Abstract
Intracranial metastases are the most frequent brain tumor with recurrence rates after treatment of around 40–60%. Age is still considered a determinant of treatment and prognosis in this pathology. Recent studies analyzing the impact of metastasectomy in elderly patients focused on reporting perioperative mortality and morbidity rates but not on the evaluation of oncological outcome parameters. Aim of this study is to determine risk factors for in-brain local recurrence after brain surgery in this sub-population. From October 2009 until September 2016 all patients aged 65 years and above with histopathologically confirmed metastasis after surgical resection were retrospectively studied. Clinical, radiological and perioperative information was collected and statistically analysed. Follow-up consisted of clinical and radiological assessment every 3-months following surgery. 78 patients were included, of these 50% were female (39 patients). Median age was 71 years (66–83). Early postoperative-MRI verified a complete surgical resection in 41 patients (52.6%) and showed a tumor-remnant in 15 patients (19.2%). In 22 patients the MRI result was inconclusive (28.2%). None of the patients experienced severe complications due to surgery. The median postoperative NIHSS was adequate 1 ± 1.4 (0–6), nonetheless, insignificantly improved in comparison to the preoperative NIHSS (p = 0.16). A total of 20 patients (25.6%) presented local recurrence. The only statistically significant factor for development of local in-brain recurrence after resection of cerebral metastases in patients above 65 years of age was a tumor-remnant in the early postoperative MRI (p = 0.00005). Median overall survival was 13 months. Local in-brain recurrence after surgical resection of a cerebral metastasis in patients above 65 years of age was 25.6%. In our analysis, tumor-remnant in early postoperative MRI is the only risk factor for local in-brain recurrence. Oncological parameters in the present cohort do not seem to differ from recent phase III studies with non-geriatric patients. Nevertheless, controlled studies on the impact of metastasectomy in elderly patients delivering high quality reliable data are required.
Similar content being viewed by others
Introduction
Although the exact incidence of cerebral metastases from solid cancers is unknown, intracerebral metastases are the most frequent brain tumors with a 3–5 times higher incidence than newly diagnosed primary malignant brain tumors each year1,2. Incidence of cerebral metastases was considered to increase from 2.8–11.1 per 100,000 population in the years before 1990 to an incidence of 7–14.3 per 100,000 population in more recent studies1. Cumulative incidence of cerebral metastases may be age-related as the highest cumulative incidence is observed in patients with primary breast cancer at the age between 20 and 39 years, in lung cancer patients at the fifth decade and in malignant melanoma patients at the sixth decade of life3. Cumulative incidence is considered to be lowest for all primary cancers in the age group above 70 years, with exception of melanoma3.
Despite the presumably lower incidence of cerebral metastases in elderly patients, incidence in this subgroup increases due to the high number of elderly patients, general increase of occurrence of cerebral metastases, improved diagnosis of brain metastases and better treatment of the primary cancer. Moreover, age above 60 years was one major risk factor for impaired overall survival (OS) in an early prospective randomized study comparing combined treatment of surgery and adjuvant whole-brain radiation therapy (WBRT) with an exclusive WBRT4. A recent individual patient data meta-analysis of 3 randomized trials of stereotactic radiosurgery (SRS) with or without WBRT for 1 to 4 cerebral metastases suggested that age might be a factor influencing the efficiency of an adjuvant WBRT following SRS. For patients <50 years of age, SRS alone favoured survival and an additional WBRT did not impact the distant in-brain progression rate. Adjuvant WBRT significantly decreased the risk of new cerebral metastases without affecting the OS in patients aged >50 years5. Some recent retrospective studies reported age as a risk factor for a reduced survival6. Age is therefore still considered to be a determinant of treatment and prognosis in this pathology in recent guidelines7.
The Dutch prospective and randomized study (surgery and WBRT vs. WBRT alone) identified age as a major determinant for OS4,8. However, patients included in this study were recruited between 1985 and 1991, a preoperative MRI to diagnose single intracerebral metastases was not mandatory and histological confirmation of the presumed metastasis was not necessarily required prior to treatment. Since the end of the 1980s, advancements in pre- and postoperative diagnosis and surgical techniques have been made9,10,11,12,13,14,15,16,17,18,19. Recent studies analyzing the impact of metastasectomy in elderly patients focused on reporting perioperative mortality and morbidity rates but not on evaluation of oncological outcome parameters.
