Abstract
In recent years, sea ice cover along coasts of East Antarctica has tended to increase. To understand ecological implications of these environmental changes, we studied benthic food web structure on the coasts of Adélie Land during an event of unusually high sea ice cover (i.e. two successive austral summers without seasonal breakup). We used integrative trophic markers (stable isotope ratios of carbon, nitrogen and sulfur) to build ecological models and explored feeding habits of macroinvertebrates. In total, 28 taxa spanning most present animal groups and functional guilds were investigated. Our results indicate that the absence of seasonal sea ice breakup deeply influenced benthic food webs. Sympagic algae dominated the diet of many key consumers, and the trophic levels of invertebrates were low, suggesting omnivore consumers did not rely much on predation and/or scavenging. Our results provide insights about how Antarctic benthic consumers, which typically live in an extremely stable environment, might adapt their feeding habits in response to sudden changes in environmental conditions and trophic resource availability. They also show that local and/or global trends of sea ice increase in Antarctica have the potential to cause drastic changes in food web structure, and therefore to impact benthic communities.
Similar content being viewed by others
Introduction
Antarctica is one of the most productive and biodiverse regions of the marine realm, and species living there have to cope with extreme, yet stable environmental conditions such as low temperatures or intense seasonality. These narrow environmental limits likely persisted over most of the last million years, and could have been a defining factor for Antarctic fauna’s evolutionary history1,2. Environmental stability in Antarctica is jeopardized by anthropogenic climate change that has fast and contrasting impacts on Southern regions1. While the western Antarctic Peninsula is one of the most rapidly warming regions in the world, resulting in sea ice cover decrease, the sea ice cover tends to increase in some parts of East Antarctica, possibly in relation with changes in atmospheric circulation3,4. These contrasting changes affect both the spatial (i.e. the sea ice concentration at a given location) and the temporal extent (i.e. the duration of the season during which sea ice is present) of the Antarctic sea ice cover5,6. Moreover, high inter-annual variability and occurrence of global change-induced extreme events7 complicate assessment of these environmental changes and understanding of their effects on biological organisms.
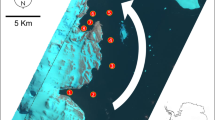
The surroundings of the Dumont-d’Urville research station (Adélie Land, East Antarctica) provide a spectacular example of sea ice increase along the Antarctic littoral. In the Western Pacific Ocean Sector of East Antarctica (90° E–160° E), landfast ice typically starts to form in April and reaches maximum extent in mid- to late September. Seasonal breakup generally occurs in November, and most coastal zones of this sector are free from landfast ice from December to March8. Local observations around Dumont-d’Urville station confirm this pattern, although there is inter-annual variability in the period at which the seasonal breakup occurs, which can range from early November to early January9,10. Regardless, sea ice season length in Adélie Land tended to increase from 1980 to 200211. Besides those long-term trends, events of extreme sea ice development have been recorded in recent years, culminating in austral summers during which no breakup occurred in the surroundings of Dumont-d’Urville station. While a classic seasonal breakup, corresponding to the situation described above, was observed during all summers from 1980 to 2013, total absence of breakup was noted in 3 of the 5 last years (austral summers 2013–2014, 2014–2015 and 2016–2017), during which islands of the Géologie archipelago remained connected by sea ice throughout summer (French Polar Institute Paul-Emile Victor – IPEV, pers. comm.). These recent trends could be linked with regional features such as calving of the Mertz glacier in 2010, as ice shelves, sea ice and fast ice seem to be bound by complex oceanic processes in coastal Adélie and George V lands12.
Impacts of such a dramatic increase in sea ice cover on benthic ecosystems are poorly understood, as ecological effects of sea ice loss have received more attention than effects of sea ice expansion13. Sea ice is nevertheless a major environmental driver of marine ecological processes in Antarctica, where food web dynamics are strongly intertwined with sea ice conditions14,15. Sea ice exerts direct (through export of sympagic primary production) and indirect (through coupling with oceanographic processes) influence on the nature and abundance of food items available for benthic organisms. Feeding habits of benthic consumers have accordingly been shown to vary between locations featuring different sea ice extent and persistence14,16. Moreover, seasonal sea-ice breakups cause strong and highly predictable food pulses of which the benthic organisms are able to take advantage by shifting their dietary niche17. Overall, it seems likely that changes in sea ice conditions of the magnitude of those encountered around Dumont-d’Urville station in recent years could influence trophic interactions among Antarctic zoobenthos.
In this context, the main objective of this study was to understand how absence of seasonal breakup could impact the structure of benthic food webs. Marine food webs are complex networks of ecological interactions, but their essential parameters can be summarized using two dimensions, leading to the traditional depiction of food webs as bi-dimensional diagrams18. The horizontal dimension of these diagrams encompasses the diversity of producers sustaining the food web. Their vertical structure is dictated by the trophic position of the consumers (e.g. primary consumers, secondary consumers, omnivores, etc.). Here, we sampled biomass-dominant invertebrates around Dumont-d’Urville station, as well as their food items, during two successive austral summers (2013–14 and 2014–15) where no seasonal breakup occurred. We used integrative trophic markers (stable isotope ratios of carbon, nitrogen and sulfur) to build ecological models that were used as proxies of both fundamental food web dimensions. The horizontal dimension was depicted using a mixing model19,20 to delineate diets of primary consumers, identify food items supporting benthic communities, and quantify the relative importance of production originating from different compartments (sympagic, pelagic and benthic) for invertebrate nutrition under those peculiar sea ice conditions. The vertical structure of the food web was recreated through a trophic position model21 that was used to provide continuous estimates of the distance between benthic consumers and the baseline items supporting them during austral summers without breakup.
Results
Stable isotope ratios of food sources and consumers (Table 1)
δ13C of food sources covered a wide interval, ranging from −34.6 ± 1.6‰ (Phyllophora antarctica blades) to −12.5 ± 1.7‰ (sympagic algae). Pygoscelis adeliae guano (δ13C = −28.5 ± 0.9‰) and suspended particulate organic matter (δ13C = −26.7 ± 0.6‰) also had quite negative δ13C. Benthic biofilm had second least δ13C of food items (δ13C = −20.0 ± 1.4‰). Its value was nevertheless clearly distinct from sympagic algae (Dunn’s post-hoc test, p < 0.0001). Himantothallus grandifolius showed inter-organ variation, as blades (δ13C = −23.6 ± 1.8‰) were more 13C-depleted than stipes (δ13C = −21.5 ± 1.1‰; Dunn’s post-hoc test, p = 0.0047) or holdfasts (δ13C = −21.9 ± 2.0‰; Dunn’s post-hoc test, p = 0.0203). All consumers δ13C were comprised between values of SPOM and sympagic algae, and most mean values were found between −14 and −20‰ (Table 1).
