder, G. Can ear postures reliably measure the positive emotional state of cows? Applied Animal Behaviour Science 161, 20–27, https://doi.org/10.1016/j.applanim.2014.09.015 (2014).
Dawkins, M. S. From an animal’s point of view: motivation, fitness, and animal welfare. Behavioral and brain sciences 13, 1–9 (1990).
Broom, D. M. Animal welfare defined in terms of attempts to cope with the environment. Acta Agriculturae Scandinavica. Section A. Animal Science. Supplementum (Denmark) (1996).
Fraser, D., Weary, D. M., Pajor, E. A. & Milligan, B. N. A Scientific Conception of Animal Welfare that Reflects Ethical Concerns. Anim Welfare 6, 187–205 (1997).
Hubrecht, R. The Welfare of Animals Used in Research: Practice and Ethics. 284 (UFAW Animal Welfare Series, 2014).
Baumans, V. Science-based assessment of animal welfare: laboratory animals. Revue scientifique et technique (International Office of Epizootics) 24, 503–513 (2005).
Geist, M. R. Using the Delphi method to engage stakeholders: A comparison of two studies. Evaluation and Program Planning 33, 147–154, https://doi.org/10.1016/j.evalprogplan.2009.06.006 (2010).
Collins, J., Hanlon, A., More, S. J., Wall, P. G. & Duggan, V. Policy Delphi with vignette methodology as a tool to evaluate the perception of equine welfare. The Veterinary Journal 181, 63–69, https://doi.org/10.1016/j.tvjl.2009.03.012 (2009).
Bracke, M. B. M. Expert opinion regarding environmental enrichment materials for pigs. Anim Welfare 15, 67–70 (2006).
Whaytt, H. R., Main, D. C. J., Greent, L. E. & Webster, A. J. F. Animal-based measures for the assessment of welfare state of dairy cattle, pigs and laying hens: consensus of expert opinion. Anim Welfare 12, 205–217 (2003).
Bennett, R. M., Broom, D. M., Henson, S. J., Blaney, S. J. P. & Harper, G. Assessment of the impact of government animal welfare policy on farm animal welfare in the UK. Anim Welfare 13, 1–11 (2004).
Rikkonen, P. Scenarios for future agriculture in Finland: a Delphi study among agri-food sector stakeholders. Agricultural and Food Science 14, 205–223 (2008).
More, S. J. et al. Setting priorities for non-regulatory animal health in Ireland: results from an expert Policy Delphi study and a farmer priority identification survey. Prev Vet Med 95, 198–207 (2010).
Adler, M. & Ziglio, E. Gazing into the oracle: The Delphi method and its application to social policy and public health, (Jessica Kingsley Publishers, 1996).
Keeney, S., McKenna, H. & Hasson, F. The Delphi technique in nursing and health research, (John Wiley & Sons, 2010).
Linstone, H. A. & Turoff, M. The Delphi Method. Techniques and applications 53 (2002).
Der Fels-Klerx, V., Ine, H. J., Goossens, L. H. J., Saatkamp, H. W. & Horst, S. H. S. Elicitation of quantitative data from a heterogeneous expert panel: formal process and application in animal health. Risk Analysis 22, 67–81 (2002).
Hess, G. R. & King, T. J. Planning open spaces for wildlife: I. Selecting focal species using a Delphi survey approach. Landscape and Urban Planning 58, 25–40, https://doi.org/10.1016/S0169-2046(01)00230-4 (2002).
Blokhuis, H. J. Improving farm animal welfare: science and society working together: the welfare quality approach, (Springer, 2013).
Nevo, B. Face Validity Revisited. Journal of Educational Measurement 22, 287–293 (1985).
Sireci, S. G. The construct of content validity. Social indicators research 45, 83–117 (1998).
Hasson, F., Keeney, S. & McKenna, H. Research guidelines for the Delphi survey technique. Journal of advanced nursing 32, 1008–1015 (2000).