Aim of the present retrospective study was therefore to analyze the progression-free and overall survival, rate of local in-brain progression and complications after surgery of brain metastases in elderly patients over the age of 65.
Methods and Materials
Study design, inclusion and exclusion criteria
This study represents a clinical and radiological retrospective analysis of a consecutive series of patients treated for intracranial metastases at a large European tertiary care centre. This study involved the review of clinical records as part of medical care. We retrospectively studied and analysed medical records and their corresponding radiological diagnostic tests of every patient presenting with histologically confirmed brain metastases from October 2009 until September 2016.
Inclusion criteria were: (1) histological diagnosis of an intracranial secondary tumor, (2) operated only at our institution, (3) between October 2009 and September 2016, (4) pre- and postoperative MRI (pre- and post gadolinium-enhanced T1-weighted sequences, T2-weighted sequences and Fluid Attenuated Inverse Recovery (FLAIR)), (5) clinical and radiological follow-up at our institution, (6) age older than 65 years.
Exclusion criteria included: (1) other tumor than cerebral metastases (primary brain tumor, small cell lung cancer, neuroendocrine or sarcoma metastases and lymphoma) (2) prior surgical treatment at a different institution, (3) exclusively palliative or no neuro-oncological treatment, (4) previous treatment with the following: biopsy, stereotactic biopsy, radiotherapy and/or SRS, and (5) preoperative diagnosis of leptomeningeal carcinomatosis (LC).
Surgery
All patients included in this study received surgical treatment for one cerebral metastases, although patients with more than one metastasis were also considered for surgery. Indication for surgical treatment of one cerebral metastasis in patients with 2 or more cerebral metastases was (1) symptomatic lesions, (2) mass effects (3) no history of systemic disease or unclear diagnosis, (4) intratumoral hemorrhage, and (5) large posterior fossa tumors.
Intraoperative frozen sections were obtained in all patients. After the histological diagnosis of a cerebral metastasis by frozen section, standard white-light assisted – and if possible – en bloc circumferential resection was performed. Surgery integrated the intraoperative use of ultrasound (ProSound alpha7, Hitachi Aloka Medical America Inc., U.S.A.), neuro-navigation (Brainlab Navigation System, Brainlab AG, München, Germany) and awake surgery using an asleep-awake-asleep protocol as described before for patients with eloquent located cerebral metastases11. An eloquent brain region was defined as a cortical or subcortical brain area where we expect intraoperative stimulation to elicit changes in neurologic conditions (particularly regarding speech, movement and tactile sensation) or to elicit a response in electrophysiological recordings in corresponding areas11,20. Adjuvant therapy was individually decided upon in every case after histological diagnosis in an interdisciplinary tumour board. Recommendations for adjuvant radiation depended on various parameters such as number of cerebral lesions, degree of resection, Karnofsky Performance Scale (KPS) and the patient preference.
Data collection, follow-up and definition of outcome measures
Additionally, pre- and postoperative clinical characteristics of the patients, preoperative performance scale, localization, number of metastases, characteristics and classification of the tumor, treatment and incidence of each primary tumor, extent of surgical resection, fluorescence of the tumor, use of intraoperative monitoring, perioperative complications, periodically follow-up visits, recurrence, time to recurrence, loco-regional or distant metastases, neoadjuvant/adjuvant/palliative therapy, survival, cause and date of death (if applicable) were collected from charts and electronic records and analyzed.
Pre-, postoperative and follow up clinical assessments were standardized using the National Health Institute of Stroke Scale (NIHSS). Degree of surgical resection was verified by early postoperative 1.5T-MRI as described before16. A senior neurosurgeon and neurological radiologist performed the radiological analysis. After surgery, patients were followed-up including a contrast-enhanced MRI every 3 months.