Most food items had mean δ15N ranging from 3 to 6.5‰ (Table 1), with the exception of H. grandifolius holdfasts (δ15N = 9.6 ± 1.2‰) and P. adeliae guano (δ15N = 22.0 ± 1.4‰). H. grandifolius tissues showed δ15N differences (Table 1). However, they were not consistent with δ13C variation, as holdfasts were more 15N-enriched than stipes (δ15N = 3.6 ± 1.3‰; Dunn’s post-hoc test, p < 0.0001) and blades (δ15N = 3.2 ± 0.4‰; Dunn’s post-hoc test, p < 0.0001). Mean consumer δ15N ranged from 4.4 ± 0.4‰ for the bivalve Laternula elliptica to 10.7 ± 0.8‰ for the sea anemone Isotealia antarctica. Inside this interval, values were widely overlapping (Table 1).
Sympagic algae had the lowest δ34S of food sources (5.6 ± 2.7‰; Table 1), followed by benthic biofilm (10.5 ± 3.0‰). Like for carbon, sulfur isotopic ratios of H. grandifolius blades (δ34S = 17.6 ± 2.7‰) differed from those of stipes (δ34S = 16.1 ± 1.0‰; Dunn’s post-hoc test, p = 0.0035) and holdfasts (δ34S = 13.1 ± 3.3‰; Dunn’s post-hoc test, p < 0.0001). Finally, SPOM had the highest measured δ34S, with values of 18.5 ± 0.8‰. Mean δ34S of the vast majority of consumers was mostly overlapping in an interval ranging from 13.5 to 17.5‰ (Table 1). Only four taxa were more 34S-depleted, i.e. the sipunculid Golfingia sp. (δ34S = 12.7 ± 0.7‰), the sea anemone Isotealia antarctica (δ34S = 11.7 ± 1.5‰), the sponge Hemigellius sp. (δ34S = 10.9 ± 2.0‰) and the polychaete Flabegraviera mundata (δ34S = 9.5 ± 2.1‰).
Resource use by consumers - SIAR mixing model (Fig. 1)
SIAR outputs (Fig. 1) indicated that sympagic algae were the main food item of 8 of the 18 investigated taxa: polychaetes Flabegraviera mundata, Harmothoe sp., Perkinsiana sp. and Polycirrus sp.; sea stars Odontaster validus and Diplasterias brucei; the sea urchin Sterechinus neumayeri; and the brittle star Ophiura sp. Contribution of sympagic algae was the highest for Odontaster validus, whose 95% credibility interval (CI95) ranged from 0.73 to 0.87 with a mode of 0.81 (Fig. 1). Generally speaking, dominance of sympagic algae in the diet was more marked in echinoderms than in polychaetes, where contributions could be moderate (e.g. mode = 0.42 and CI95 = [0.30, 0.54] for Perkinsiana sp.; Fig. 1).
Boxplots of relative contributions of (A) sympagic algae, (B) suspended particulate organic matter, (C) pooled benthic sources (sum of contributions of benthic biofilm and Himantothallus grandifolius blades) to invertebrates’ diet, computed using the SIAR model. Orange: Porifera; red: Polychaeta and Sipunculida; blue: Mollusca; purple: Echinodermata. Black dots, boxes and error bars are respectively the modes, 50% and 95% credibility intervals of posterior probability distributions of model solutions.
Four taxa relied on sympagic algae and suspended particulate organic matter (SPOM) in similar proportions: two sponges (Homaxinella balfourensis and an unidentified species of Demospongiae), the sipunculid Golfingia sp. and the gastropod Margarella sp. (Fig. 1). Model outputs suggested that the main resource supporting the gastropods Marseniopsis sp. and Trophonella longstaffi was SPOM (Fig. 1). Diet of both Dendrochirota sea cucumbers (Heterocucumis sp. and Staurocucumis sp.) was co-dominated by SPOM and benthic sources (Fig. 1). Difference in the feeding habits of these two species were nonetheless visible, as Heterocucumis sp. consumed more SPOM (mode = 0.59; CI95 = [0.39, 0.77]) than Staurocucumis sp. (mode = 0.39; CI95 = [0.27, 0.54]) in 95.98% of model solutions. Accordingly, SPOM was Heterocucumis sp.’s main food item, while benthic production was more important than SPOM for Staurocucumis sp. (Fig. 1). Finally, benthic food items largely dominated the diet of bivalves Adamussium colbecki (mode = 0.75; CI95 = [0.52, 0.84]) and Laternula elliptica (mode = 0.95; CI95 = [0.82, 0.99]: Fig. 1).
Trophic position estimates (Fig. 2)
The modal trophic position (Fig. 2) of studied organisms varied between 1.12 for Adamussium colbecki (CI95 = [1.04, 1.21]) and 3.18 for Isotealia antarctica (CI95 = [2.91, 3.58]). Many invertebrates had low trophic positions (TP), as eight taxa had a modal trophic level inferior to 2 (Adamussium colbecki, Heterocucumis sp., Laternula elliptica, Golfingia sp., Trophonella longstaffi, Staurocucumis sp., Margarella sp. and Sterechinus neumayeri; Fig. 2). Only six taxa had a modal trophic position close to or higher than 3 (Hemigellius sp., Saliasterias brachiata, Acodontaster sp., Mycale acerata, Ammothea carolinensis and Isotealia antarctica; Fig. 2). Trophic position of the remaining taxa oscillated between 2 and 3. Striking intra-group differences were found in sponges (Fig. 2), as trophic positions of Hemigellius sp. (modal TP = 2.91; CI95 = [2.56, 3.38]) and Mycale acerata (modal TP = 2.99; CI95 = [2.50, 3.65]) were higher than those of Homaxinella balfourensis (modal TP = 2.02; CI95 = [1.69, 2.35]) and the unidentified species of Demospongiae (modal TP = 2.04; CI95 = [1.86, 2.29]) in more than 95% of model solutions.
Boxplots of consumers’ trophic positions, estimated using the tRophicPosition model. Orange: Porifera; brown: Cnidaria; red: Nemertea, Nematoda, Polychaeta and Sipunculida; green: Arthropoda; blue: Mollusca; purple: Echinodermata. Black dots, boxes and error bars are respectively the modes, 50% and 95% credibility intervals of posterior probability distributions of model solutions.