Keeney, S., Hasson, F. & McKenna, H. Consulting the oracle: ten lessons from using the Delphi technique in nursing research. Journal of advanced nursing 53, 205–212 (2006).
Leach, M. C. & Main, D. C. J. An assessment of laboratory mouse welfare in UK animal units. Anim Welfare 17, 171–187 (2008).
McKenna, H. P. The essential elements of a practitioners’ nursing model: a survey of psychiatric nurse managers. Journal of Advanced Nursing 19, 870–877 (1994).
McKenna, H. P., Bradley, M. & Keeney, S. Primary care nursing: a study exploring key issues for future developments. Coleraine: University of Ulster (2001).
McKenna, H. & Hasson, F. A study of skill mix issues in midwifery: a multimethod approach. Journal of Advanced Nursing 37, 52–61 (2002).
Wentholt, M. T. A. et al. Defining European preparedness and research needs regarding emerging infectious animal diseases: Results from a Delphi expert consultation. Prev Vet Med 103, 81–92, https://doi.org/10.1016/j.prevetmed.2011.09.021 (2012).
Van de Weerd, H. A., Van Loo, P. L. P., Van Zutphen, L. F. M., Koolhaas, J. M. & Baumans, V. Preferences for nesting material as environmental enrichment for laboratory mice. Lab Anim-Uk 31, 133–143, https://doi.org/10.1258/002367797780600152 (1997).
Barnett, J. L. & Hemsworth, P. H. The validity of physiological and behavioural measures of animal welfare. Applied Animal Behaviour Science 25, 177–187, https://doi.org/10.1016/0168-1591(90)90079-S (1990).
Würbel, H., Stauffacher, M. & Holst, D. Stereotypies in Laboratory Mice—Quantitative and Qualitative Description of the Ontogeny of ‘Wire‐gnawing’and ‘Jumping’in Zur: ICR and Zur: ICR nu. Ethology 102, 371–385 (1996).
Augustsson, H. & Meyerson, B. J. Exploration and risk assessment: a comparative study of male house mice (Mus musculus musculus) and two laboratory strains. Physiol Behav 81, 685–698, https://doi.org/10.1016/j.physbeh.2004.03.014 (2004).
Hawkins, P. et al. A guide to defining and implementing protocols for the welfare assessment of laboratory animals: eleventh report of the BVAAWF/FRAME/RSPCA/UFAW Joint Working Group on Refinement. Lab Anim-Uk 45, 1–13 (2011).
Gonyou, H. W., Hemsworth, P. H. & Barnett, J. L. Effects of frequent interactions with humans on growing pigs. Applied Animal Behaviour Science 16, 269–278, https://doi.org/10.1016/0168-1591(86)90119-X (1986).
Spangenberg, E. M. F. & Keeling, L. J. Assessing the welfare of laboratory mice in their home environment using animal-based measures – a benchmarking tool. Lab Anim-Uk, https://doi.org/10.1177/0023677215577298 (2015).
Langford, D. J. et al. Coding of facial expressions of pain in the laboratory mouse. Nature methods 7, 447–449 (2010).
Faller, K. M., McAndrew, D. J., Schneider, J. E. & Lygate, C. A. Refinement of analgesia following thoracotomy and experimental myocardial infarction using the Mouse Grimace Scale. Experimental physiology 100, 164–172 (2015).
Miller, A. L. & Leach, M. C. The mouse grimace scale: a clinically useful tool? PloS one 10, e0136000 (2015).
Defensor, E. B., Corley, M. J., Blanchard, R. J. & Blanchard, D. C. Facial expressions of mice in aggressive and fearful contexts. Physiol Behav 107, 680–685 (2012).
Leach, M. C. et al. The assessment of post-vasectomy pain in mice using behaviour and the Mouse Grimace Scale. PloS one 7, e35656 (2012).
Ullman-Culleré, M. H. & Foltz, C. J. Body condition scoring: a rapid and accurate method for assessing health status in mice. Comparative Medicine 49, 319–323 (1999).