Local in-brain-progression/recurrence was defined according to the RANO criteria21, as an increase by 20% from the initial longest diameter of the target lesion with an absolute 5 mm growth, as measured in contrast-enhanced T1, T2 and diffusion sequences. Radiological postoperative evaluation of the resection cavity was defined as inconclusive when characteristics such as postoperative blood residues, pronounced vessels, reactive tissue, suboptimal image quality were present16. Distant in-brain-progression was defined as appearance of new metastasis distant to the site of the resected metastasis (distance to the resection cavity of at least 2 cm). Dural inclusion of cerebral metastasis and leptomeningeal carcinomatosis (LC) were interpreted as different radiological entities. Dural inclusion explicitly represented radiological or intraoperatively verified contact of the dura with the brain metastases (BM) with no additional radiological signs of LC. LC was diagnosed by cranial MRI showing a diffuse enhancement of meninges or by lumbar puncture and confirmation of malignant tumour cells in the cerebrospinal fluid (CSF). Time to in-brain-progression was defined as time between surgery and diagnosis of the in-brain-tumour progression. The overall survival was considered as time span between surgery and death.
Statistical analysis
Follow-up ended in April 2018 and the database was finalized shortly thereafter. All statistical analyses were performed with SPSS software (Version 22.0, -IBM-, USA). Data is presented as the median and standard deviation. Descriptive statistics including mean and standard error of mean were calculated for all continuous variables. The Chi-Square-test was used in nominal variables to identify significant differences. Contingency tables were performed according to the number of possible answers. As multiple statistical testing was performed, the significance level according to Šidák’s and Bonferroni’s correction: Šidák’s and Bonferroni-correction revealed an adjusted p-value of 0.0051 or 0.005, respectively.
Ethical statement
Data acquisition, radiological interpretation, as well as an analysis of both, were approved by the institutional research ethics board (Medical Faculty, Heinrich-Heine- University, Nr. 5713). For every patient treated at our institution with any brain pathology we obtained an informed consent allowing a retrospective analysis of the data, as well as the inclusion of the pathological specimen in an institutional tumor-bank.
Results
Patient’s characteristics
A total of 78 patients aged 65 or above were included; of these 50% were female (39 patients). The median age was 71 years (66–83). In our series, 50 patients (64.1%) presented a single metastasis, 18 patients (23.1%) had two or three cerebral metastases and 10 patients (12.8%) presented more than three metastases. All patients were required to be in good clinical condition to be eligible for surgery. Every patient had a preoperative Karnofsky Performance Scale (KPS) of 70 or more. The median preoperative KPS was 90 ± 9.8 and the median pre-operative NIHSS was 1 ± 1.9 (0–10).
The most common primary tumor type was non-small cell lung carcinoma (NSCLC) in 35 patients (44.9%). Adenocarcinoma was the most frequent histological diagnosis and was present in 58 patients (74.4%). Epidemiological features of the present patient population are summarized in Table 1.
Treatment
Surgery was performed as an en-bloc resection in 40 patients (51.2%) and as piece-meal resection in 38 patients (48.7%). Early postoperative-MRI verified a complete removal in 41 patients (52.6%) and showed a tumor-remnant in 15 patients (19.2%). In 22 patients the MRI result was inconclusive (28.2%).
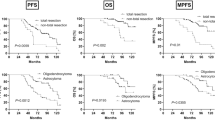
No patient experienced a severe complication due to surgery, such as surgery-associated death or cardio-pulmonary complications. The median post-operative NIHSS was 1 ± 1.4 (0–6). The median post-operative NIHSS was 1 ± 1.4 (0–6). Therefore, median post-operative NIHSS was not significantly improved in comparison to the pre-operative NIHSS (p = 0.16; Fig. 1) The NIHSS improved after surgery in 28 patients (35.9%), decreased in 9 patients (11.6%) and was unchanged in 41 patients (52.6%).
As local adjuvant treatment, almost half of the patients received whole brain radiation therapy (37 patients, 47.4%) and in 15 patients no adjuvant therapy was performed (summarized in Table 1).
Local in-brain progression and overall survival
A total of 20 patients above 65 years of age (25.6%) presented a local recurrence with a median time-to-local recurrence of 3 ± 2.9 months (0–10 months). 23 patients (26.9%) developed distant metastases and 13 patients (16.7%) carcinomatous meningitis. (See Table 2).