Inter-annual comparison (Fig. 3)
At site 1 (Anse du Lion), the isotopic composition of suspended particulate organic matter, Himantothallus grandifolius blades and sympagic algae was similar for both carbon and nitrogen between austral summers 2013–2014 and 2014–2015 (Mann-Whitney U test, p > 0.05 in each case; Fig. 3). Biofilm δ15N did not change over time (Mann-Whitney U test, p = 0.0837; Fig. 3), but its δ13C showed significant variation (Mann-Whitney U test, p = 0.0012), shifting from −17.6 ± 1.1‰ in 2013–2014 to −19.9 ± 0.9‰ in 2014–2015 (Fig. 3).
Contrastingly, the carbon and nitrogen isotopic ratios of all consumers that could be sampled at site 1 in both years varied strongly. Both δ13C and δ15N of Sterechinus neumayeri, Odontaster validus and Ophiura sp. significantly shifted over time (Mann-Whitney U test, p < 0.05 in each case; Fig. 3). Most of these changes were striking, sometimes as much as 3‰. Although magnitude of changes was variable, all taxa showed a δ15N decrease and a δ13C increase (Fig. 3). Adamussium colbecki also exhibited considerable δ15N decrease (6.8 ± 0.3‰ in 2013–2014 vs. 4.4 ± 0.3‰ in 2014–2015; Mann-Whitney U test, p = 0.0013; Fig. 3), but its δ13C remained constant over time (Mann-Whitney U test, p = 0.1909; Fig. 3).
Discussion
Most of the complexity of trophic interactions can be captured using two fundamental dimensions, leading to the traditional depiction of food webs as bi-dimensional diagrams18. Here, we used state-of-the-art models based on well-established trophic tracers (i.e. stable isotope ratios of light biogenic elements) as proxies of both of these food web dimensions. Use of a mixing model allowed us to quantify the relative importance of primary producers for animal nutrition at our study site, and therefore to estimate the diversity of producers sustaining the food web. The trophic position model, on the other hand, provided information on the vertical structure of the food web. Our results suggest that, in zones that typically undergo a sea ice breakup during austral summer (hereafter referred to as “normal conditions”), year-long persistence of sea ice is likely to influence both food web dimensions, as reliance of consumers on sympagic algae seemed higher (change in horizontal structure), while many consumers seemed to occupy lower trophic positions (change in vertical structure) than in normal conditions.
Co-occurrence of three groups of producers, each associated with an ecosystem compartment (i.e. sympagic, pelagic and benthic producers) is a ubiquitous feature of coastal marine systems of Antarctica22. Here, mixing model outputs (Fig. 1) pointed out that sympagic algae were very important for the food web, as they dominated or co-dominated the diet of 12 out of 18 investigated taxa. In comparison, pelagic food items dominated or co-dominated the diet of 8 taxa, and benthic producers (macroalgae and biofilm), despite the seemingly huge available biomass, were among the main food items of only 4 out of 18 taxa. This widespread reliance of benthic consumers on sympagic algae strikingly spanned multiple feeding guilds (suspension and deposit feeders, grazers, browsers, and omnivores) and seemed to extend to sessile taxa such as sedentary polychaetes or sponges. During sampling dives, large (several cm in length) algal filaments were observed at the interface between ice and seawater (Supplementary Information S1). Algal aggregates are known to sink quickly upon detachment23, and can likely reach the water bottom of shallow zones such as our study areas in a few hours. Aggregates can therefore be available for consumption by benthic animals directly on the substrate, but also during their sinking, or as re-suspended material. Preferential consumption of sympagic algae might be explained by their higher nutritional value and/or palatability. In the Arctic, ice algae are readily consumed by benthic taxa, and may be preferentially selected by some species, possibly due to their high essential fatty acid content24. Here, the ratio between elemental contents of carbon and nitrogen (C/N ratio), a commonly used proxy for nutritional quality of a food item, was very high for Himantothallus grandifolius blades (14.0 ± 2.2; mean ± SD), which might limit suitability of this food item for some consumers. Moreover, H. grandifolius has thick and robust blades, and large Phaeophyceae commonly contain high amounts of poorly digestible structural compounds25, as well as herbivore deterrents26. They might therefore be avoided by some of the studied fauna. C/N ratios were comparable for biofilm (6.2 ± 0.6; mean ± SD) and sympagic algae (6.7 ± 1.2; mean ± SD), suggesting similar nutritional value for both food items. Preferential consumption of sympagic algae could therefore be explained by palatability differences. Microscopic examination of benthic biofilm showed that it was a highly heterogeneous mix of dead and living microalgae, prokaryotes, organic detritus from various origins, and even inorganic material. Some of these elements could act as deterrents for invertebrate consumers, which might instead feed on sea-ice derived material that is likely mostly present in the form of pure sympagic algae aggregates.
Regardless of factors driving it, this predominance of sympagic algae in benthic invertebrate diet does not seem to be a common feature of coastal Antarctic food webs. In the Antarctic Peninsula, phytoplankton, Phaeophyceae and their associated diatoms have been reported to be the main items fuelling food webs from rocky shores27. Similarly, soft-bottom consumers can rely on planktonic production and microphytobenthos and macroalgae28, that can notably be consumed under detrital form29. In East Antarctica, three major carbon pathways were reported: one based on pelagic POM, one on macroalgae and their epiflora, and a last one on sediment POM, macroalgal detritus and benthic diatoms30. Sympagic material was not identified as a dominant food item in any of those pathways. In the Ross Sea, studies based on spatial designs suggested that sea ice extent modulates resource use by benthic consumers14,16. Interestingly, feeding patterns of consumers seemed to differ from the ones we observed in this study. In the Ross Sea, sympagic inputs to the diet of other consumers such as Odontaster validus and Sterechinus neumayeri were high but did not vary according to sea ice extent or persistence16. Here, stable isotope ratios of both taxa were significantly different between the two sampling years, suggesting that they shifted towards a more sympagic-based diet between the first and the second summer without seasonal sea ice breakup (Fig. 3). Past studies showed that predominance of sediment-associated detritus in the diet of benthic consumers, including the sea urchin Sterechinus neumayeri, increased in sea ice-influenced localities14. Contrastingly, our mixing model suggests that in Adélie Land, this species relied mostly on sympagic-derived material, and that importance of benthic food items in its diet was limited (Fig. 1). On the other hand, past research pointed out that contribution of sympagic material to diet of Laternula elliptica increased in locations with higher sea ice cover16. This was not seen here, as our model suggested that this species was one of the few ones to rely quasi-exclusively on benthic production (Fig. 1). These discrepancies might be linked with the peculiar physiology of Antarctic benthic invertebrates. Many consumers studied here have long life spans (sometimes several decades) and low growth and metabolic rates31,32,33. Experimental estimates of isotopic turnover of tissues does not, to the best of our knowledge, exist for Antarctic benthic invertebrates. It has nevertheless been suggested that this turnover time is likely to be high, and could approach a year or more16,34. In this context, some of the sampled consumers might not have reached isotopic equilibrium with their food items yet. This would suggest caution when interpreting model outputs, as isotopic equilibrium between consumers and food items is one of the main assumptions underlying them. Consumers could also have a greater selectivity than what was modeled here (e.g. by selectively feeding on specific items among the biofilm). The mixing model built here represents a simplification of reality, and would not be able to detect such fine-scale processes. Nevertheless, the clear changes in isotopic ratios of dominant consumers between the first and the second year without seasonal breakup combined with the absence of consistent changes in isotopic composition of food items (Fig. 3) strongly suggest that the diet of those species actually shifted over this period. These horizontal changes in food web structure, and important reliance of multiple benthic invertebrate taxa on sympagic production could have functional implications, as they could increase competition for resources, and ultimately impact community structure. They could also have biogeochemical implications, as organic matter fluxes between the two situations could differ drastically. On the other hand, these diet shifts could indicate that dominant benthic species are able to readily adjust to changes in food item availability. This trophic plasticity, that could be an adaptive trait in the context of intense seasonality encountered in coastal Antarctica17, could also increase resilience of benthic invertebrate fauna to future environmental changes.