Clough, G. Environmental effects on animals used in biomedical research. Biological Reviews 57, 487–523 (1982).
Botreau, R., Veissier, I., Butterworth, A., Bracke, M. B. M. & Keeling, L. J. Definition of criteria for overall assessment of animal welfare. Animal welfare-potters bar then wheathampstead 16, 225 (2007).
Rushen, J. Changing concepts of farm animal welfare: bridging the gap between applied and basic research. Applied Animal Behaviour Science 81, 199–214, https://doi.org/10.1016/S0168-1591(02)00281-2 (2003).
Hubrecht, R. et al. Refining rodent husbandry: the mouse. Lab Anim 27, 301–329 (1993).
Rousing, T., Bonde, M. & Sørensen, J. T. Aggregating welfare indicators into an operational welfare assessment system: a bottom-up approach. Acta Agriculturae Scandinavica, Section A-Animal Science 51, 53–57 (2001).
Wells, D. J. et al. Assessing the welfare of genetically altered mice. Lab Anim-Uk 40, 111–114 (2006).
Van der Meer, M. et al. Behavioral and physiological effects of biotechnology procedures used for gene targeting in mice. Physiol Behav 73, 719–730, https://doi.org/10.1016/S0031-9384(01)00529-7 (2001).
van der Meer, M., Rolls, A., Baumans, V., Olivier, B. & van Zutphen, L. F. M. Use of score sheets for welfare assessment of transgenic mice. Lab Anim-Uk 35, 379–389, https://doi.org/10.1258/0023677011911859 (2001).
Russell, W. M. S., Burch, R. L. & Hume, C. W. The principles of humane experimental technique (1959).
Morton, D. B. 5–12 (London: Royal Society of Medicine Press, 1999).
Morton, D. B. The importance of non-statistical design in refining animal experiments, (ANZCCART, 1998).
Stokes, W. S. Humane endpoints for laboratory animals used in regulatory testing. Ilar J 43, S31–S38 (2002).
Leach, M. C., Thornton, P. D. & Main, D. C. J. Identification of appropriate measures for the assessment of laboratory mouse welfare. Anim Welfare 17, 161–170 (2008).
Frewer, L. J. et al. The use of Delphi methodology in agrifood policy development: Some lessons learned. Technological Forecasting and Social Change 78, 1514–1525, https://doi.org/10.1016/j.techfore.2011.05.005 (2011).
Hsu, C.-C. & Sandford, B. A. The Delphi technique: making sense of consensus. Practical assessment, research & evaluation 12, 1–8 (2007).
Schmidt, R. C. Managing Delphi Surveys Using Nonparametric Statistical Techniques*. Decision Sciences 28, 763–774, https://doi.org/10.1111/j.1540-5915.1997.tb01330.x (1997).
Acknowledgements
The authors would like to thank all of the participants who took part in the scoping meeting, pilot survey, as well as the both phases of the consultation process. Professor Lynne Frewer for her advice and feedback on using the Delphi consultation survey. This project was funded by the Colombian Administrative Department of Science Technology and Innovation (Colciencias).
Author information
Authors and Affiliations
Contributions
I.C.L., A.M., M.L. and A.B. conceived the Delphi consultation, I.C.L. and A.M. conducted the scoping meeting, I.C.L. conducted the pilot, round one and round two of Delphi, M.L., A.M. and I.C.L. analysed the results. I.C.L., A.M., M.L. and A.B. reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Campos-Luna, I., Miller, A., Beard, A. et al. Validation of mouse welfare indicators: a Delphi consultation survey. Sci Rep 9, 10249 (2019). https://doi.org/10.1038/s41598-019-45810-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-45810-y
This article is cited by
-
Construction of an index system of core competence assessment for infectious disease specialist nurse in China: a Delphi study
BMC Infectious Diseases (2021)
-
The identification of effective welfare indicators for laboratory-housed macaques using a Delphi consultation process
Scientific Reports (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.