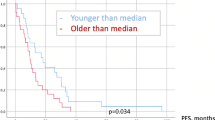
According to statistical correlation, the only factor statistically significant for the development of local in-brain recurrence after resection of cerebral metastases in patients above 65 years of age was a tumor-remnant in the early postoperative MRI (p = 0.00005). In our series no other risk factors such as sex, localization, number of metastases, preoperative KPS and NIHSS, postoperative NIHSS, primary tumor site, histology, type of surgical resection and type of adjuvant radiation, could be identified (each p > 0.05).
A total of 8 patients (10.3% on May 22nd 2018) were still alive at the end of the study and continued with the scheduled follow-up visits. Two of these 8 patients suffered from local recurrence. The median OS was 13 months. Kaplan Meier estimates for OS and local in-brain progression are shown in Fig. 2.
Discussion
The main results of our analysis are as follows: (1) the local in-brain recurrence after surgical resection of a BM in patients above 65 years of age is 25.6% with a median time to occurrence of three months; (2) in patients above 65 years of age tumor-remnant in an early postoperative MRI was the only risk factor for local in-brain recurrence and (3) the median overall survival was 13 months in the present series.
Most studies analyzing the impact of cerebral metastasis resection focus on reporting perioperative morbidity and mortality rates but omit oncological outcome parameters such as (local) in-brain progression and survival. Median overall survival was 13 months after metastasectomy in the present retrospective series of elderly patients aged 65 years and over. It ranged between 2.8 and 18 months in recent prospective randomized and controlled phase III trials including surgery as treatment of cerebral metastases (e.g. 11.6 and 12.2 months in the NCCTG N107C/CEC·3 trial by Brown et al., 2017; 17 and 18 months in the study by Mahajan et al. 2017; 2.8 months in the trial by Roos et al., 2011, 10.7 and 10.9 months in the EORTC 22952–26001 study by Kocher and coworkers, 2011, respectively). Although results from retrospective studies have limited comparability to those derived from prospective randomized and controlled phase III trials, median survival in the present analysis seems to be comparable to those in recent phase III studies. However, overall survival was related to the treatment of cerebral metastases only in early phase III trials from the 1990s (Patchell et al., 1990; Vecht et al., 1993) but not in more recent phase III trials (e.g. Kocher et al., 2011; Brown et al., 2017; Mahajan et al., 2017). Occurrence of single cerebral metastasis and the choice of their treatment modalities may therefore be insufficient to predict survival of patients – even in elderly patients. In contrast, treatment of cerebral metastases is well known to influence the local and distant in-brain progression as well as patients’ quality of life. In the present study, local recurrence rate was 25.6%, the distant development rate was 26.9%. These rates are congruent with our previous results and the recurrence rates reported in prospective randomized and controlled phase III trials. Therefore, elderly patients have comparable in-brain progression and overall survival as reported from previous oncologic patient cohorts. After thorough analysis, tumor-remnant in the early postoperative MRI as described in16 was the only statistically significant risk factor for local recurrence. Relevance of early postoperative MRI in oncological patients has already been defined16,22. Although many factors (e.g. surgical technique, number of metastases, local control) have been proposed as the cause of local recurrence and distant development or carcinomatous meningitis22,23,24,25,26,27, we could not establish another association or correlation in patients above 65 years of age.
In the present population, we observed no severe complication and no case fatalities within the first 30 days after surgery. The pre- and postoperative NIHSS, as well as KPS and follow-up visits showed no immediate or mediate deteriorations or complications. The median pre- and postoperative NIHSS was 1 without significant differences suggesting no new neurological deficits due to metastasectomy and a favorable overall surgical outcome. Perioperative morbidity and mortality were considered to be elevated in elderly patients in some neurosurgical but non-oncological series [e.g.28]. However, this may only partially be true for geriatric patients with cerebral metastases. Within a retrospective analysis of a United States inpatient sample, 4.907 patients aged 64 years and above were identified who underwent brain metastases resection. This study concluded that surgical resection of brain metastases among the elderly up to the ninth decade of life is feasible but that age above 80 years and comorbidities were important prognostic factors for inpatient outcome29. In a retrospective observational cohort-comparison study of patients with brain metastases, complication rate was 5.7% in the geriatric cohort with 174 patients aged 70 years and over30. Several further studies reported low complication rates after surgery of elderly patients with malignant brain tumors31,32,33,34.