Continuous estimates of trophic position (Fig. 2) are commonly used metrics in food web ecology. They provide simple ways to assess organisms’ functional roles in natural ecosystems and to estimate energy flow through ecological communities, while taking into account complex processes such as omnivory35,36,37. This last point is particularly critical for Antarctic zoobenthos, among which food webs are better described as a “trophic continuum” than as discrete trophic levels30. Omnivory appears to be an effective way to cope with the strong spatial and temporal changes in food availability characteristic of Antarctic coastal systems, and many consumers have multiple feeding strategies14,30,38. Estimates yielded by our trophic position model (Fig. 2) were overall rather low. Many taxa typically regarded as omnivores (e.g. polychaetes, sea stars Odontaster validus and Diplasterias brucei, the sea urchin Sterechinus neumayeri) had a trophic level inferior to 2.5, suggesting that from a functional point of view, they acted mostly as herbivores. Only a few taxa had trophic positions over 3, suggesting they were actually secondary consumers. To the best of our knowledge, none of the studied taxa significantly rely on mixotrophy. It is therefore likely that animals showing TP inferior to 2 (e.g. Adamussium colbecki, Laternula elliptica, Heterocucumis sp.) were either not at isotopic equilibrium with their food sources, or selectively feeding on low δ15N items. Nevertheless, δ15N of some abundant consumers such as S. neumayeri and O. validus showed a significant decrease between the two sampling years, while the δ15N of food items remained constant over the same period of time (Fig. 3). This demonstrates that the trophic position of some taxa decreased between the first and second year without seasonal breakup. Contrastingly, previous research lead in East Antarctica suggested that many consumers (including O. validus) had trophic levels markedly superior to 330. Comparable findings were reported from the Antarctic Peninsula27. Moreover, in the Ross Sea, δ15N of benthic invertebrates tended to be higher, and animal-derived matter contributed more to the diet of some taxa (e.g. S. neumayeri) in stations with high sea ice influence, presumably because of lower macrophyte productivity and diversity14.
Here, unusual absence of seasonal breakup could have caused a decline in animal prey availability for benthic consumers. While faunal densities were not quantified in our study, occurrence of taxa reported as abundant during previous investigations at the same site seemed moderate, forcing us to increase sampling effort to reach adequate replication. This is in agreement with other observations from East Antarctica, where, although Ophiuroidea seemed able to cope with such conditions, anomalous year-round sea ice persistence was linked with low overall abundance of fauna13. Low trophic positions of some consumers, including biomass-dominant and ecologically important omnivores, could be another hint at high trophic plasticity among Antarctic zoobenthos. They could indicate that, when faced with high availability of suitable plant-derived material, animals are able to shift their diet to take advantage of this food supply. Important availability of basal resources has accordingly been associated with a decrease of trophic level in predator39 as well as Arctic omnivore invertebrates40. Low trophic positions nevertheless raise concerns about energy flow among benthic communities in the absence of sea-ice breakup. Each animal consumer indeed needs sufficient energy to thrive, and has to cope with stoechiometrical constraints41. Some of the taxa showing surprisingly low trophic positions here might have trouble meeting their energy and/or nutrient demands. This could have adverse effects on secondary production and population dynamics, and, ultimately, community structure. In this context, alternative feeding strategies might be an efficient way to deal with nutritional stress. Here, strong intra-group differences in trophic positions were pointed out in sponges (Fig. 2). Sponges harbor a variety of symbionts capable of heterotrophic and autotrophic metabolism42, which can represent up to 35% of sponge biomass43. In abyssal plains, it has been suggested that symbiotic bacteria could be able to metabolize refractory material, which can then be assimilated by the sponge. This process leads to an elevation of δ15N of sponges44. Something similar could happen here, and the widely different trophic levels found in sponges could reflect different levels of reliance on symbiont-mediated trophic processes.
Patterns of food web structure under increased sea ice conditions in Adélie Land seem different from those suggested by previous studies from the Ross Sea14,16. These discrepancies might be driven by local variation in environmental parameters such as sea ice thickness and cover that will in turn influence under-ice light availability. Moreover, both studies report that sea ice conditions across their surveys were consistent over long time periods, suggesting that organisms had time to adapt to environmental constraints and, that communities had time reach stable structure. In our case, however, rapid (when compared to invertebrate life spans) environmental changes could cause instability in community structure and food supply to the benthos. This could explain the clear changes in feeding habits of dominant consumers between the first and second year without breakup. Timing, frequency and extent of sea ice breakup could therefore be essential parameters to consider to achieve a satisfactory understanding of how current changes in sea ice cover influence benthic food webs in Antarctica.
Conclusions
When compared with food webs described in contrasting but stable sea ice conditions, our results suggest that the absence of seasonal breakup causes simplification of the food web in both its fundamental dimensions. Horizontal trophic interactions, indicating food/energy sources, were characterized by low diversity of food items supporting animal population and high reliance on sympagic material. Vertical food web structure seemed weakly defined, with many consumers occupying low and overlapping trophic positions. In East Antarctica, zoobenthos richness and abundance is positively correlated with food availability45. Food web modifications could therefore have implications for ecosystem functioning and, ultimately, community structure. Holistic, quantitative approaches attempting to link environmental changes to the role of species in ecological networks have accordingly been suggested as an efficient way to understand impacts of global change on Antarctic ecosystems46. In addition to the processes we document here, some impressive ecosystem changes, such as unprecedented development of biofilm that overgrows macroalgae and sessile consumers (Supplementary Information S1), or discoloration of macroalgae, were observed but not quantified. These changes are akin those described in other parts of Antarctica, where abnormal year-round persistence of sea ice in relation to iceberg calving strongly impacted benthic communities, possibly triggering an ecosystem phase shift13. Results presented here suggest that changes in food supply and trophic interactions could be one of the mechanisms through which this phase shift could take place.