Limitations
Our study presents a single center experience with a reasonable number of patients, with a homogenous diagnostic and therapeutic approach allowing comparison. However, our study presents some limitations: (1) 78 geriatric patients suffering from cerebral metastases within a period of 7 years were included. This is based on a very dense net of exclusion criteria. Therefore, this cohort might not be representative for all geriatric patients. However, the present cohort is heterogeneous in terms of different primary sites and adjuvant therapies. Furthermore, a subgroup analysis for patients with urgent or acute surgery for BM was not performed. Due to the acute setting, proper planning and supplementary accessories might have been impossible to accomplish and thus have resulted in a higher probability of tumor-remnant or an increased risk of complications. (2) Extent of surgical resection was analysed by an early postoperative-MRI. In the current literature, only few retrospective studies analysed the impact of this method in diagnosing residual tumor tissue16,35. A definite conclusion regarding the resection degree was not possible in 28.2% for several reasons, e.g. residual tumor tissue could not reliably be differentiated from dilated vessels in the wall of the resection cavity, poor image quality (e.g. due to patient motion), blood in the resection. The early postoperative MRI revealed an incomplete surgical resection is in 19.2%. The comparatively high rate of incompletely and questionably completely resected metastases is in line with previous non-geriatric series16. (3) A median time-to-local recurrence of 3 ± 2.9 months (0–10 months) is fairly low. Time-to-local in brain-progression was therefore lower as in the recent phase III trials (e.g. 7.6 months and not reached in the recent phase III trial by Mahajan et al.)36. The reason for the comparatively low time-to-local in-brain progression remains unclear. One explanation might be the high rate of incomplete surgical resection or patients without any adjuvant radiation therapy (19.2% each) and the significant correlation between verification of tumor remnants on an early MRI and a later local in-brain progression. We are not aware of any studies directly analyzing a potential correlation between local recurrence and death. Several recent phase III studies addressed the local control and/or the overall survival after treatment of 1–4 cerebral metastases. Except the study by El Gantery and coworkers (2014), none of the studies observed an effect of the therapy modality on the overall survival but nearly all studies showed a significant effect on the local control9,12,29,30,37. (4) Current prognostic indicators were not performed or analyzed in our population. A comparative analysis between our data and known literature cannot be performed. (5) Moreover, preoperative evaluation of the geriatric population should be required in order to increase quality of care, identify unknown entities, reduce complications and improve outcome38. There are many tools to preoperatively assess geriatric patients. Although there are still some discrepancies39 and new innovative tools are being studied40, the most common and thorough tool utilized to identify those patients with higher risk for worse outcome or a greater benefit from surgical treatment is the comprehensive geriatric assessment (CGA)41. Interestingly, most of the tools (if not all) use the KPS described in 1948 as a base42. Still, a unified guideline for this subgroup of patients has yet to be established. In our study, we did not perform any geriatric assessment. Nonetheless, the pre- and postoperative NIHSS, as well as KPS and follow-up visits showed no immediate or mediate deteriorations or complications, and more importantly, they represent an adequate parameter. However designed for other reasons and purposes37,43,44, the NIHSS seems a feasible tool. (5) The influence of comorbidities, multi-organ metastatic disease, current medication and other elective or palliative surgeries was not taken into account. (7) Controlled or absent systemic neoplastic disease was assumed according to the clinical status and routine blood work. No evidence was established to prove that fact. (8) Another subgroup analysis of patients, in whom a palliative intervention therapy was performed, was also not analyzed separately. How this affects the course of disease, the type of therapy received additionally or the influence on overall survival remains unknown. (9) Patients’ adherence, compliance, complications or changes altogether regarding adjuvant therapy were not analyzed. (10) Preoperative elaborate analysis of geriatric patients was not performed.
Conclusion
Local in-brain recurrence after surgical resection of a BM in patients above 65 years of age was 25.6%. Tumor-remnant in early postoperative MRI is the only risk factor for local in-brain recurrence. Mean overall survival was 13 months. Oncological parameters in the present cohort seem not to differ from recent phase III studies with non-geriatric patients. As reliable data are lacking, controlled studies analyzing the impact of metastasectomy in elderly patients are required.
Disclosure of potential conflicts of interest
Prof. Sabel and PD Dr. Rapp work as consultants for Johnson & Johnson Company and Integra Company. Dr. Dibué-Adjei is an employee of LivaNova PLC, manufacturer of vagus nerve stimulators. All other authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
References
Kong, X.-T., Alexandru, D. & Bota, D. A. Epidemiology of Central Nervous System Metastases. In: Hayat MA (ed) Brain Metastases from Primary Tumors. Epidemiology, Biology, and Therapy, vol 1. Academic Press, pp. 11–23 (2014).
Sawaya, R. Considerations in the diagnosis and management of brain metastases. Oncology (Williston Park) 15, 1144–1154, 1157–1148; discussion 1158, 1163–1145 (2001).
Barnholtz-Sloan, J. S. et al. Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. J Clin Oncol 22, 2865–2872, https://doi.org/10.1200/JCO.2004.12.149 (2004).
Noordijk, E. M. et al. The choice of treatment of single brain metastasis should be based on extracranial tumor activity and age. Int J Radiat Oncol Biol Phys 29, 711–717 (1994).
Sahgal, A. et al. Phase 3 trials of stereotactic radiosurgery with or without whole-brain radiation therapy for 1 to 4 brain metastases: individual patient data meta-analysis. Int J Radiat Oncol Biol Phys 91, 710–717, https://doi.org/10.1016/j.ijrobp.2014.10.024 (2015).
Stark, A. M., Stohring, C., Hedderich, J., Held-Feindt, J. & Mehdorn, H. M. Surgical treatment for brain metastases: Prognostic factors and survival in 309 patients with regard to patient age. Journal of clinical neuroscience: official journal of the Neurosurgical Society of Australasia 18, 34–38, https://doi.org/10.1016/j.jocn.2010.03.046 (2011).
Soffietti, R. et al. Diagnosis and treatment of brain metastases from solid tumors: guidelines from the European Association of Neuro-Oncology (EANO). Neuro Oncol 19, 162–174, https://doi.org/10.1093/neuonc/now241 (2017).
Vecht, C. J. et al. Treatment of single brain metastasis: radiotherapy alone or combined with neurosurgery? Ann Neurol 33, 583–590, https://doi.org/10.1002/ana.410330605 (1993).
Chacko, A. G., Kumar, N. K., Chacko, G., Athyal, R. & Rajshekhar, V. Intraoperative ultrasound in determining the extent of resection of parenchymal brain tumours–a comparative study with computed tomography and histopathology. Acta Neurochir (Wien) 145, 743–748; discussion 748, https://doi.org/10.1007/s00701-003-0009-2 (2003).
Coburger, J. et al. Tumor detection with 5-aminolevulinic acid fluorescence and Gd-DTPA-enhanced intraoperative MRI at the border of contrast-enhancing lesions: a prospective study based on histopathological assessment. Neurosurgical focus 36, E3, https://doi.org/10.3171/2013.11.FOCUS13463 (2014).
Kamp, M. A. et al. Proof of principle: supramarginal resection of cerebral metastases in eloquent brain areas. Acta Neurochir (Wien) 154, 1981–1986, https://doi.org/10.1007/s00701-012-1463-5 (2012).
Kamp, M. A. et al. The tumour is not enough or is it? Problems and new concepts in the surgery of cerebral metastases. Ecancermedicalscience 7, 306, https://doi.org/10.3332/ecancer.2013.306 (2013).
Kamp, M. A. et al. 5-ALA fluorescence of cerebral metastases and its impact for the local-in-brain progression. Oncotarget, https://doi.org/10.18632/oncotarget.11488 (2016)
Kamp, M. A. et al. Predictors for a further local in-brain progression after re-craniotomy of locally recurrent cerebral metastases. Neurosurgical review, https://doi.org/10.1007/s10143-017-0931-z (2017).
Kamp, M. A. et al. 5-aminolevulinic acid (5-ALA)-induced fluorescence in intracerebral metastases: a retrospective study. Acta Neurochir (Wien) 154, 223–228; discussion 228, https://doi.org/10.1007/s00701-011-1200-5 (2012).