When faced with such substantial environmental changes, animals can either adapt, migrate or disappear47. Migration capabilities of Antarctic zoobenthos are often limited due to low mobility and/or absence of dispersing larval stages48. On the other hand, our results support the hypothesis that absence of seasonal sea ice breakup caused benthic invertebrates to shift their diet. At the seasonal timescale, trophic plasticity of coastal Antarctic zoobenthos is regarded as beneficial17. It could allow organisms to take advantage of the short-term shifts in resource availability, which translates to an increase in metabolic activity49, in nutritional status and energy content50, and in reproductive activity51. In the longer term, trophic plasticity of Antarctic invertebrates could mediate adaptation to future environmental changes. Integrative studies attempting to relate dietary changes to individual and/or population-level assessments of fitness would provide valuable insights regarding how taxa cope with to their new environmental constraints. Consumer identity will likely modulate response to environmental changes, as feeding habits could show important variation among some studied taxonomical groups (e.g. sponges, bivalves, pycnogonids, sea stars and sea cucumbers; Figs 1 and 2). Similarly, the shift in isotopic values between the two sampled years differed between some consumers in both magnitude and direction (Fig. 3).
More research is needed to understand how the magnitude of environmental changes (e.g. varying levels of sea ice thickness and/or snow cover) could further modulate food web architecture. How community resilience could help benthic ecosystems to recover from such extreme sea ice events also remains an open question. However, many ecological processes are slow in Antarctica52, while the recent frequency of austral summers without sea ice breakup in coastal Adélie Land has been high, with normal breakup conditions happening only twice over the past 5 years. In this context, local or global trends of temporal or spatial increase of sea ice cover might have strong impacts on benthic invertebrate communities.
Methods
Sampling
Sampling took place in the surroundings of Dumont-d’Urville station (French Polar Institute Paul-Emile Victor - IPEV), on Petrels Island (Adélie Land, East Antarctica) during the austral summers of 2013–2014 (22–26/01/2014) and 2014–2015 (17/12/2014–12/01/2015). During the sampling period, fast ice did not undergo seasonal breakup for two successive years (ice thickness fluctuating between 40 and over 200 cm during summer, cf. Supplementary Information S2). Snowfall was negligible (i.e. too low to be measured) throughout all the period (Supplementary Information S2). Two sampling sites were chosen. Site 1 (“Anse du Lion”; 140.003°E, 66.661°S) was visited during both campaigns, while site 2 (“Cap des Eléphants”; 139.997°E, 66.667°S) was sampled only in 2014–2015. In both sampling campaigns, both sites were covered by a thick layer of fast ice (from around 100 to over 200 cm; Supplementary Information S2), and holes were drilled to allow access to the sea.
In total, 28 invertebrate taxa (Table 1) spanning 9 phyla and most common functional guilds in coastal Antarctica were sampled at depths ranging from 10 to 20 m. Most invertebrate samples were collected by SCUBA divers, either by hand or using small landing nets, but some specimens were caught using small traps baited with fish tissues (Table 1).
Six items (producers or organic matter pools, Table 1) were identified as potential food sources for primary consumers. Sympagic algae, which mostly occurred as several cm long filaments (Supplementary Information S1), were sampled by SCUBA divers by scraping the lower surface of fast ice. The dominant macroalgae, the large Phaeophyceae Himantothallus grandifolius and the Rhodophyceae Phyllophora antarctica, were hand-collected by SCUBA divers. Benthic biofilm was collected by scraping rocks in situ. It was scarce in 2013–2014 but extremely abundant in 2014–2015 (thick layer of several cm covering rocks but also macroalgae and sponges, Supplementary Information S1). Seawater for suspended particulate organic matter (SPOM) was collected through the diving holes, at 10 m depth, using a Niskin bottle. Seawater was then pre-sieved to remove items larger than 5 mm, and filtered on pre-combusted (4 h at 400 °C) glass fibre filters (Whatman GF/F, sieve size 0.7 µm). For each SPOM sample, 20 litres of seawater were filtered. Finally, samples of the abundant deposits of guano surrounding the extensive colonies of Adélie penguins (Pygoscelis adeliae) were hand collected on land, in the vicinity of the diving holes.
Stable isotope analysis
At Dumont-d’Urville station, animal samples were placed in aerated seawater tanks immediately after collection and processed as soon as possible. Animals were dissected to separate soft and non-metabolically active tissues53. Due to important differences in invertebrate body structure and size, selected tissues were not the same for each taxon (Table 1). Most food items were processed whole, with the exception of Himantothallus grandifolius for which holdfasts, stipes and blades were separated. All samples were oven-dried at 60 °C for 72 h, then placed in airtight containers and kept at room temperature before further treatment once back from the expedition. They were subsequently ground to a homogeneous powder using mortar and pestle or, when required, a MM301 mixer mill (Retsch GmBH, Haan, Germany) (cycles of 60 seconds at 25 Hz).
Inorganic carbon present in samples can be a source of bias in carbon stable isotope analysis. “Champagne tests” where therefore used to highlight presence of carbonates in tissues54. They revealed that tissues of Margarella sp., Acodontaster sp., Diplasterias brucei, Odontaster validus, Saliasterias brachiata, Sterechinus neumayeri, Ophiura sp., Heterocucumis sp. and Staurocucumis sp. contained moderate yet significant amounts of carbonates. They were therefore acidified by exposing them to HCl vapours for 48 h in an airtight container55. After acidification, a second series of “champagne tests” were run. They indicated that the procedure successfully removed all carbonates from samples. Since acidification can alter N53 and S56 isotopic ratios, acidified samples were analysed twice: once for C isotopic ratios, using decarbonated material, and once for N and S isotopic ratios, using native material.