Kamp, M. A. et al. Early postoperative magnet resonance tomography after resection of cerebral metastases. Acta Neurochir (Wien) 157, 1573–1580, https://doi.org/10.1007/s00701-015-2479-4 (2015).
Kamp, M. A. et al. Incidence of local in-brain progression after supramarginal resection of cerebral metastases. Acta Neurochir (Wien) 157, 905–910; discussion 910–901, https://doi.org/10.1007/s00701-015-2405-9 (2015).
Krieg, S. M. et al. Reliability of intraoperative neurophysiological monitoring using motor evoked potentials during resection of metastases in motor-eloquent brain regions: clinical article. Journal of neurosurgery 118, 1269–1278, https://doi.org/10.3171/2013.2.JNS121752 (2013).
Unsgaard, G. et al. Intra-operative 3D ultrasound in neurosurgery. Acta Neurochir (Wien) 148, 235–253; discussion 253, https://doi.org/10.1007/s00701-005-0688-y (2006).
Duffau, H. et al. Usefulness of intraoperative electrical subcortical mapping during surgery for low-grade gliomas located within eloquent brain regions: functional results in a consecutive series of 103 patients. Journal of neurosurgery 98, 764–778, https://doi.org/10.3171/jns.2003.98.4.0764 (2003).
Lin, N. U. et al. Response Assessment in Neuro-Oncology g Response assessment criteria for brain metastases: proposal from the RANO group. Lancet Oncol 16, e270–278, https://doi.org/10.1016/S1470-2045(15)70057-4 (2015).
Soffietti, R. et al. Diagnosis and treatment of brain metastases from solid tumors: guidelines from the European Association of Neuro-Oncology (EANO). Neuro-Oncology 19, 162–174, https://doi.org/10.1093/neuonc/now241 (2017).
Patel, A. J. et al. Factors influencing the risk of local recurrence after resection of a single brain metastasis. J Neurosurg 113, 181–189, https://doi.org/10.3171/2009.11.JNS09659 (2010).
Patel, A. J. et al. Impact of surgical methodology on the complication rate and functional outcome of patients with a single brain metastasis. J Neurosurg 122, 1132–1143, https://doi.org/10.3171/2014.9.JNS13939 (2015).
Suki, D. et al. Comparative risk of leptomeningeal disease after resection or stereotactic radiosurgery for solid tumor metastasis to the posterior fossa. J Neurosurg 108, 248–257, https://doi.org/10.3171/JNS/2008/108/2/0248 (2008).
Suki, D. et al. Comparative risk of leptomeningeal dissemination of cancer after surgery or stereotactic radiosurgery for a single supratentorial solid tumor metastasis. Neurosurgery 64, 664–674; discussion 674–666, https://doi.org/10.1227/01.NEU.0000341535.53720.3E (2009).
Vogelbaum, M. A. & Suh, J. H. Resectable brain metastases. Journal of clinical oncology: official journal of the American Society of Clinical Oncology 24, 1289–1294, https://doi.org/10.1200/JCO.2005.04.6235 (2006).
Munoz-Bendix, C. et al. Outcome following surgical treatment of chronic subdural hematoma in the oldest-old population. Neurosurgical review 40, 461–468, https://doi.org/10.1007/s10143-016-0803-y (2017).
Grossman, R. et al. Predictors of inpatient death and complications among postoperative elderly patients with metastatic brain tumors. Ann Surg Oncol 18, 521–528, https://doi.org/10.1245/s10434-010-1299-2 (2011).
Frati, A. et al. Surgical Treatment of the Septuagenarian Patients Suffering From Brain Metastases: A Large Retrospective Observational Analytic Cohort-Comparison Study. World neurosurgery 114, e565–e572, https://doi.org/10.1016/j.wneu.2018.03.034 (2018).
Brandes, A. A. & Monfardini, S. The treatment of elderly patients with high-grade gliomas. Semin Oncol 30, 58–62 (2003).
Mukerji, N. et al. Treating high grade gliomas in the elderly: the end of ageism? J Neurooncol 86, 329–336, https://doi.org/10.1007/s11060-007-9476-2 (2008).
Pirracchio, R. et al. One-year outcome after neurosurgery for intracranial tumor in elderly patients. Journal of neurosurgical anesthesiology 22, 342–346, https://doi.org/10.1097/ANA.0b013e3181e6daa2 (2010).
Rogne, S. G. et al. Intracranial tumor surgery in patients >70 years of age: is clinical practice worthwhile or futile? Acta Neurol Scand 120, 288–294, https://doi.org/10.1111/j.1600-0404.2009.01157.x (2009).
Benveniste, R. J., Ferraro, N. & Tsimpas, A. Yield and utility of routine postoperative imaging after resection of brain metastases. J Neurooncol 118, 363–367, https://doi.org/10.1007/s11060-014-1440-3 (2014).
Mahajan, A. et al. Post-operative stereotactic radiosurgery versus observation for completely resected brain metastases: a single-centre, randomised, controlled, phase 3 trial. The lancet oncology 18, 1040–1048, https://doi.org/10.1016/S1470-2045(17)30414-X (2017).
Brott, T. et al. Measurements of acute cerebral infarction: a clinical examination scale. Stroke; a journal of cerebral circulation 20, 864–870 (1989).
Tan, H. J., Saliba, D., Kwan, L., Moore, A. A. & Litwin, M. S. Burden of Geriatric Events Among Older Adults Undergoing Major Cancer Surgery. Journal of clinical oncology: official journal of the American Society of Clinical Oncology 34, 1231–1238, https://doi.org/10.1200/JCO.2015.63.4592 (2016).
Extermann, M. et al. Task Force on CGAotISoGOUse of comprehensive geriatric assessment in older cancer patients: recommendations from the task force on CGA of the International Society of Geriatric Oncology (SIOG). Crit Rev Oncol Hematol 55, 241–252, https://doi.org/10.1016/j.critrevonc.2005.06.003 (2005).
Min L. et al. Estimating Risk of Postsurgical General and Geriatric Complications Using the VESPA Preoperative Tool. JAMA Surg. https://doi.org/10.1001/jamasurg.2017.2635 (2017).
National Institutes of Health Consensus Development Conference Statement: geriatric assessment methods for clinical decision-making. Journal of the American Geriatrics Society 36, 342–347 (1988).
Karnofsky, D. A. A. W. H., Craver, L. F. & Burchenal, J. H. The use of the nitrogen mustards in the palliative treatment of carcinoma. Cancer 1, 634–656 (1948).
Appelros, P. & Terent, A. Characteristics of the National Institute of Health Stroke Scale: results from a population-based stroke cohort at baseline and after one year. Cerebrovasc Dis 17, 21–27, https://doi.org/10.1159/000073894 (2004).
Kasner, S. E. Clinical interpretation and use of stroke scales. Lancet neurology 5, 603–612, https://doi.org/10.1016/S1474-4422(06)70495-1 (2006).
Author information
Authors and Affiliations
Contributions
C.M.-B. and M.A.K. were involved in the conception and design of research approach, data acquisition, as well as drafting the manuscript. C.M.-B., M.A.K., M.R., H.-J.M., C.v.S., M.D.-A., J.F.C., H.-J.S., B.T. and M.S. participated in the analysis and interpretation of the data, revision of the manuscript and commented on the final version of the manuscript.
Corresponding author
Ethics declarations
Competing Interests
Prof. Sabel and PD Dr. Rapp work as consultants for Johnson & Johnson Company and Integra Company. Dr. Dibué-Adjei is an employee of LivaNova PLC, manufacturer of vagus nerve stimulators. All other authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Munoz-Bendix, C., Rapp, M., Mijderwijk, HJ. et al. Risk factors for in-brain local progression in elderly patients after resection of cerebral metastases. Sci Rep 9, 7431 (2019). https://doi.org/10.1038/s41598-019-43942-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-43942-9
This article is cited by
-
Clinical characteristics and risk factors of perioperative outcomes in elderly patients with intracranial tumors
Neurosurgical Review (2021)
-
Age-stratified clinical performance and survival of patients with IDH-wildtype glioblastoma homogeneously treated by radiotherapy with concomitant and maintenance temozolomide
Journal of Cancer Research and Clinical Oncology (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.