Stable isotope ratios measurements were performed via continuous flow - elemental analysis - isotope ratio mass spectrometry (CF-EA-IRMS) at University of Liège (Belgium), using a vario MICRO cube C-N-S elemental analyser (Elementar Analysensysteme GMBH, Hanau, Germany) coupled to an IsoPrime100 isotope ratio mass spectrometer (Isoprime, Cheadle, United Kingdom). Isotopic ratios were expressed using the widespread δ notation57, in ‰ and relative to the international references Vienna Pee Dee Belemnite (for carbon), Atmospheric Air (for nitrogen) and Vienna Canyon Diablo Troilite (for sulfur). IAEA (International Atomic Energy Agency, Vienna, Austria) certified reference materials sucrose (IAEA-C-6; δ13C = −10.8 ± 0.5‰; mean ± SD), ammonium sulphate (IAEA-N-2; δ15N = 20.3 ± 0.2‰; mean ± SD) and silver sulfide (IAEA-S-1; δ34S = −0.3‰) were used as primary analytical standards. Sulfanilic acid (Sigma-Aldrich; δ13C = −25.6 ± 0.4‰; δ15N = −0.13 ± 0.4‰; δ34S = 5.9 ± 0.5‰; means ± SD) was used as secondary analytical standard. Standard deviations on multi-batch replicate measurements of secondary and internal lab standards (amphipod crustacean muscle) analysed interspersed with samples (one replicate of each standard every 15 analyses) were 0.2‰ for both δ13C and δ15N and 0.5‰ for δ34S.
Data treatment
Unless noted otherwise, all values are presented as mean ± SD. Differences in δ13C, δ15N and δ34S were tested using hypothesis-based comparison procedures. D′Agostino & Pearson and Shapiro-Wilk normality tests revealed that several datasets did not follow a Gaussian distribution. Non-parametric procedures (Mann-Whitney U test when 2 groups were compared, Kruskal-Wallis one-way analysis of variance followed by Dunn’s post-hoc test when 3 groups or more were compared) were therefore applied. All statistical analyses were conducted using Prism 6.05 (GraphPad Software, La Jolla, U.S.A.). In the vast majority of cases, no inter-site difference in δ13C, δ15N or δ34S of consumers or food items was found, motivating our decision to pool all measurements made in 2014–2015 (Supplementary Information S3; Table 1).
To quantify resource use by primary consumers and omnivores, we used the Bayesian SIAR (Stable Isotope Analysis in R) mixing model20. Models were built only for 2014–2015 data, as replication for the 2013–2014 samples was low (Table 1). Four food sources were held for modelling purposes: suspended particulate organic matter, benthic biofilm, sympagic algae and Himantothallus grandifolius blades. Phyllophora antarctica blades and Pygoscelis adeliae guano were discarded because of their extreme δ13C and δ15N values, respectively (see “Results” section for details). Himantothallus grandifolius stipes and holdfasts were discarded because their tough tissues likely renders them unpalatable for most marine invertebrates. Primary consumers and omnivores were selected by analysing their δ15N values (see “Results” section for more details). Considering the highest mean δ15N found in food items (suspended particulate organic matter; δ15N = 6.71‰) and a mean N trophic enrichment factor (TEF) of 2.30‰58, we considered that organisms were primary consumers or omnivores when their δ15N was less than 2.30‰ over suspended particulate organic matter, i.e. equal or inferior to 8.71‰. It was the case for 18 invertebrate taxa (Table 1). Since there are no specific TEFs for the taxa studied here, we used widely applicable values, i.e. 0.4 ± 1.2‰ for C, 2.3 ± 1.6‰ for N and 0.5 ± 1.9‰ for S (mean ± SD in each case)58. Models were run using the SIAR 4.2 package in R 3.3.259. Iteration numbers and burn-in size were set at 106 and 105, respectively. Two modelling scenarios were considered: one using δ13C, δ15N and δ34S, and the other using only δ13C and δ15N. Model diagnostics surprisingly indicated that the scenario using all three isotopic ratios showed poorer performance. This might be caused by a combination of factors: 1) δ34S values of food items showed a high variability (Table 1); 2) δ34S values of sympagic algae and biofilm, as well as those of H. grandifolius blades and SPOM were statistically identical (Dunn’s post-hoc test, p = 0.2387 and p > 0.9999, respectively), leading to poor discrimination of those sources by the model; and 3) experimental measurements of sulfur trophic fractionation in aquatic consumers are extremely scarce, which forced us to use a supposedly generally applicable TEF that might not be fully suitable.
The scenario using only δ13C and δ15N was retained and presented here. Model solutions were presented using credibility intervals of probability density function distributions20. Benthic biofilm and H. grandifolius were treated as different food items for modelling. However, in order to have a proxy of total benthic production inputs to invertebrate diet, their contributions were summed a posteriori and referred to as “benthic sources”. When relevant, direct pairwise comparisons of model-estimated contributions were performed. Those comparisons were considered meaningful when probability of occurrence exceeded 95%.
To estimate trophic position (TP) of invertebrates, we used the Bayesian model tRophicPosition 0.5.0.1000 20 in R 3.3.221. Trophic position estimates were performed only for 2014–2015 data, as replication for 2013–2014 samples was low (Table 1). Models were run using δ13C and δ15N values of consumers, TEFs of 0.4 ± 1.2‰ for C and 2.3 ± 1.6‰ for N, and taking into account two baseline items directly60. For taxa for which SIAR modelling had been performed, model output was used to select the two most relevant baseline items (i.e. the two items contributing the most to animal diet). For others, sympagic algae and suspended particulate organic matter were used as baseline items. Trophic position of basal food items was set to 1.0, meaning that a TP of 2.0 represents a primary consumer, 3.0 a secondary consumer, etc. For each taxon, two parallel chains were sampled with 10000 adaptive iterations. Model solutions were presented using credibility intervals of probability density function distributions. When relevant, direct pairwise comparisons of model-estimated trophic positions were performed. Those comparisons were considered meaningful when probability of occurrence exceeded 95%.
Data Availability
All data supporting this article are openly available via the Antarctic Biodiversity Information Facility (ANTABIF, www.biodiversity.aq) at https://ipt.biodiversity.aq/resource?r=ddu_isotopes_verso_2013_2015. They are also registered at the Global Biodiversity Information Facility (GBIF) under UUID 90f2713a-79ac-4d96–9a66-889a5fb9abb1, and freely accessible at https://doi.org/10.15468/wgfw0h.
References
Barnes, D. K. A. & Peck, L. S. Vulnerability of Antarctic shelf biodiversity to predicted regional warming. Clim. Res. 37, 149–163 (2008).
Portner, H. O., Peck, L. & Somero, G. Thermal limits and adaptation in marine Antarctic ectotherms: an integrative view. Philos. Trans. R. Soc. B Biol. Sci. 362, 2233–2258 (2007).
Parkinson, C. L. & Cavalieri, D. J. Antarctic sea ice variability and trends, 1979–2010. Cryosphere 6, 871–880 (2012).
Turner, J. et al. Antarctic climate change during the last 50 years. Int. J. Climatol. 25, 279–294 (2005).
Massom, R. A. & Stammerjohn, S. E. Antarctic sea ice change and variability - Physical and ecological implications. Polar Sci. 4, 149–186 (2010).
King, J. A. Resolution of the Antarctic paradox. Nature 505, 491–492 (2014).
Turner, J., Harangozo, S. A., Marshall, G. J., King, J. C. & Colwell, S. R. Anomalous atmospheric circulation over the Weddell Sea, Antarctica during the Austral summer of 2001/02 resulting in extreme sea ice conditions. Geophys. Res. Lett. 29, 13-1–13–4 (2002).
Fraser, A. D., Massom, R. A., Michael, K. J., Galton-Fenzi, B. K. & Lieser, J. L. East antarctic landfast sea ice distribution and variability, 2000-08. J. Clim. 25, 1137–1156 (2012).
Fripiat, F., Sigman, D. M., Massé, G. & Tison, J. L. High turnover rates indicated by changes in the fixed N forms and their stable isotopes in Antarctic landfast sea ice. J. Geophys. Res. Ocean. 120, 3079–3097 (2015).
Massom, R. A. et al. Fast ice distribution in Adélie Land, East Antarctica: Interannual variability and implications for emperor penguins Aptenodytes forsteri. Mar. Ecol. Prog. Ser. 374, 243–257 (2009).
Barbraud, C. & Weimerskirch, H. Antarctic birds breed later in response to climate change. Proc. Natl. Acad. Sci. 103, 6248–6251 (2006).
Kusahara, K. et al. Modeling Ocean–Cryosphere Interactions off Adélie and George V Land, East Antarctica. J. Clim. 30, 163–188 (2017).
Clark, G. F., Marzinelli, E. M., Fogwill, C. J., Turney, C. S. M. & Johnston, E. L. Effects of sea-ice cover on marine benthic communities: a natural experiment in Commonwealth Bay, East Antarctica. Polar Biol. 38, 1213–1222 (2015).
Norkko, A. et al. Trophic structure of coastal Antarctic food webs associated with changes in sea ice and food supply. Ecology 88, 2810–2820 (2007).
Michel, L. N., David, B., Dubois, P., Lepoint, G. & De Ridder, C. Trophic plasticity of Antarctic echinoids under contrasted environmental conditions. Polar Biol. 39, 913–923 (2016).
Wing, S. R., McLeod, R. J., Leichter, J. J., Frew, R. D. & Lamare, M. D. Sea ice microbial production supports Ross Sea benthic communities: Influence of a small but stable subsidy. Ecology 93, 314–323 (2012).
Calizza, E., Careddu, G., Sporta Caputi, S., Rossi, L. & Costantini, M. L. Time- and depth-wise trophic niche shifts in Antarctic benthos. PLoS One 13, e0194796 (2018).
Elton, C. S. Animal Ecology, https://doi.org/10.1098/rstb.2010.0107 Animal ecology (MacMillan, 1927).
Parnell, A. C. et al. Bayesian stable isotope mixing models. Environmetrics 24, 387–399 (2013).
Parnell, A. C., Inger, R., Bearhop, S. & Jackson, A. L. Source partitioning using stable isotopes: Coping with too much variation. PLoS One 5, e9672 (2010).
Quezada-Romegialli, C. et al. tRophicPosition, an R package for the Bayesian estimation of trophic position from consumer stable isotope ratios. Methods Ecol. Evol. 9, 1592–1599 (2018).
Knox, G. A. Primary Production and Consumption in McMurdo Sound, Antarctica. In Antarctic Ecosystems 115–128, https://doi.org/10.1007/978-3-642-84074-6_11 (Springer, 1990).
Riebesell, U., Schloss, I. & Smetacek, V. Aggregation of algae released from melting sea ice: implications for seeding and sedimentation. Polar Biol. 11, 239–248 (1991).
McMahon, K. W. et al. Benthic community response to ice algae and phytoplankton in Ny Ålesund, Svalbard. Mar. Ecol. Prog. Ser. 310, 1–14 (2006).
Bruhn, A. et al. Crude fucoidan content in two North Atlantic kelp species, Saccharina latissima and Laminaria digitata—seasonal variation and impact of environmental factors. J. Appl. Phycol. 29, 3121–3137 (2017).
Duggins, D. O. & Eckman, J. E. Is kelp detritus a good food for suspension feeders? Effects of kelp species, age and secondary metabolites. Mar. Biol. 128, 489–495 (1997).
Dunton, K. H. δ15N and δ13C Measurements of Antarctic Peninsula Fauna: Trophic Relationships and Assimilation of Benthic Seaweeds. Am. Zool. 41, 99–112 (2001).
Corbisier, T. N., Petti, M. A. V., Skowronski, R. S. P. & Brito, T. A. S. Trophic relationships in the nearshore zone of Martel Inlet (King George Island, Antarctica): δ13C stable-isotope analysis. Polar Biol. 27, 75–82 (2004).
Pasotti, F. et al. Benthic trophic interactions in an Antarctic shallow water ecosystem affected by recent glacier retreat. PLoS One 10, e0141742 (2015).
Gillies, C. L., Stark, J. S., Johnstone, G. J. & Smith, S. D. A. Carbon flow and trophic structure of an Antarctic coastal benthic community as determined by δ13C and δ15N. Estuar. Coast. Shelf Sci. 97, 44–57 (2012).
Peck, L. S., Convey, P. & Barnes, D. K. A. Environmental constraints on life histories in Antarctic ecosystems: Tempos, timings and predictability. Biol. Rev. Camb. Philos. Soc. 81, 75–109 (2006).
Agüera, A., Collard, M., Jossart, Q., Moreau, C. & Danis, B. Parameter estimations of Dynamic Energy Budget (DEB) model over the life history of a key Antarctic species: The Antarctic sea star Odontaster validus Koehler, 1906. PLoS One 10, e0140078 (2015).
Agüera, A., Ahn, I.-Y., Guillaumot, C. & Danis, B. A Dynamic Energy Budget (DEB) model to describe Laternula elliptica (King, 1832) seasonal feeding and metabolism. PLoS One 12, e0183848 (2017).
Fry, B. Stable Isotope Ecology. (Springer, 2006).
Hairston, N. G. & Hairston, N. G. Cause-Effect Relationships in Energy Flow, Trophic Structure, and Interspecific Interactions. Am. Nat. 142, 379–411 (1993).
Post, D. M. Using stable isotopes to estimate trophic position: models, methods, and assumptions. Ecology 83, 703–718 (2002).
Vander Zanden, M. J. & Rasmussen, J. B. Primary consumers δ13C and δ15N and the trophic position of aquatic consumers. Ecology 80, 1395–1404 (1999).
Smale, D. A., Barnes, D. K. A., Fraser, K. P. P., Mann, P. J. & Brown, M. P. Scavenging in Antarctica: Intense variation between sites and seasons in shallow benthic necrophagy. J. Exp. Mar. Bio. Ecol. 349, 405–417 (2007).
Calizza, E., Costantini, M. L., Rossi, D., Carlino, P. & Rossi, L. Effects of disturbance on an urban river food web. Freshw. Biol. 57, 2613–2628 (2012).
McMeans, B. C., McCann, K. S., Humphries, M., Rooney, N. & Fisk, A. T. Food Web Structure in Temporally-Forced Ecosystems. Trends Ecol. Evol. 30, 662–672 (2015).
Sterner, R. W. & Elser, J. J. Ecological Stoichiometry: The Biology of Elements from Molecules to the Biosphere. (Princeton University Press, 2002).
Webster, N. S. & Thomas, T. The Sponge Hologenome. MBio 7, 1–14 (2016).
Weisz, J. B., Lindquist, N. & Martens, C. S. Do associated microbial abundances impact marine demosponge pumping rates and tissue densities? Oecologia 155, 367–376 (2008).
Iken, K., Brey, T., Wand, U., Voigt, J. & Junghans, P. Food web structure of the benthic community at the Porcupine Abyssal Plain (NE Atlantic): A stable isotope analysis. Prog. Oceanogr. 50, 383–405 (2001).
Jansen, J. et al. Abundance and richness of key Antarctic seafloor fauna correlates with modelled food availability. Nat. Ecol. Evol. 2, 71–80 (2018).
Ortiz, M. et al. Quantifying keystone species complexes: Ecosystem-based conservation management in the King George Island (Antarctic Peninsula). Ecol. Indic. 81, 453–460 (2017).
Williams, S. E., Shoo, L. P., Isaac, J. L., Hoffmann, A. A. & Langham, G. Towards an Integrated Framework for Assessing the Vulnerability of Species to Climate Change. PLoS Biol. 6, e325 (2008).
Barnes, D. K. A. & Conlan, K. E. Disturbance, colonization and development of Antarctic benthic communities. Philos. Trans. R. Soc. B Biol. Sci. 362, 11–38 (2007).
Brockington, S. & Peck, L. Seasonality of respiration and ammonium excretion in the Antarctic echinoid Sterechinus neumayeri. Mar. Ecol. Prog. Ser. 219, 159–168 (2001).
Brockington, S., Clarke, A. & Chapman, A. L. G. Seasonality of feeding and nutritional status during the austral winter in the Antarctic sea urchin Sterechinus neumayeri. Mar. Biol. 139, 127–138 (2001).
Pearse, J. S., McClintock, J. B. & Bosch, I. Reproduction of Antarctic Benthic Marine Invertebrates: Tempos, Modes, and Timing. Am. Zool. 31, 65–80 (1991).
Stanwell-Smith, D. & Barnes, D. K. Benthic community development in Antarctica: recruitment and growth on settlement panels at Signy Island. J. Exp. Mar. Bio. Ecol. 212, 61–79 (1997).
Mateo, M. A., Serrano, O., Serrano, L. & Michener, R. H. Effects of sample preparation on stable isotope ratios of carbon and nitrogen in marine invertebrates: implications for food web studies using stable isotopes. Oecologia 157, 105–115 (2008).
Jaschinski, S., Hansen, T. & Sommer, U. Effects of acidification in multiple stable isotope analyses. Limnol. Oceanogr. Methods 6, 12–15 (2008).
Hedges, J. I. & Stern, J. H. Carbon and nitrogen determinations of carbonate-containing solids. Limnol. Oceanogr. 29, 657–663 (1984).
Connolly, R. M. & Schlacher, T. A. Sample acidification significantly alters stable isotope ratios of sulfur in aquatic plants and animals. Mar. Ecol. Prog. Ser. 493, 1–8 (2013).
Coplen, T. B. Guidelines and recommended terms for expression of stable-isotope-ratio and gas-ratio measurement results. Rapid Commun. Mass Spectrom. 25, 2538–2560 (2011).
McCutchan, J. H. J., Lewis, W. M. Jr., Kendall, C. & McGrath, C. C. Variation in trophic shift for stable isotope ratios of carbon, nitrogen, and sulfur. Oikos 102, 378–390 (2003).
R Core Team, N. R: A Language and Environment for Statistical Computing. (R Foundation for Statistical Computing, 2016).
Cabana, G. & Rasmussen, J. B. Modelling food chain structure and contaminant bioaccumulation using stable nitrogen isotopes. Nature 372, 255–257 (1994).
Acknowledgements
This research was funded by the Belgian Science Policy Office (BELSPO) through the vERSO project (http://www.rectoversoprojects.be.be, contract nr. BR/132/A1/vERSO). Access to Dumont-d’Urville station was granted through REVOLTA 1124, a research program co-funded by the French Polar Institute Paul-Emile Victor (IPEV), MNHN, Sorbonne Université and CNRS. P.D. and G.L. are respectively Belgian Fund for Scientific Research (F.R.S-FNRS) research director and research associate. Authors warmly thank all involved IPEV staff for their efficient logistical support during the planning and execution of the field campaigns. They also thank Eduardo da Forno and Elsa Freschet for sample collection during the 2013–14 sampling campaign, Svetlana Vasic for her help during sample preparation, Daniela Zeppilli (Laboratoire Environnement Profond – Ifremer) for identifying the nematodes and Anton P. Van de Putte (OD Nature - Royal Belgian Institute of Natural Sciences) for his help with data archiving. This paper is contribution nr. 30 to the vERSO project and MARE publication nr. 388.
Author information
Authors and Affiliations
Contributions
L.M., B.D., M.E., C.G. and G.L. conceived the study. L.M., P.D., P.J., C.G. and J.F. performed the sampling. L.M. and G.L. performed the stable isotope analysis. L.M. analysed the data and prepared the figures with input from all authors. All authors significantly contributed to writing the manuscript and approved its final version.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Michel, L.N., Danis, B., Dubois, P. et al. Increased sea ice cover alters food web structure in East Antarctica. Sci Rep 9, 8062 (2019). https://doi.org/10.1038/s41598-019-44605-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-44605-5
This article is cited by
-
Using latent behavior analysis to identify key foraging areas for Adélie penguins in a declining colony in West Antarctic Peninsula
Marine Biology (2024)
-
Extreme El Niño southern oscillation conditions have contrasting effects on the body condition of five euphausiid species around the northern Antarctic Peninsula during winter
Polar Biology (2023)
-
Distribution of trace elements in benthic infralittoral organisms from the western Antarctic Peninsula reveals no latitudinal gradient of pollution
Scientific Reports (2021)
-
A novel approach to quantifying trophic interaction strengths and impact of invasive species in food webs
Biological Invasions (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.